Implementation of Safe-by-Design for Nanomaterial Development and Safe Innovation: Why We Need a Comprehensive Approach
Abstract
:1. Introduction
Safe-by-Design: A Concept for Safe Innovations
- comprehensive knowledge questioning what property makes a nanomaterial or nanoproduct more or less safe;
- means to implement this knowledge in a structured way into industrial innovation processes; and
- information exchange between the involved stakeholders.
2. Design of Safe Nanomaterials and Nanoproducts
3. Bottom-Up Approach to Safe-by-Design
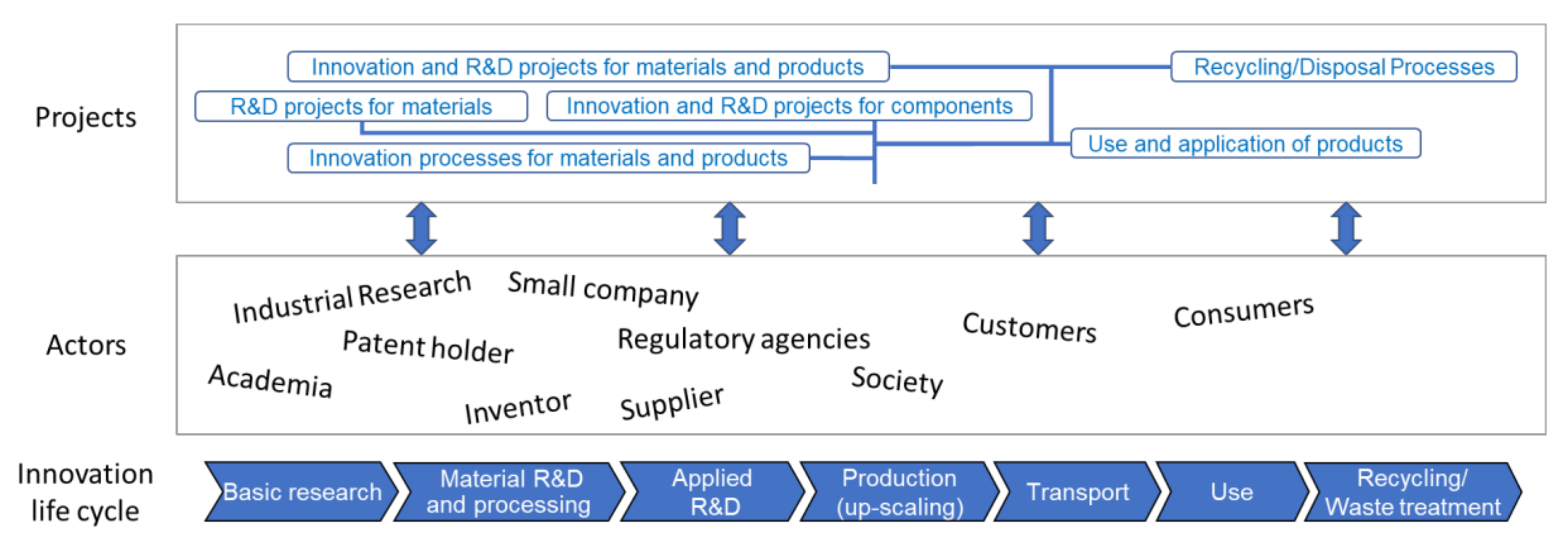
3.1. The NANoREG SbD Concept
- (1)
- the innovation project, including all participating actors;
- (2)
- the safety dossier;
- (3)
- the safety profile; and
- (4)
- the harmonised inventory of the state of the art of SbD protocols and procedures.
3.2. Elements of the SbD Concept
4. Trusted Environments
4.1. Definition of a Trusted Environment
4.2. Governance and Building of Trust
- -
- it has to be possible to share information in confidentiality, this confidentiality should have a stronger basis than just a non-disclosure agreement;
- -
- it has to be possible to share information in anonymity, meaning that the role of a participant may be visible to other participant but not the name or the exact identity; and
- -
- it has to be possible to make “non-binding statements”, meaning that a participant will not be liable for the statements he or she made during the process of information sharing; this will be particularly relevant for regulators.
