Spatially Correlated, Single Nanomaterial-Level Structural and Optical Profiling of Cu-Doped ZnO Nanorods Synthesized via Multifunctional Silicides
Abstract
:1. Introduction
2. Experimental Methods
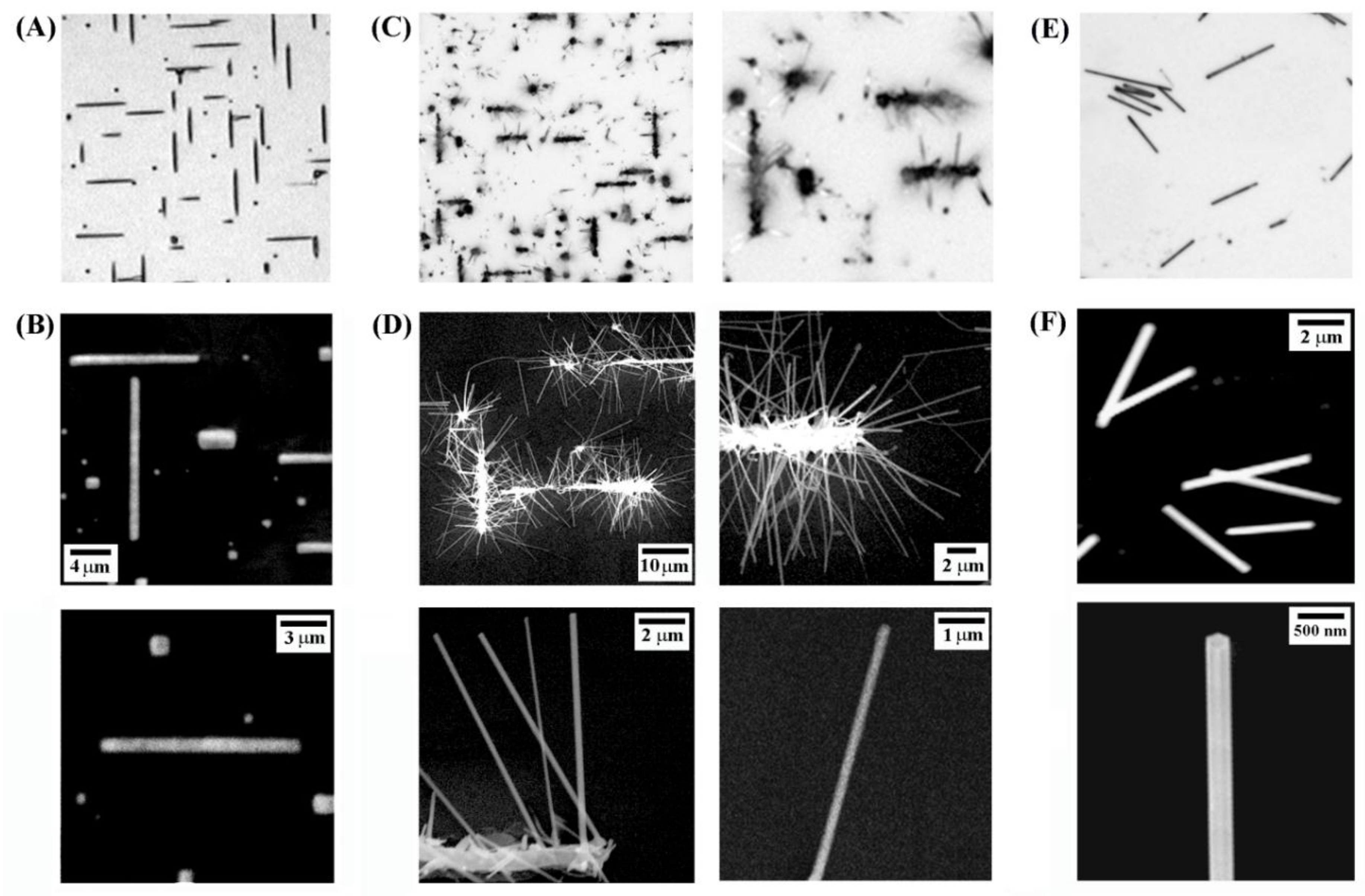
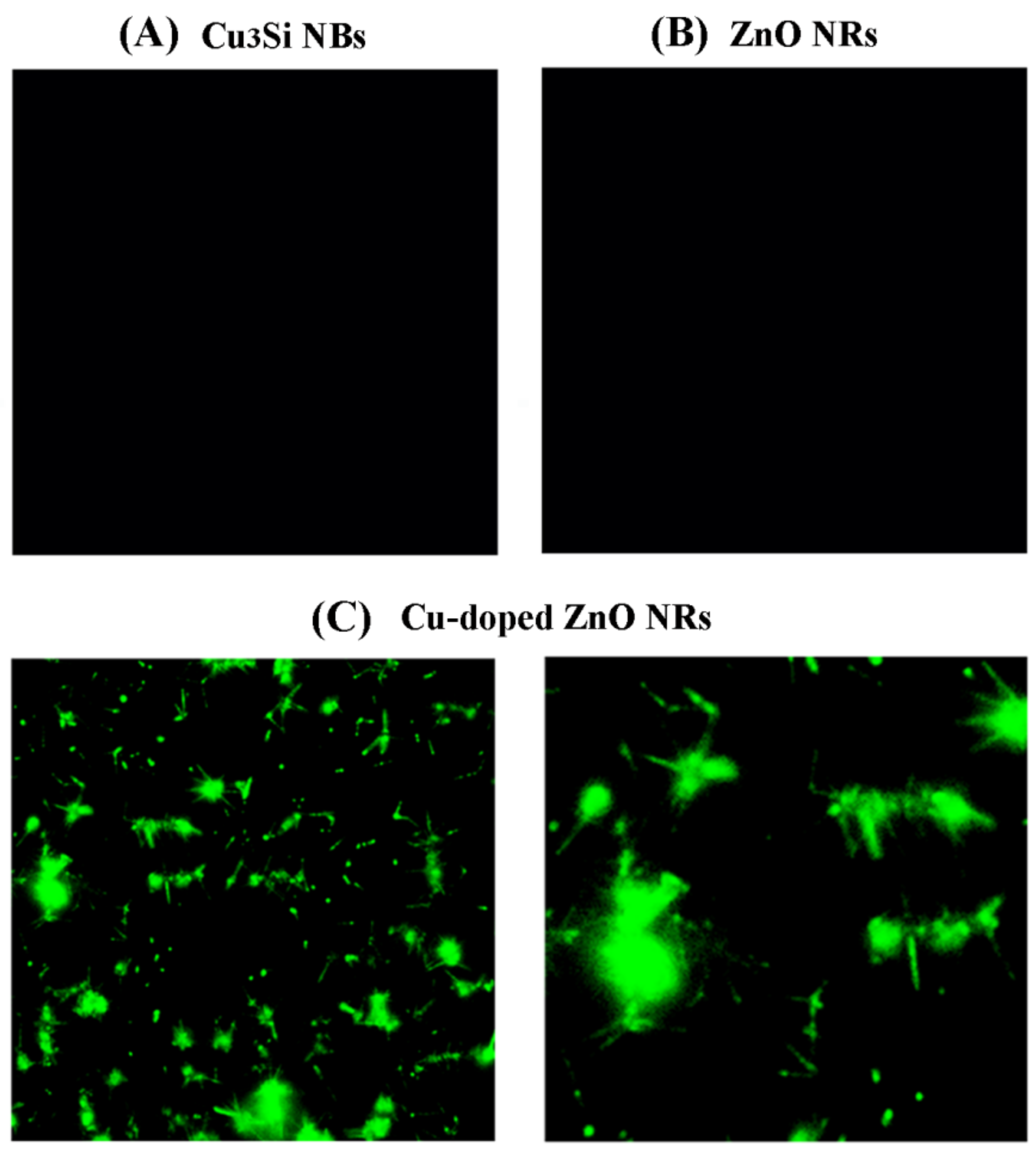
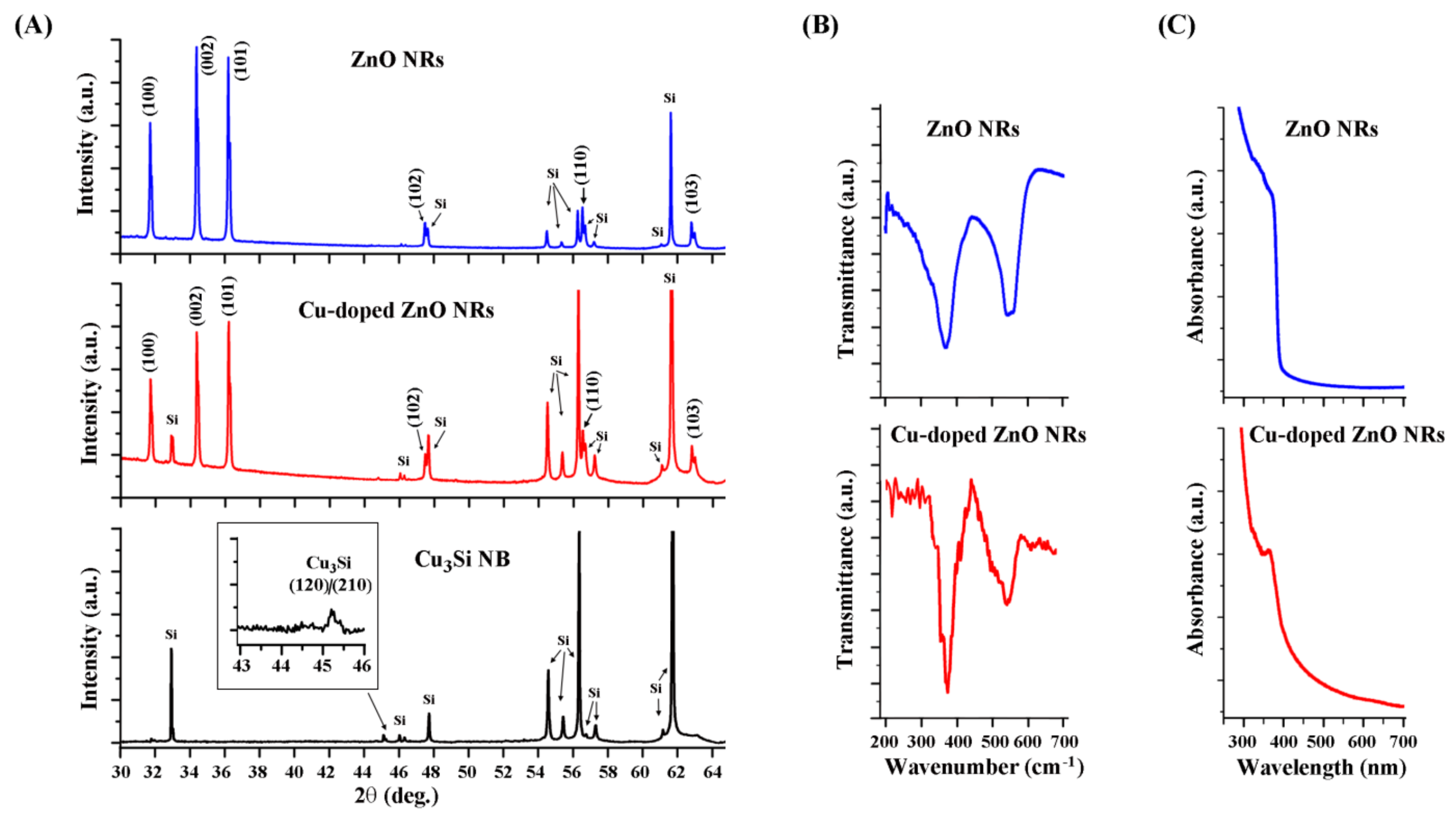
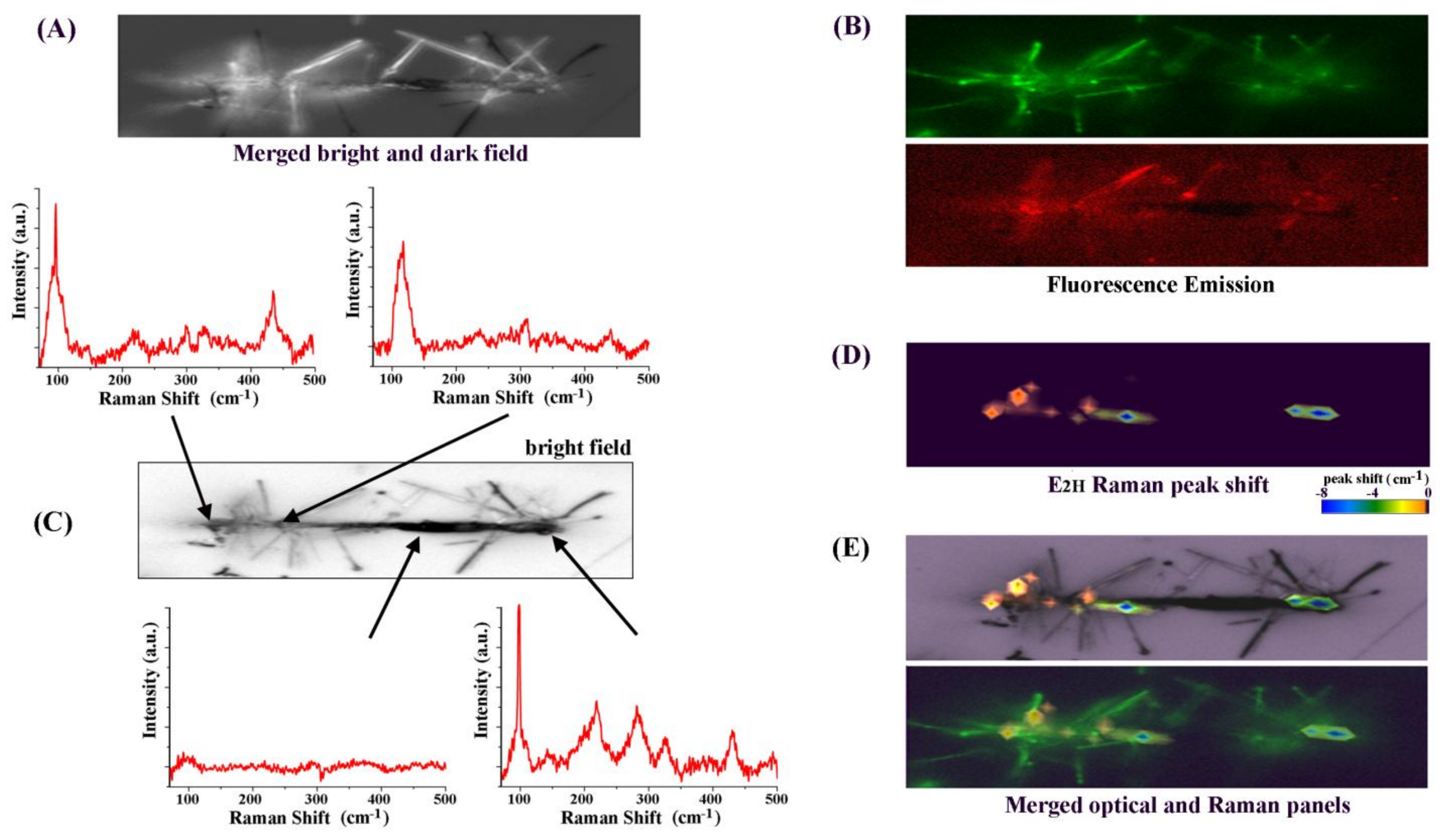
3. Results and Discussion
4. Summary
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Law, M.; Sirbuly, D.J.; Johnson, J.C.; Goldberger, J.; Saykally, R.J.; Yang, P. Nanoribbon waveguides for subwavelength photonics integration. Science 2004, 305, 1269–1273. [Google Scholar] [CrossRef] [PubMed]
- Sirbuly, D.J.; Law, M.; Pauzauskie, P.; Yan, H.; Maslov, A.V.; Knutsen, K.; Ning, C.Z.; Saykally, R.J.; Yang, P. Optical routing and sensing with nanowire assemblies. Proc. Natl. Acad. Sci. USA 2005, 102, 7800–7805. [Google Scholar] [CrossRef] [PubMed]
- Sirbuly, D.J.; Law, M.; Yan, H.; Yang, P. Semiconductor nanowires for subwavelength photonics integration. J. Phys. Chem. B 2005, 109, 15190–15213. [Google Scholar] [CrossRef] [PubMed]
- Willander, M.; Nur, O.; Zhao, Q.X.; Yang, L.L.; Lorenz, M.; Cao, B.Q.; Zúñiga Pérez, J.; Czekalla, C.; Zimmermann, G.; Grundmann, M.; et al. Zinc oxide nanorod based photonic devices: Recent progress in growth, light emitting diodes and lasers. Nanotechnology 2009, 20, 332001. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.C.; Yan, H.; Schaller, R.D.; Haber, L.H.; Saykally, R.J.; Yang, P. Single nanowire lasers. J. Phys. Chem. B 2001, 105, 11387–11390. [Google Scholar] [CrossRef]
