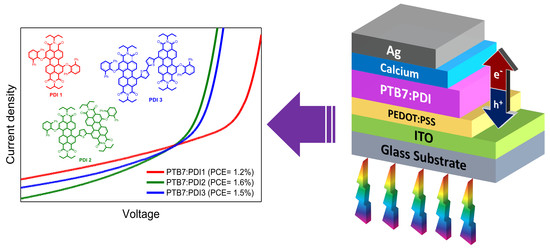
Diphenylphenoxy-Thiophene-PDI Dimers as Acceptors for OPV Applications with Open Circuit Voltage Approaching 1 Volt
Abstract
:1. Introduction
2. Results
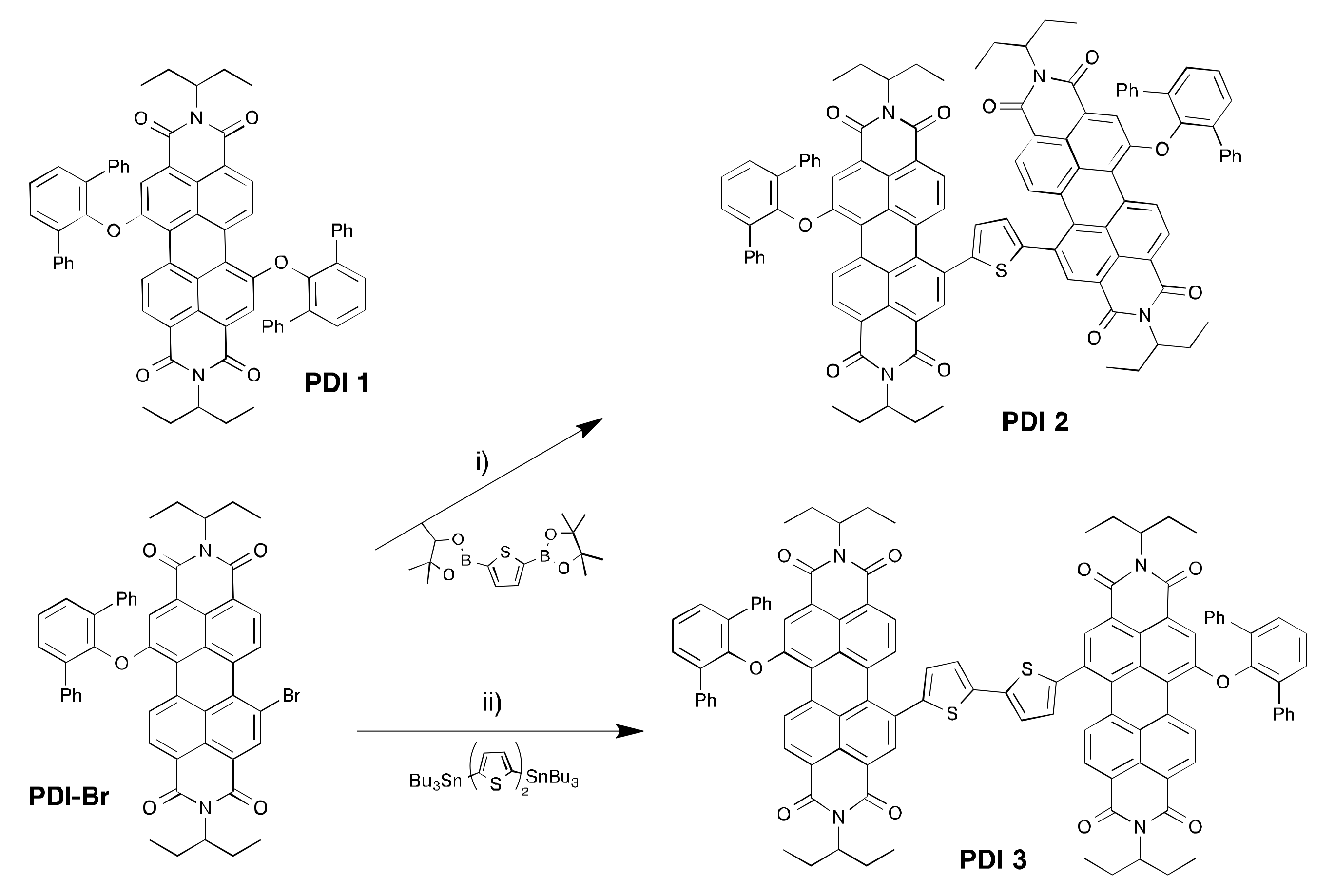
2.1. Synthesis and Characterization of the PDI-Acceptors
2.2. Solar Cell Devices Fabrication and Characterization
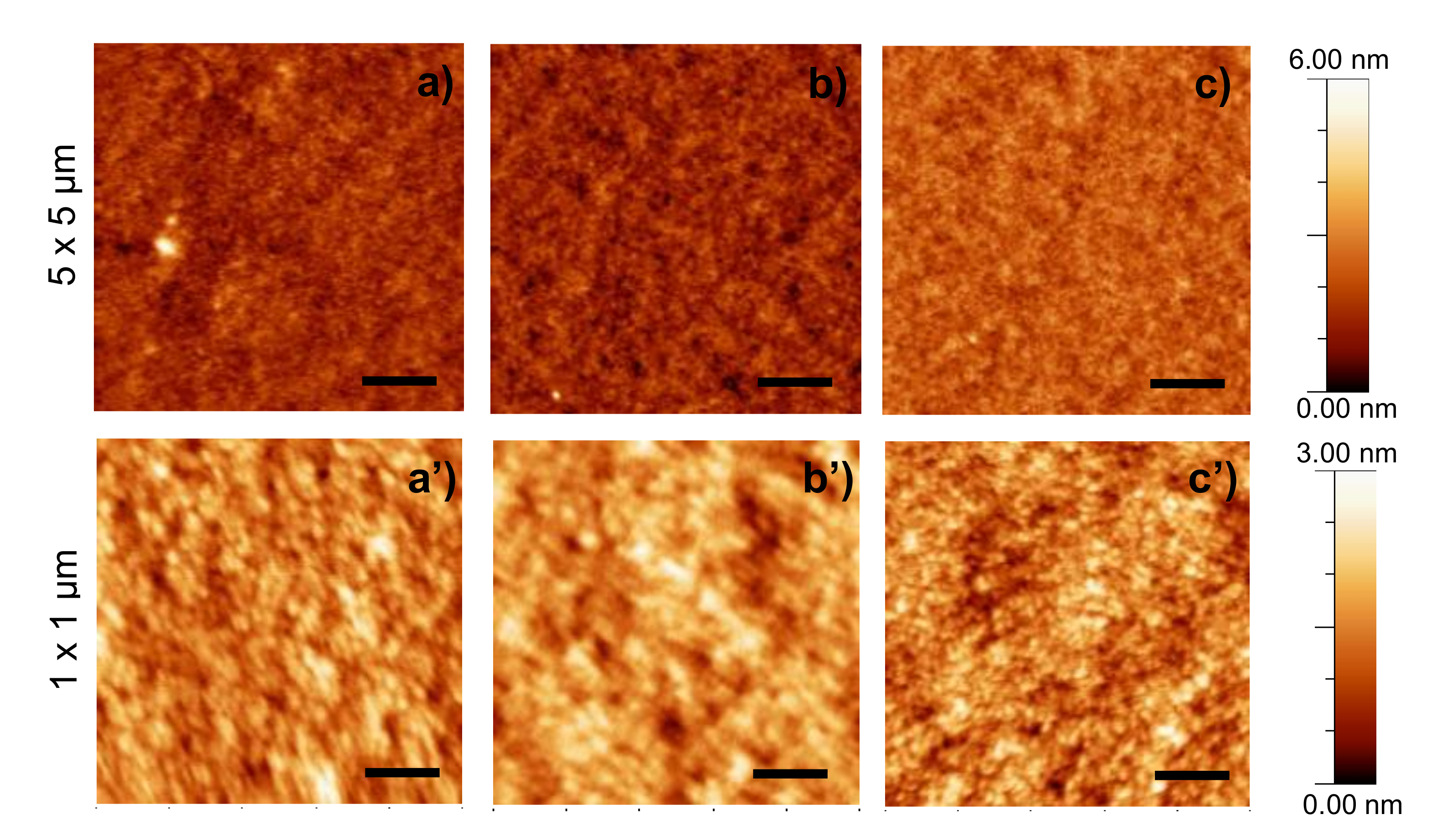
2.3. Morphologival Characterization
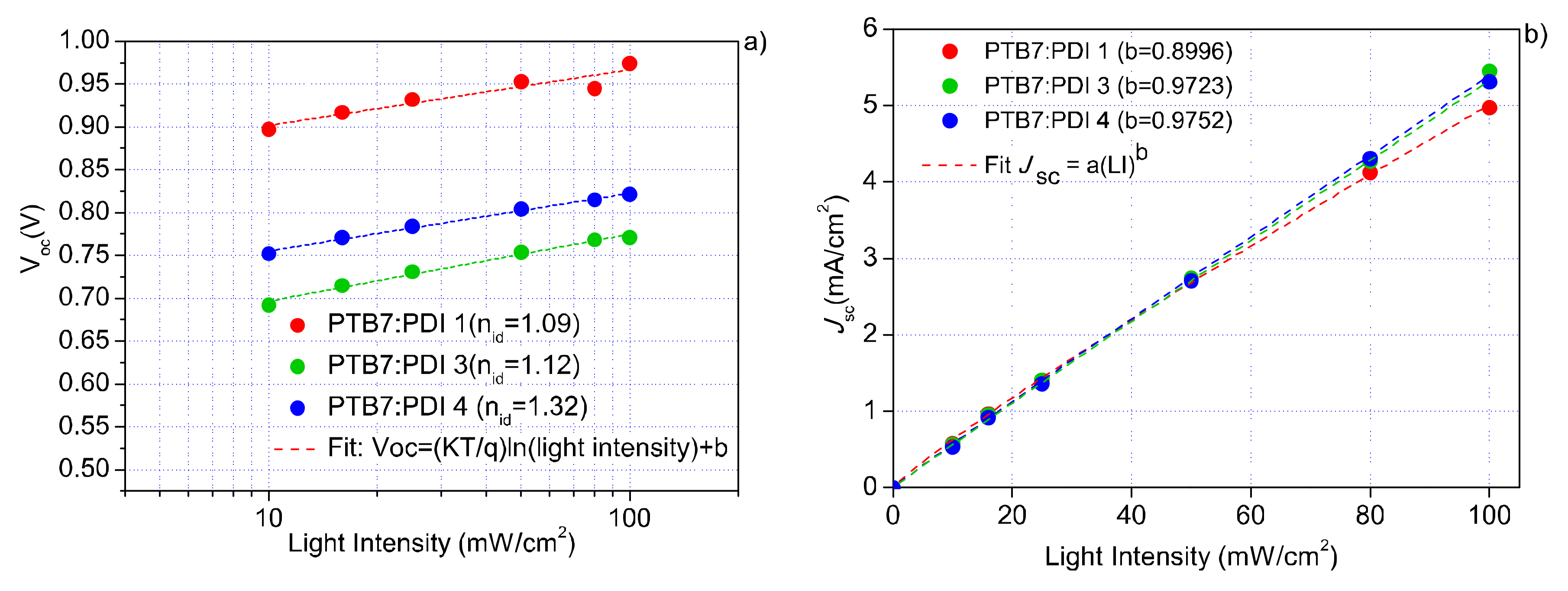
2.4. Electrical and Photophysical Characterization
3. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhao, C.X.; Wang, X.; Zeng, W.; Chen, Z.K.; Ong, B.S.; Wang, K.; Deng, L.; Xu, G. Organic photovoltaic power conversion efficiency improved by AC electric field alignment during fabrication. Appl. Phys. Lett. 2011, 99, 053305. [Google Scholar] [CrossRef]
- Chen, J.-D.; Cui, C.; Li, Y.-Q.; Zhou, L.; Ou, Q.-D.; Li, C.; Li, Y.; Tang, J.-X. Single-Junction Polymer Solar Cells Exceeding 10% Power Conversion Efficiency. Adv. Mater. 2015, 27, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.-H.; Jhuo, H.-J.; Yeh, P.-N.; Cheng, Y.-S.; Li, Y.-L.; Lee, Y.-H.; Sharma, S.; Chen, S.-A. Single Junction Inverted Polymer Solar Cell Reaching Power Conversion Efficiency 10.31% by Employing Dual-Doped Zinc Oxide Nano-Film as Cathode Interlayer. Sci. Rep. 2014, 4, 6813. [Google Scholar] [CrossRef] [PubMed]
- Kan, B.; Zhang, Q.; Li, M.; Wan, X.; Ni, W.; Long, G.; Wang, Y.; Yang, X.; Feng, H.; Chen, Y. Solution-Processed Organic Solar Cells Based on Dialkylthiol-Substituted Benzodithiophene Unit with Efficiency near 10%. J. Am. Chem. Soc. 2014, 136, 15529–15532. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Kan, B.; Liu, F.; Long, G.; Wan, X.; Chen, X.; Zuo, Y.; Ni, W.; Zhang, H.; Li, M.; et al. Small-molecule solar cells with efficiency over 9%. Nat. Photonics 2015, 9, 35–41. [Google Scholar] [CrossRef]
- Facchetti, A. Polymer donor–polymer acceptor (all-polymer) solar cells. Mater. Today 2013, 16, 123–132. [Google Scholar] [CrossRef]
- Guo, X.; Tu, D.; Liu, X. Recent advances in rylene diimide polymer acceptors for all-polymer solar cells. J. Energy Chem. 2015, 24, 675–685. [Google Scholar] [CrossRef]