5. Conclusions
- -
- adopting a pragmatic product-oriented Responsible Research & Innovation (RR&I) approach in the whole innovation chain, starting from academia-based start-ups and spin-offs, balancing functionality and safety from the beginning of the innovation project;
- -
- up-to-date centralized inventory of reliable tools and methods for nanomaterial characterization, safety, and sustainability assessment;
- -
- safe and trustworthy information exchange and management system, with a one-stop-shop for pertinent information retrieval;
- -
- reduction of overall costs due to intelligent testing strategy, and faster technological and regulatory screening of promising innovation projects;
- -
- generation of higher quality regulatory safety information;
- -
- structured and trustworthy interaction of innovators with regulators, reducing regulatory uncertainty for companies and allowing faster authorization processes;
- -
- early-on assessment by regulators of the suitability of existing regulations for the innovation;
- -
- timely regulatory development reducing uncertainty for companies and making the development of the innovation sector faster and safer, transferring the benefits to the society; and
- -
- increasing the transparency of the overall process and thus the trust of the public.
Acknowledgments
Conflicts of Interest
References
- Xia, T.; Zhao, Y.; Sager, T.; George, S.; Pokhrel, S.; Li, N.; Schoenfeld, D.; Meng, H.; Lin, S.; Wang, X.; et al. Decreased dissolution of ZnO by iron doping yields nanoparticles with reduced toxicity in the rodent lung and zebrafish embryos. ACS Nano 2011, 5, 1223–1235. [Google Scholar] [CrossRef] [PubMed]
- Naatz, H.; Lin, S.; Li, R.; Jiang, W.; Ji, Z.; Chang, C.H.; Köser, J.; Thöming, J.; Xia, T.; Nel, A.E.; et al. Safe-by-Design CuO Nanoparticles via Fe-Doping, Cu-O Bond Length Variation, and Biological Assessment in Cells and Zebrafish Embryos. ACS Nano 2017, 11, 501–515. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Yu, T.; Yu, Z.; Hu, X.; Yin, D. Nanomaterials Safer-by-Design: An Environmental Safety Perspective. Adv. Mater. 2018, 1705691. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Mercer, R.R.; Barger, M.; Schwegler-Berry, D.; Cohen, J.M.; Demokritou, P.; Castranova, V. Effects of amorphous silica coating on cerium oxide nanoparticles induced pulmonary responses. Toxicol. Appl. Pharmacol. 2015, 288, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Sotiriou, G.A.; Singh, D.; Zhang, F.; Wohlleben, W.; Chalbot, M.-C.G.; Kavouras, I.G.; Demokritou, P. An integrated methodology for the assessment of environmental health implications during thermal decomposition of nano-enabled products. Environ. Sci. Nano 2015, 2, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Truffier-Boutry, D.; Fiorentino, B.; Bartolomei, V.; Soulas, R.; Sicardy, O.; Benayad, A.; Damlencourt, J.F.; Pépin-Donat, B.; Lombard, C.; Gandolfo, A.; et al. Characterization of photocatalytic paints: A relationship between the photocatalytic properties—Release of nanoparticles and volatile organic compounds. Environ. Sci. Nano 2017, 4, 1998–2009. [Google Scholar] [CrossRef]
- Reinosa, J.J.; Docio, C.M.Á.; Ramírez, V.Z.; Lozano, J.F.F. Hierarchical nano ZnO-micro TiO2 composites: High UV protection yield lowering photodegradation in sunscreens. Ceram. Int. 2018, 44, 2827–2834. [Google Scholar] [CrossRef]
- Boulanger, P.; Belkadi, L.; Descarpentries, J.; Porterat, D.; Hibert, E.; Brouzes, A.; Mille, M.; Patel, S.; Pinault, M.; Reynaud, C. Towards large scale aligned carbon nanotube composites: An industrial safe-by-design and sustainable approach. J. Phys. Conf. Ser. 2013, 429, 012050. [Google Scholar] [CrossRef]
- Libralato, G.; Galdiero, E.; Falanga, A.; Carotenuto, R.; de Alteriis, E.; Guida, M. Toxicity Effects of Functionalized Quantum Dots, Gold and Polystyrene Nanoparticles on Target Aquatic Biological Models: A Review. Molecules 2017, 22, 1439. [Google Scholar] [CrossRef] [PubMed]