- Johnson, J.C.; Yan, H.; Yang, P.; Saykally, R.J. Optical cavity effects in ZnO nanowire lasers and waveguides. J. Phys. Chem. B 2003, 107, 8816–8828. [Google Scholar] [CrossRef]
- Law, M.; Greene, L.E.; Johnson, J.C.; Saykally, R.; Yang, P. Nanowire dye-sensitized solar cells. Nat. Mater. 2005, 4, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Soci, C.; Zhang, A.; Xiang, B.; Dayeh, S.A.; Aplin, D.P.R.; Park, J.; Bao, X.Y.; Lo, Y.H.; Wang, D. ZnO nanowire UV photodetectors with high internal gain. Nano Lett. 2007, 7, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Chu, S.; Zhan, N.; Lin, Y.; Chernyak, L.; Liu, J. ZnO homojunction photodiodes based on Sb-doped p-type nanowire array and n-type film for ultraviolet detection. Appl. Phys. Lett. 2011, 98, 041107. [Google Scholar] [CrossRef]
- Yan, H.; Johnson, J.; Law, M.; He, R.; Knutsen, K.; McKinney, J.R.; Pham, J.; Saykally, R.; Yang, P. ZnO nanoribbon microcavity lasers. Adv. Mater. 2003, 15, 1907–1911. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, F.; Qian, S.; Kumar, N.; Hahm, J.-I.; Xu, J. Multiphoton absorption induced amplified spontaneous emission from biocatalyst-synthesized ZnO nanorods. Appl. Phys. Lett. 2008, 92, 233116. [Google Scholar] [CrossRef]
- Adalsteinsson, V.; Parajuli, O.; Kepics, S.; Gupta, A.; Reeves, W.B.; Hahm, J. Ultrasensitive detection of cytokines enabled by nanoscale ZnO arrays. Anal. Chem. 2008, 80, 6594–6601. [Google Scholar] [CrossRef] [PubMed]
- Hahm, J. Zinc oxide nanomaterials for biomedical fluorescence detection. J. Nanosci. Nanotechnol. 2014, 14, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Truong, J.; Reeves, W.B.; Hahm, J.-I. Emerging cytokine biosensors with optical detection modalities and nanomaterial-enabled signal enhancement. Sensors 2017, 17, 428. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.-S.; Deutsch, T.; Yan, Y.; Jiang, C.-S.; Perkins, C.L.; Turner, J.; Al-Jassim, M. Synthesis of band-gap-reduced p-type ZnO films by Cu incorporation. J. Appl. Phys. 2007, 102, 023517. [Google Scholar] [CrossRef]
- Hu, L.; Zhu, L.; He, H.; Guo, Y.; Pan, G.; Jiang, J.; Jin, Y.; Sun, L.; Ye, Z. Colloidal chemically fabricated ZnO: Cu-based photodetector with extended UV-visible detection waveband. Nanoscale 2013, 5, 9577–9581. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, D.B.; Chang, R.P.H.; Song, J.-Y.; Ketterson, J.B. Room-temperature ferromagnetism in Cu-doped ZnO thin films. Appl. Phys. Lett. 2005, 87, 082504. [Google Scholar] [CrossRef]
- Herng, T.S.; Wong, M.F.; Qi, D.; Yi, J.; Kumar, A.; Huang, A.; Kartawidjaja, F.C.; Smadici, S.; Abbamonte, P.; Sánchez-Hanke, C.; et al. Mutual ferromagnetic–ferroelectric coupling in multiferroic copper-doped ZnO. Adv. Mater. 2011, 23, 1635–1640. [Google Scholar] [CrossRef] [PubMed]
- Kutty, T.R.N.; Raghu, N. Varistors based on polycrystalline ZnO:Cu. Appl. Phys. Lett. 1989, 54, 1796–1798. [Google Scholar] [CrossRef]
- Kryshtab, T.G.; Khomchenko, V.S.; Papusha, V.P.; Mazin, M.O.; Tzyrkunov, Y.A. Thin ZnS:Cu,Ga and ZnO:Cu,Ga film phosphors. Thin Solid Films 2002, 403–404, 76–80. [Google Scholar] [CrossRef]
- Elżbieta, R.; Emerson, C.; Michał, K.; Stefan, J.; Karol, Z.; Henryk, D. The effect of Cu doping on the mechanical and optical properties of zinc oxide nanowires synthesized by hydrothermal route. Nanotechnology 2016, 27, 175706. [Google Scholar]