- Kang, H.; Lee, W.; Oh, J.; Kim, T.; Lee, C.; Kim, B.J. From Fullerene-Polymer to All-Polymer Solar Cells: The Importance of Molecular Packing, Orientation, and Morphology Control. Acc. Chem. Res. 2016, 49, 2424–2434. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lim, E. Development of Polymer Acceptors for Organic Photovoltaic Cells. Polymers 2014, 6, 382–407. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, Q. Recent progress in non-fullerene small molecule acceptors in organic solar cells (OSCs). J. Mater. Chem. C 2017, 5, 1275–1302. [Google Scholar] [CrossRef]
- Fernandez-Lazaro, F.; Zink-Lorre, N.; Sastre-Santos, A. Perylenediimides as non-fullerene acceptors in bulk-heterojunction solar cells (BHJSCs). J. Mater. Chem. A 2016, 4, 9336–9346. [Google Scholar] [CrossRef]
- Kozma, E.; Catellani, M. Perylene diimides based materials for organic solar cells. Dyes Pigment. 2013, 98, 160–179. [Google Scholar] [CrossRef]
- Lin, Y.; Zhan, X. Non-fullerene acceptors for organic photovoltaics: An emerging horizon. Mater. Horiz. 2014, 1, 470–488. [Google Scholar] [CrossRef]
- McAfee, S.M.; Topple, J.M.; Hill, I.G.; Welch, G.C. Key components to the recent performance increases of solution processed non-fullerene small molecule acceptors. J. Mater. Chem. A 2015, 3, 16393–16408. [Google Scholar] [CrossRef]
- Nielsen, C.B.; Holliday, S.; Chen, H.-Y.; Cryer, S.J.; McCulloch, I. Non-Fullerene Electron Acceptors for Use in Organic Solar Cells. Acc. Chem. Res. 2015, 48, 2803–2812. [Google Scholar] [CrossRef] [PubMed]
- Sonar, P.; Lim, J.P.F.; Chan, K.L. Organic non-fullerene acceptors for organic photovoltaics. Energy Environ. Sci. 2011, 4, 1558–1574. [Google Scholar] [CrossRef]
- Zhang, S.; Ye, L.; Hou, J. Breaking the 10% Efficiency Barrier in Organic Photovoltaics: Morphology and Device Optimization of Well-Known PBDTTT Polymers. Adv. Energy Mater. 2016, 6. [Google Scholar] [CrossRef]