- Mantecca, P.; Kasemets, K.; Deokar, A.; Perelshtein, I.; Gedanken, A.; Bahk, Y.K.; Kianfar, B.; Wang, J. Airborne Nanoparticle Release and Toxicological Risk from Metal-Oxide-Coated Textiles: Toward a Multiscale Safe-by-Design Approach. Environ. Sci. Technol. 2017, 51, 9305–9317. [Google Scholar] [CrossRef] [PubMed]
- Starost, K.; Frijns, E.; Van Laer, J.; Faisal, N.; Egizabal, A.; Elizetxea, C.; Nelissen, I.; Blázquez, M.; Njuguna, J. The effect of nanosilica (SiO2) and nanoalumina (Al2O3) reinforced polyester nanocomposites on aerosol nanoparticle emissions into the environment during automated drilling. Aerosol Sci. Technol. 2017, 51, 1035–1046. [Google Scholar] [CrossRef]
- Vlasova, I.I.; Kapralov, A.A.; Michael, Z.P.; Burkert, S.C.; Shurin, M.R.; Star, A.; Shvedova, A.A.; Kagan, V.E. Enzymatic oxidative biodegradation of nanoparticles: Mechanisms, significance and applications. Toxicol. Appl. Pharmacol. 2016, 299, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, E.; Benetti, F. Approach for in vitro testing of oral nanocarrier toxicity. Chim. Oggi/Chem. Today 2017, 35, 52–55. [Google Scholar]
- Gonzalez, L.; Cundari, E.; Leyns, L.; Kirsch-Volders, M. Towards a New Paradigm in Nano-Genotoxicology: Facing Complexity of Nanomaterials’ Cellular Interactions and Effects. Basic Clin. Pharmacol. Toxicol. 2017, 121, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.E. Implementation of alternative test strategies for the safety assessment of engineered nanomaterials. J. Int. Med. 2013, 274, 561–577. [Google Scholar] [CrossRef] [PubMed]
- Damoiseaux, R.; George, S.; Li, M.; Pokhrel, S.; Ji, Z.; France, B.; Xia, T.; Suarez, E.; Rallo, R.; Mädler, L.; et al. No time to lose—High throughput screening to assess nanomaterial safety. Nanoscale 2011, 3, 1345–1360. [Google Scholar] [CrossRef] [PubMed]
- Cobaleda-Siles, M.; Guillamon, A.P.; Delpivo, C.; Vázquez-Campos, S.; Puntes, V.F. Safer by design strategies. J. Phys. Conf. Ser. 2017, 838, 012016. [Google Scholar] [CrossRef]
- Singh, D.; Sotiriou, G.A.; Zhang, F.; Mead, J.; Bello, D.; Wohlleben, W.; Demokritou, P. End-of-life thermal decomposition of nano-enabled polymers: Effect of nanofiller loading and polymer matrix on by-products. Environ. Sci. Nano 2016, 3, 1293–1305. [Google Scholar] [CrossRef]
- Bottero, J.Y.; Rose, J.; De Garidel, C.; Masion, A.; Deutsch, Th.; Brochard, G.; Carrière, M.; Gontard, N.; Wortham, H.; Rabilloud, T.; et al. SERENADE: Safer and ecodesign research and education applied to nanomaterial development, the new generation of materials safer by design. Environ. Sci. Nano 2017, 4, 526–538. [Google Scholar] [CrossRef]
- López De Ipina, J.M.; Hernan, A.; Cenigaonaindia, X.; Insunza, M.; Florez, S.; Seddon, R.; Vavouliotis, A.; Kostopoulos, V.; Latko, P.; Duralek, P.; et al. Implementation of a safe-by-design approach in the development of new open pilot lines for the manufacture of carbon nanotube-based nano-enabled products. J. Phys. Conf. Ser. 2017, 838, 012018. [Google Scholar] [CrossRef]
- Rivera-Gil, P.; Jimenez De Aberasturi, D.; Wulf, V.; Pelaz, B.; Del Pino, P.; Zhao, Y.; Parak, W.J. The Challenge to Relate the Physicochemical Properties of Colloidal Nanoparticles to Their Cytotoxicity. Acc. Chem. Res. 2013, 46, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, C.; Goesmann, H. Nanoparticulate Functional Materials. Angew. Chem. Int. 2010, 49, 1362–1395. [Google Scholar] [CrossRef]
- Freund, R.; Lächelt, U.; Gruber, T.; Rühle, B.; Wuttke, S. Multifunctional Efficiency: Extending the Concept of Atom Economy to Functional Nanomaterials. ACS Nano 2018, 12, 2094–2105. [Google Scholar] [CrossRef] [PubMed]
- Duffus, J.; Worth, H. The Science of Chemical Safety, Essential Toxicology 4, Hazard and Risk, IUPAC Educators’ Resource Material. Available online: https://old.iupac.org/publications/cd/essential_toxicology/IUPACTOX4.pdf (accessed on 5 March 2018).