- Xu, C.; Yang, K.; Huang, L.; Wang, H. Cu-doping induced ferromagnetism in ZnO nanowires. J. Chem. Phys. 2009, 130, 124711. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.X.; Sun, X.W.; Zhang, X.H.; Ke, L.; Chua, S.J. Photoluminescent properties of copper-doped zinc oxide nanowires. Nanotechnology 2004, 15, 856. [Google Scholar] [CrossRef]
- Zhou, S.-M.; Zhang, X.-H.; Meng, X.-M.; Zou, K.; Fan, X.; Wu, S.-K.; Lee, S.-T. The fabrication and optical properties of highly crystalline ultra-long Cu-doped ZnO nanowires. Nanotechnology 2004, 15, 1152. [Google Scholar] [CrossRef]
- Kouklin, N. Cu-doped ZnO nanowires for efficient and multispectral photodetection applications. Adv. Mater. 2008, 20, 2190–2194. [Google Scholar] [CrossRef]
- Zhu, H.; Iqbal, J.; Xu, H.; Yu, D. Raman and photoluminescence properties of highly Cu doped ZnO nanowires fabricated by vapor-liquid-solid process. J. Chem. Phys. 2008, 129, 124713. [Google Scholar] [CrossRef] [PubMed]
- The Long, P.; Roger, V.; David, C.; Nguyen Xuan, N.; Ursaki, V.V. Raman scattering in Me-doped ZnO nanorods (Me = Mn, Co, Cu and Ni) prepared by thermal diffusion. Nanotechnology 2008, 19, 475702. [Google Scholar]
- Lupan, O.; Pauporté, T.; Le Bahers, T.; Viana, B.; Ciofini, I. Wavelength-emission tuning of ZnO nanowire-based light-emitting diodes by Cu doping: Experimental and computational insights. Adv. Funct. Mater. 2011, 21, 3564–3572. [Google Scholar] [CrossRef]
- Singh, M.; Jiang, R.; Coia, H.; Choi, D.S.; Alabanza, A.; Chang, J.Y.; Wang, J.; Hahm, J.-I. Insight into factors affecting the presence, degree, and temporal stability of fluorescence intensification on ZnO nanorod ends. Nanoscale 2015, 7, 1424–1436. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Song, S.; Hahm, J. Unique temporal and spatial biomolecular emission profile on individual zinc oxide nanorods. Nanoscale 2014, 6, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Hahm, J.-I. Fundamental properties of one-dimensional zinc oxide nanomaterials and implementations in various detection modes of enhanced biosensing. Ann. Rev. Phys. Chem. 2016, 67, 691–717. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Zhuo, X.; Choi, D.S.; Gonzalez, L.E.; Wang, J.; Hahm, J.-I. Effects of crystallographic facet-specific peptide adsorption along single ZnO nanorods on the characteristic fluorescence intensification on nanorod ends (FINE) phenomenon. Nanoscale 2015, 7, 18813–18826. [Google Scholar] [CrossRef] [PubMed]
- Truong, J.; Singh, M.; Hansen, M.; Hahm, J.-I. Polarization-resolved mechanistic investigation of fluorescence signal intensification on zinc oxide nanorod ends. Nanoscale 2017, 9, 8164–8175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wong, L.M.; Ong, H.G.; Wang, X.J.; Wang, J.L.; Wang, S.J.; Chen, H.; Wu, T. Self-assembled shape- and orientation-controlled synthesis of nanoscale Cu3Si triangles, squares, and wires. Nano Lett. 2008, 8, 3205–3210. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.-W.; Wang, C.-Y.; Li, G.-A.; Chang, S.-H.; Chu, L.-W.; Chen, L.-J.; Tuan, H.-Y. Solution-phase synthesis of single-crystal Cu3Si nanowire arrays on diverse substrates with dual functions as high-performance field emitters and efficient anti-reflective layers. Nanoscale 2013, 5, 9875–9881. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Zhu, L.; He, H.; Zhang, L.; Ye, Z. Acceptor defect-participating magnetic exchange in ZnO: Cu nanocrystalline film: Defect structure evolution, Cu–N synergetic role and magnetic control. J. Mater. Chem. C 2015, 3, 1330–1346. [Google Scholar] [CrossRef]