- Liu, T.; Guo, Y.; Yi, Y.; Huo, L.; Xue, X.; Sun, X.; Fu, H.; Xiong, W.; Meng, D.; Wang, Z.; et al. Ternary Organic Solar Cells Based on Two Compatible Nonfullerene Acceptors with Power Conversion Efficiency >10%. Adv. Mater. 2016, 28, 10008–10015. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, Z.-G.; Bin, H.; Chen, S.; Gao, L.; Xue, L.; Yang, C.; Li, Y. Side-Chain Isomerization on an n-type Organic Semiconductor ITIC Acceptor Makes 11.77% High Efficiency Polymer Solar Cells. J. Am. Chem. Soc. 2016, 138, 15011–15018. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Dai, S.; Wu, Y.; Zhang, Q.; Wang, J.; Jiang, L.; Ling, Q.; Wei, Z.; Ma, W.; You, W.; et al. Single-Junction Binary-Blend Nonfullerene Polymer Solar Cells with 12.1% Efficiency. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Qian, D.; Zhang, S.; Li, S.; Inganas, O.; Gao, F.; Hou, J. Fullerene-Free Polymer Solar Cells with over 11% Efficiency and Excellent Thermal Stability. Adv. Mater. 2016, 28, 4734–4739. [Google Scholar] [CrossRef] [PubMed]
- Bin, H.; Zhang, Z.-G.; Gao, L.; Chen, S.; Zhong, L.; Xue, L.; Yang, C.; Li, Y. Non-Fullerene Polymer Solar Cells Based on Alkylthio and Fluorine Substituted 2D-Conjugated Polymers Reach 9.5% Efficiency. J. Am. Chem. Soc. 2016, 138, 4657–4664. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ye, L.; Zhao, W.; Zhang, S.; Mukherjee, S.; Ade, H.; Hou, J. Energy-Level Modulation of Small-Molecule Electron Acceptors to Achieve over 12% Efficiency in Polymer Solar Cells. Adv. Mater. 2016, 28, 9423–9429. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Li, S.; Yao, H.; Zhang, S.; Zhang, Y.; Yang, B.; Hou, J. Molecular Optimization Enables over 13% Efficiency in Organic Solar Cells. J. Am. Chem. Soc. 2017, 139, 7148–7151. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Hwang, Y.-J.; Courtright, B.A.E.; Eberle, F.N.; Subramaniyan, S.; Jenekhe, S.A. Fine-Tuning the 3D Structure of Nonfullerene Electron Acceptors Toward High-Performance Polymer Solar Cells. Adv. Mater. 2015, 27, 3266–3272. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Mu, C.; Jiang, K.; Zhao, J.; Li, Y.; Zhang, L.; Li, Z.; Lai, J.Y.L.; Hu, H.; Ma, T.; et al. A Tetraphenylethylene Core-Based 3D Structure Small Molecular Acceptor Enabling Efficient Non-Fullerene Organic Solar Cells. Adv. Mater. 2015, 27, 1015–1020. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Meng, D.; Cai, Y.; Fan, B.; Li, Y.; Jiang, W.; Huo, L.; Sun, Y.; Wang, Z. Non-Fullerene-Acceptor-Based Bulk-Heterojunction Organic Solar Cells with Efficiency over 7%. J. Am. Chem. Soc. 2015, 137, 11156–11162. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Sun, K.; Jiang, W.; Zhang, S.; Zhao, W.; Yao, H.; Wang, Z.; Hou, J. Enhanced Efficiency in Fullerene-Free Polymer Solar Cell by Incorporating Fine-designed Donor and Acceptor Materials. ACS Appl. Mater. Interfaces 2015, 7, 9274–9280. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Li, C.-Z.; Chueh, C.-C.; Williams, S.T.; Jiang, W.; Wang, Z.-H.; Yu, J.-S.; Jen, A.K.Y. Integrated Molecular, Interfacial, and Device Engineering towards High-Performance Non-Fullerene Based Organic Solar Cells. Adv. Mater. 2014, 26, 5708–5714. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhan, C.; Yao, J. Non-Fullerene Organic Solar Cells with 6.1% Efficiency through Fine-Tuning Parameters of the Film-Forming Process. Chem. Mater. 2015, 27, 166–173. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Y.; Lin, H.; Liu, Y.; Jiang, K.; Mu, C.; Ma, T.; Lin Lai, J.Y.; Hu, H.; Yu, D.; et al. High-efficiency non-fullerene organic solar cells enabled by a difluorobenzothiadiazole-based donor polymer combined with a properly matched small molecule acceptor. Energy Environ. Sci. 2015, 8, 520–525. [Google Scholar] [CrossRef]
- Zhong, Y.; Trinh, M.T.; Chen, R.; Wang, W.; Khlyabich, P.P.; Kumar, B.; Xu, Q.; Nam, C.-Y.; Sfeir, M.Y.; Black, C.; et al. Efficient Organic Solar Cells with Helical Perylene Diimide Electron Acceptors. J. Am. Chem. Soc. 2014, 136, 15215–15221. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Sun, D.; Zhong, C.; Liu, T.; Fan, B.; Huo, L.; Li, Y.; Jiang, W.; Choi, H.; Kim, T.; et al. High-Performance Solution-Processed Non-Fullerene Organic Solar Cells Based on Selenophene-Containing Perylene Bisimide Acceptor. J. Am. Chem. Soc. 2016, 138, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Earmme, T.; Ren, G.; Saeki, A.; Yoshikawa, S.; Murari, N.M.; Subramaniyan, S.; Crane, M.J.; Seki, S.; Jenekhe, S.A. Beyond Fullerenes: Design of Nonfullerene Acceptors for Efficient Organic Photovoltaics. J. Am. Chem. Soc. 2014, 136, 14589–14597. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Zhou, Y.; Zheng, Y.-Q.; Pei, J.; Zhao, D. Towards rational design of organic electron acceptors for photovoltaics: A study based on perylenediimide derivatives. Chem. Sci. 2013, 4, 4389–4394. [Google Scholar] [CrossRef]
- Margulies, E.A.; Shoer, L.E.; Eaton, S.W.; Wasielewski, M.R. Excimer formation in cofacial and slip-stacked perylene-3,4:9,10-bis(dicarboximide) dimers on a redox-inactive triptycene scaffold. Phys. Chem. Chem. Phys. 2014, 16, 23735–23742. [Google Scholar] [CrossRef] [PubMed]
- Hartnett, P.E.; Timalsina, A.; Matte, H.S.S.R.; Zhou, N.; Guo, X.; Zhao, W.; Facchetti, A.; Chang, R.P.H.; Hersam, M.C.; Wasielewski, M.R.; et al. Slip-Stacked Perylenediimides as an Alternative Strategy for High Efficiency Nonfullerene Acceptors in Organic Photovoltaics. J. Am. Chem. Soc. 2014, 136, 16345–16356. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-Y.; Wu, C.-H.; Li, C.-Z.; Liu, S.-Q.; Wei, K.-H.; Chen, H.-Z.; Jen, A.K.Y. A Tetraperylene Diimides Based 3D Nonfullerene Acceptor for Efficient Organic Photovoltaics. Adv. Sci. 2015, 2, 1500014. [Google Scholar] [CrossRef] [PubMed]
- Shivanna, R.; Shoaee, S.; Dimitrov, S.; Kandappa, S.K.; Rajaram, S.; Durrant, J.R.; Narayan, K.S. Charge generation and transport in efficient organic bulk heterojunction solar cells with a perylene acceptor. Energy Environ. Sci. 2014, 7, 435–441. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, Z.; Ye, L.; Zhan, C.; Hou, J.; Zhang, S.; Jiang, B.; Zhao, Y.; Huang, J.; Zhang, S.; et al. A Potential Perylene Diimide Dimer-Based Acceptor Material for Highly Efficient Solution-Processed Non-Fullerene Organic Solar Cells with 4.03% Efficiency. Adv. Mater. 2013, 25, 5791–5797. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.-J.; Jimenez, A.J.; Burschka, C.; Wurthner, F. Bay-substituted perylene bisimide dye with an undistorted planar scaffold and outstanding solid state fluorescence properties. Chem. Commun. 2012, 48, 12050–12052. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, M.G.; Pla, S.; Boj, P.G.; Villalvilla, J.M.; Quintana, J.A.; Diaz-Garcia, M.A.; Fernandez-Lazaro, F.; Sastre-Santos, A. 1,7-Bay-Substituted Perylenediimide Derivative with Outstanding Laser Performance. Adv. Opt. Mater. 2013, 1, 933–938. [Google Scholar] [CrossRef] [Green Version]