- Nel, A.E.; Parak, W.J.; Chan, W.C.W.; Xia, T.; Hersam, M.C.; Brinker, C.J.; Weiss, P.S. Where Are We Heading in Nanotechnology Environmental Health and Safety and Materials Characterization? ACS Nano 2015, 9, 5627–5630. [Google Scholar] [CrossRef] [PubMed]
- Roco, M.C.; Harthorn, B.; Guston, D.; Shapira, P. Innovative and Responsible Governance of Nanotechnology for Societal Development. In Nanotechnology Research Directions for Societal Needs in 2020; Science Policy Reports; Springer: Dordrecht, Germany, 2011; Volume 1. [Google Scholar]
- RIVM ProSafe Repository. The ProSafe Safe-by-Design Implementation Concept. Available online: https://rivm.nl/en/About_RIVM/International_Affairs/International_Projects/Completed/ProSafe/ProSafe_Deliverables%3AxvVbzWzuS8eQnl9AI07Qmw/ProSafe_Safe_by_Design_SbD_implementation_concept_final.org (accessed on 4 April 2018).
- Parker, G.G.; Van Alstyne, M.W.; Choudary, S.P. Platform Revolution: How Networked Markets Are Transforming the Economy and How to Make Them Work for You; W.W. Norton & Company: New York, NY, USA, 2016. [Google Scholar]
- Justo-Hanani, R.; Dayan, T. European Risk Governance of Nanotechnology: Explaining Emerging Regulatory Policy. Res. Policy 2015, 44, 1527–1536. [Google Scholar] [CrossRef]
- George, S.; Pokhrel, S.; Xia, T.; Gilbert, B.; Ji, Z.; Schowalter, M.; Rosenauer, A.; Damoiseaux, R.; Bradley, K.A.; Mädler, L.; et al. Use of a Rapid Cytotoxicity Screening Approach to Engineer a Safer Zinc Oxide Nanoparticle through Iron Doping. ACS Nano 2010, 4, 15–19. [Google Scholar] [CrossRef] [PubMed]


| SbD Strategy | Measure | Source |
|---|---|---|
| Design out hazard (direct and indirect effects of nanomaterials) | NanoParticle (NP) doping | [1,2] |
| Surface passivation | [3,4,5] | |
| NP coating | ||
| Reduction of photo-catalytic efficiency | [6] | |
| Formation of composites | [7,8] | |
| Surface functionalisation | [9] | |
| Reduce release | Adaptation of the processing | [10] |
| Selection of nanofiller | [11] | |
| Reduce bio-persistence | Carbon NanoTubes (CNT) Doping | [12] |
| Testing strategies for safety evaluation | High throughput screening, alternative testing strategies and biological mechanisms | [13,14,15,16] |
| Material characterisation | [17] | |
| Identification of risk hotspots for potential SbD approaches | End of life cycle: thermal decomposition Life cycle assessment | [5,18,19] |
| Pilot plant development | Risk mitigation | [20] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kraegeloh, A.; Suarez-Merino, B.; Sluijters, T.; Micheletti, C. Implementation of Safe-by-Design for Nanomaterial Development and Safe Innovation: Why We Need a Comprehensive Approach. Nanomaterials 2018, 8, 239. https://doi.org/10.3390/nano8040239
Kraegeloh A, Suarez-Merino B, Sluijters T, Micheletti C. Implementation of Safe-by-Design for Nanomaterial Development and Safe Innovation: Why We Need a Comprehensive Approach. Nanomaterials. 2018; 8(4):239. https://doi.org/10.3390/nano8040239
Chicago/Turabian StyleKraegeloh, Annette, Blanca Suarez-Merino, Teun Sluijters, and Christian Micheletti. 2018. "Implementation of Safe-by-Design for Nanomaterial Development and Safe Innovation: Why We Need a Comprehensive Approach" Nanomaterials 8, no. 4: 239. https://doi.org/10.3390/nano8040239