- Park, M.S.; Min, B.I. Ferromagnetism in ZnO codoped with transition metals. Phys. Rev. B 2003, 68, 224436. [Google Scholar] [CrossRef]
- Pashchanka, M.; Hoffmann, R.C.; Gurlo, A.; Swarbrick, J.C.; Khanderi, J.; Engstler, J.; Issanin, A.; Schneider, J.J. A molecular approach to Cu doped ZnO nanorods with tunable dopant content. Dalton Trans. 2011, 40, 4307–4314. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.F.; Ansari, A.H.; Hameedullah, M.; Ahmad, E.; Husain, F.M.; Zia, Q.; Baig, U.; Zaheer, M.R.; Alam, M.M.; Khan, A.M.; et al. Sol-gel synthesis of thorn-like ZnO nanoparticles endorsing mechanical stirring effect and their antimicrobial activities: Potential role as nano-antibiotics. Sci. Rep. 2016, 6, 27689. [Google Scholar] [CrossRef] [PubMed]
- Daniel, S.C.; Matthew, H.; Edward Van, K.; Jong-in, H. Highly photoresponsive, ZnO nanorod-based photodetector for operation in the visible spectral range. Nanotechnology 2017, 28, 145203. [Google Scholar]
- Calleja, J.M.; Cardona, M. Resonant raman scattering in ZnO. Phys. Rev. B 1977, 16, 3753–3761. [Google Scholar] [CrossRef]
- Arguello, C.A.; Rousseau, D.L.; Porto, S.P.S. First-order raman effect in wurtzite-type crystals. Phys. Rev. 1969, 181, 1351–1363. [Google Scholar] [CrossRef]
- Cuscó, R.; Alarcón-Lladó, E.; Ibáñez, J.; Artús, L.; Jiménez, J.; Wang, B.; Callahan, M.J. Temperature dependence of raman scattering in ZnO. Phys. Rev. B 2007, 75, 165202. [Google Scholar] [CrossRef]