- Han, P.L.; Viterisi, A.; Ferre-Borrull, J.; Pallares, J.; Marsal, L.F. Morphology-driven photocurrent enhancement in PTB7/PC71BM bulk heterojunction solar cells via the use of ternary solvent processing blends. Org. Electron. 2017, 41, 229–236. [Google Scholar] [CrossRef]
- Deng, L.; Wang, K.; Zhao, C.X.; Yan, H.; Britten, J.F.; Xu, G. Phase and Texture of Solution-Processed Copper Phthalocyanine Thin Films Investigated by Two-Dimensional Grazing Incidence X-ray Diffraction. Crystals 2011, 1, 112–119. [Google Scholar] [CrossRef]
- Blom, P.W.M.; Mihailetchi, V.D.; Koster, L.J.A.; Markov, D.E. Device Physics of Polymer:Fullerene Bulk Heterojunction Solar Cells. Adv. Mater. 2007, 19, 1551–1566. [Google Scholar] [CrossRef]
- Mihailetchi, V.D.; van Duren, J.K.J.; Blom, P.W.M.; Hummelen, J.C.; Janssen, R.A.J.; Kroon, J.M.; Rispens, M.T.; Verhees, W.J.H.; Wienk, M.M. Electron Transport in a Methanofullerene. Adv. Funct. Mater. 2003, 13, 43–46. [Google Scholar] [CrossRef]
- Credgington, D.; Durrant, J.R. Insights from Transient Optoelectronic Analyses on the Open-Circuit Voltage of Organic Solar Cells. J. Phys. Chem. Lett. 2012, 3, 1465–1478. [Google Scholar] [CrossRef] [PubMed]
- Maurano, A.; Hamilton, R.; Shuttle, C.G.; Ballantyne, A.M.; Nelson, J.; O’Regan, B.; Zhang, W.M.; McCulloch, I.; Azimi, H.; Morana, M.; et al. Recombination Dynamics as a Key Determinant of Open Circuit Voltage in Organic Bulk Heterojunction Solar Cells: A Comparison of Four Different Donor Polymers. Adv. Mater. 2010, 22, 4987–4992. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Belmonte, G.; Bisquert, J. Open-circuit voltage limit caused by recombination through tail states in bulk heterojunction polymer-fullerene solar cells. Appl. Phys. Lett. 2010, 96, 113301–113303. [Google Scholar] [CrossRef]
- Etxebarria, I.; Guerrero, A.; Albero, J.; Garcia-Belmonte, G.; Palomares, E.; Pacios, R. Inverted vs standard PTB7:PC70BM organic photovoltaic devices. The benefit of highly selective and extracting contacts in device performance. Org. Electron. 2014, 15, 2756–2762. [Google Scholar] [CrossRef]
- Foertig, A.; Kniepert, J.; Gluecker, M.; Brenner, T.; Dyakonov, V.; Neher, D.; Deibel, C. Nongeminate and Geminate Recombination in PTB7: PCBM Solar Cells. Adv. Funct. Mater. 2014, 24, 1306–1311. [Google Scholar] [CrossRef]
- Rauh, D.; Deibel, C.; Dyakonov, V. Charge Density Dependent Nongeminate Recombination in Organic Bulk Heterojunction Solar Cells. Adv. Funct. Mater. 2012, 22, 3371–3377. [Google Scholar] [CrossRef]
- Guerrero, A.; Montcada, N.F.; Ajuria, J.; Etxebarria, I.; Pacios, R.; Garcia-Belmonte, G.; Palomares, E. Charge carrier transport and contact selectivity limit the operation of PTB7-based organic solar cells of varying active layer thickness. J. Mater. Chem. A 2013, 1, 12345–12354. [Google Scholar] [CrossRef]
- Eng, M.P.; Barnes, P.R.F.; Durrant, J.R. Concentration-Dependent Hole Mobility and Recombination Coefficient in Bulk Heterojunctions Determined from Transient Absorption Spectroscopy. J. Phys. Chem. Lett. 2010, 1, 3096–3100. [Google Scholar] [CrossRef]
- Shuttle, C.G.; Hamilton, R.; Nelson, J.; O’Regan, B.C.; Durrant, J.R. Measurement of Charge-Density Dependence of Carrier Mobility in an Organic Semiconductor Blend. Adv. Funct. Mater. 2010, 20, 698–702. [Google Scholar] [CrossRef]
- Shuttle, C.G.; Hamilton, R.; O’Regan, B.C.; Nelson, J.; Durrant, J.R. Charge-density-based analysis of the current–voltage response of polythiophene/fullerene photovoltaic devices. Proc. Natl. Acad. Sci. USA 2010, 107, 16448–16452. [Google Scholar] [CrossRef] [PubMed]
- Spoltore, D.; Oosterbaan, W.D.; Khelifi, S.; Clifford, J.N.; Viterisi, A.; Palomares, E.; Burgelman, M.; Lutsen, L.; Vanderzande, D.; Manca, J. Effect of Polymer Crystallinity in P3HT:PCBM Solar Cells on Band Gap Trap States and Apparent Recombination Order. Adv. Energy Mater. 2013, 3, 466–471. [Google Scholar] [CrossRef]