- Powell, D.; Compaan, A.; Macdonald, J.R.; Forman, R.A. Raman-scattering study of ion-implantation-produced damage in Cu2O. Phys. Rev. B 1975, 12, 20–25. [Google Scholar] [CrossRef]
- Simas, R.; Albert, G.N.; Hua, J.; Ying, T.; Victor, I.K.; Jani, S.; Elena, D.O.; Sofia, N.B.; Alexander, N.O.; Esko, I.K. A novel method for metal oxide nanowire synthesis. Nanotechnology 2009, 20, 165603. [Google Scholar]
- Van Vugt, L.K.; Piccione, B.; Cho, C.-H.; Nukala, P.; Agarwal, R. One-dimensional polaritons with size-tunable and enhanced coupling strengths in semiconductor nanowires. Proc. Natl. Sci. Acad. USA 2011, 108, 10050–10055. [Google Scholar] [CrossRef] [PubMed]
- Na, Y.-R.; Kim, S.Y.; Gaublomme, J.T.; Shalek, A.K.; Jorgolli, M.; Park, H.; Yang, E.G. Probing enzymatic activity inside living cells using a nanowire–cell “sandwich” assay. Nano Lett. 2013, 13, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Hällström, W.; Mårtensson, T.; Prinz, C.; Gustavsson, P.; Montelius, L.; Samuelson, L.; Kanje, M. Gallium phosphide nanowires as a substrate for cultured neurons. Nano Lett. 2007, 7, 2960–2965. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Song, J.; Mantini, G.; Lu, M.-Y.; Fang, H.; Falconi, C.; Chen, L.-J.; Wang, Z.L. Quantifying the traction force of a single cell by aligned silicon nanowire array. Nano Lett. 2009, 9, 3575–3580. [Google Scholar] [CrossRef] [PubMed]
- Bucaro, M.A.; Vasquez, Y.; Hatton, B.D.; Aizenberg, J. Fine-tuning the degree of stem cell polarization and alignment on ordered arrays of high-aspect-ratio nanopillars. ACS Nano 2012, 6, 6222–6230. [Google Scholar] [CrossRef] [PubMed]
- Trine, B.; Sara, B.; Katrine, R.R.; Morten Hannibal, M.; Claus, B.S.; Jesper, N.; Karen, L.M. Cell membrane conformation at vertical nanowire array interface revealed by fluorescence imaging. Nanotechnology 2012, 23, 415102. [Google Scholar]
- Selvin, P.R. The renaissance of fluorescence resonance energy transfer. Nat. Struct. Mol. Biol. 2000, 7, 730–734. [Google Scholar] [CrossRef] [PubMed]
- Forster, T. 10th spiers memorial lecture. Transfer mechanisms of electronic excitation. Discuss. Faraday Soc. 1959, 27, 7–17. [Google Scholar] [CrossRef]
- Yan, Y.; Marriott, G. Analysis of protein interactions using fluorescence technologies. Curr. Opin. Chem. Biol. 2003, 7, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Jameson, D.M.; Ross, J.A. Fluorescence polarization/anisotropy in diagnostics and imaging. Chem. Rev. 2010, 110, 2685–2708. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Truong, J.; Hansen, M.; Szychowski, B.; Xie, T.; Daniel, M.-C.; Hahm, J.-i. Spatially Correlated, Single Nanomaterial-Level Structural and Optical Profiling of Cu-Doped ZnO Nanorods Synthesized via Multifunctional Silicides. Nanomaterials 2018, 8, 222. https://doi.org/10.3390/nano8040222
Truong J, Hansen M, Szychowski B, Xie T, Daniel M-C, Hahm J-i. Spatially Correlated, Single Nanomaterial-Level Structural and Optical Profiling of Cu-Doped ZnO Nanorods Synthesized via Multifunctional Silicides. Nanomaterials. 2018; 8(4):222. https://doi.org/10.3390/nano8040222
Chicago/Turabian StyleTruong, Johnson, Matthew Hansen, Brian Szychowski, Tian Xie, Marie-Christine Daniel, and Jong-in Hahm. 2018. "Spatially Correlated, Single Nanomaterial-Level Structural and Optical Profiling of Cu-Doped ZnO Nanorods Synthesized via Multifunctional Silicides" Nanomaterials 8, no. 4: 222. https://doi.org/10.3390/nano8040222