- Maurano, A.; Shuttle, C.C.; Hamilton, R.; Ballantyne, A.M.; Nelson, J.; Zhang, W.; Heeney, M.; Durrant, J.R. Transient Optoelectronic Analysis of Charge Carrier Losses in a Selenophene/Fullerene Blend Solar Cell. J. Phys. Chem. C 2011, 115, 5947–5957. [Google Scholar] [CrossRef]
- Fernandez, D.; Viterisi, A.; Challuri, V.; Ryan, J.W.; Martinez-Ferrero, E.; Gispert-Guirado, F.; Martinez, M.; Escudero, E.; Stenta, C.; Marsal, L.F.; et al. Understanding the Limiting Factors of Solvent-Annealed Small-Molecule Bulk-Heterojunction Organic Solar Cells from a Chemical Perspective. Chemsuschem 2017, 10, 3118–3134. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, D.; Viterisi, A.; William Ryan, J.; Gispert-Guirado, F.; Vidal, S.; Filippone, S.; Martin, N.; Palomares, E. Small molecule BHJ solar cells based on DPP(TBFu)(2) and diphenylmethanofullerenes (DPM): Linking morphology, transport, recombination and crystallinity. Nanoscale 2014, 6, 5871–5878. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.W.; Kirchartz, T.; Viterisi, A.; Nelson, J.; Palomares, E. Understanding the Effect of Donor Layer Thickness and a MoO3 Hole Transport Layer on the Open-Circuit Voltage in Squaraine/C-60 Bilayer Solar Cells. J. Phys. Chem. C 2013, 117, 19866–19874. [Google Scholar] [CrossRef]
- Montcada, N.F.; Pelado, B.; Viterisi, A.; Albero, J.; Coro, J.; de la Cruz, P.; Langa, F.; Palomares, E. High open circuit voltage in efficient thiophene-based small molecule solution processed organic solar cells. Org. Electron. 2013, 14, 2826–2832. [Google Scholar] [CrossRef]
- MacKenzie, R.C.I.; Kirchartz, T.; Dibb, G.F.A.; Nelson, J. Modeling Nongeminate Recombination in P3HT:PCBM Solar Cells. J. Phys. Chem. C 2011, 115, 9806–9813. [Google Scholar] [CrossRef]
- Kirchartz, T.; Pieters, B.E.; Kirkpatrick, J.; Rau, U.; Nelson, J. Recombination via tail states in polythiophene:fullerene solar cells. Phys. Rev. B Condens. Matter Mater. Phys. 2011, 83, 115209. [Google Scholar] [CrossRef] [Green Version]
- Kirchartz, T.; Nelson, J. Meaning of reaction orders in polymer:fullerene solar cells. Phys. Rev. B Condens. Matter Mater. Phys. 2012, 86, 165201. [Google Scholar] [CrossRef] [Green Version]
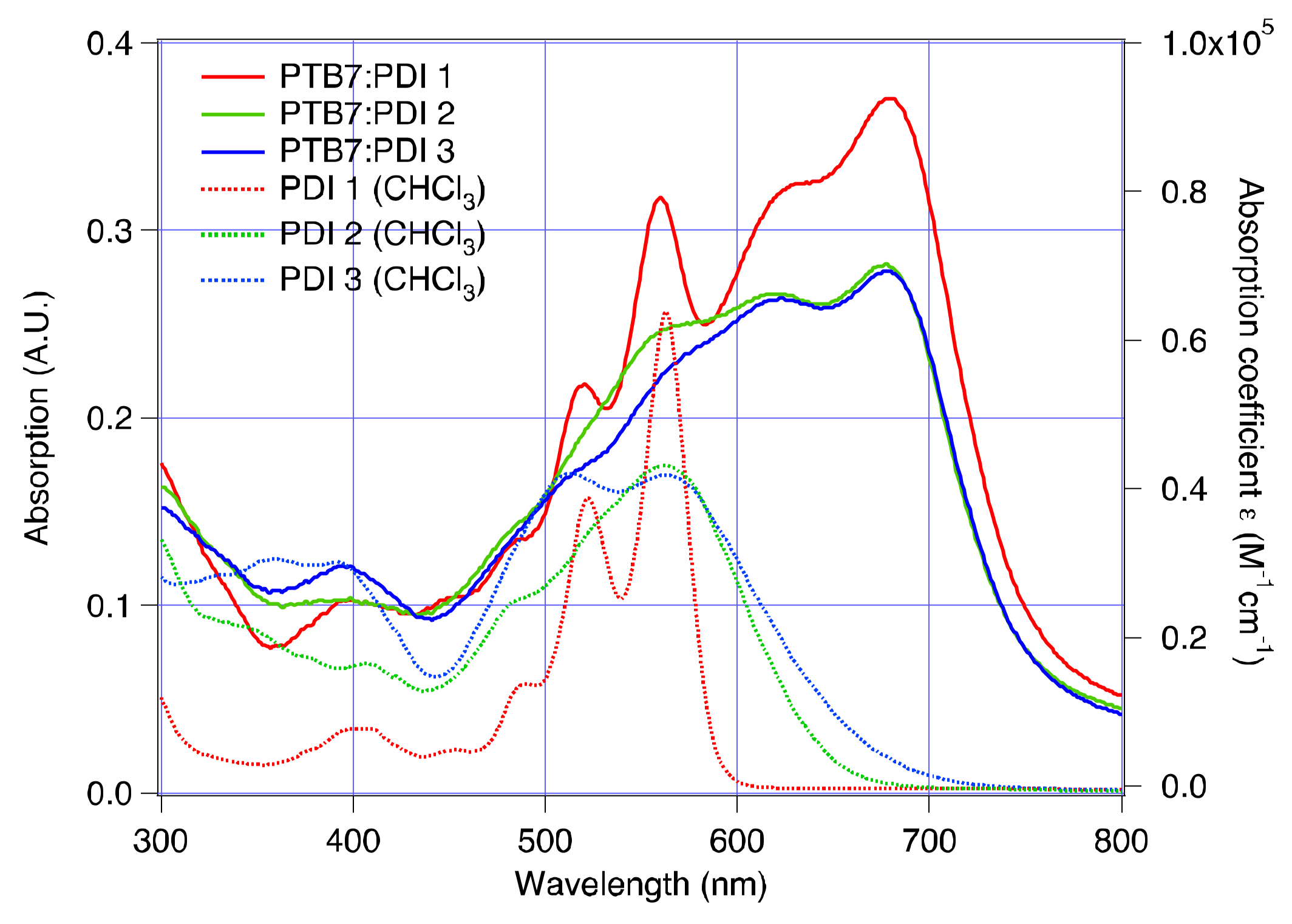
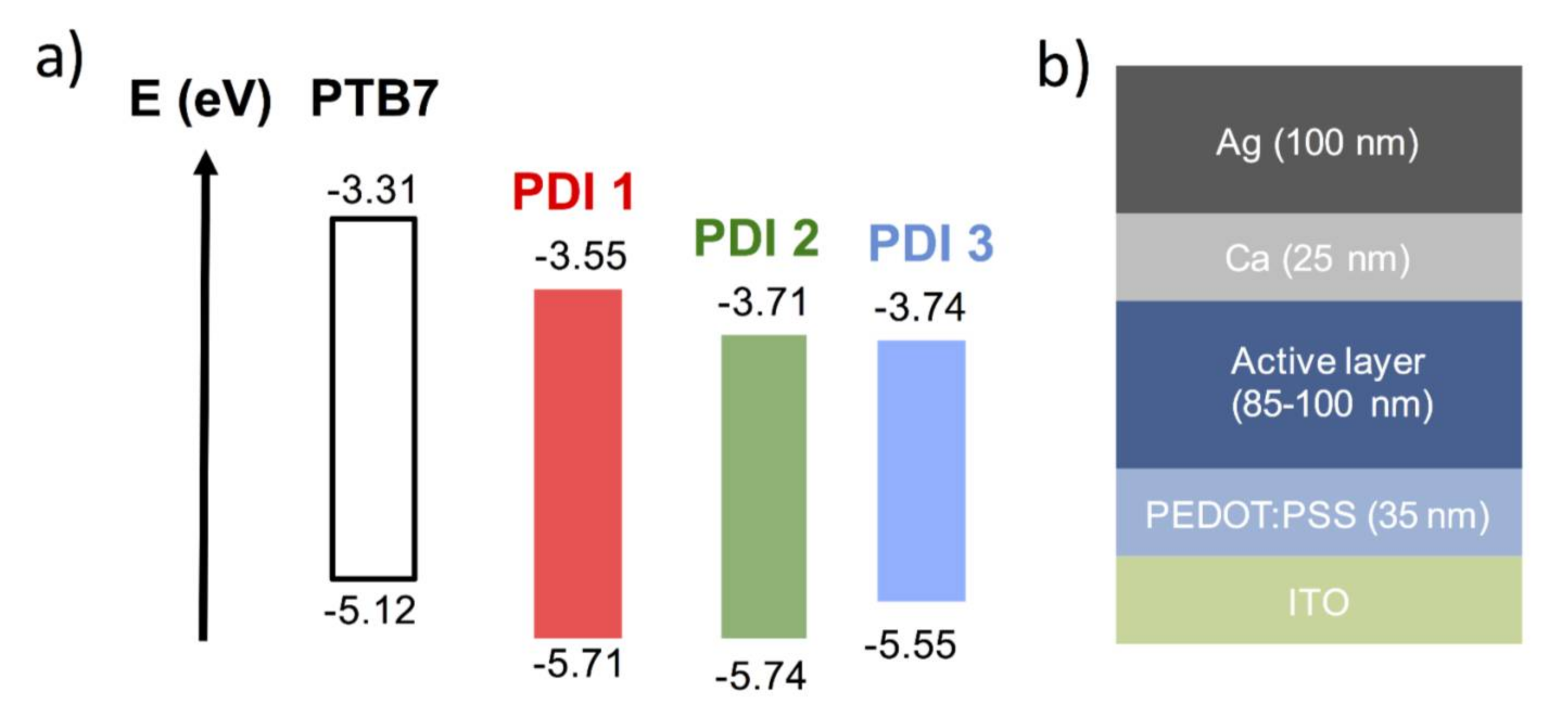
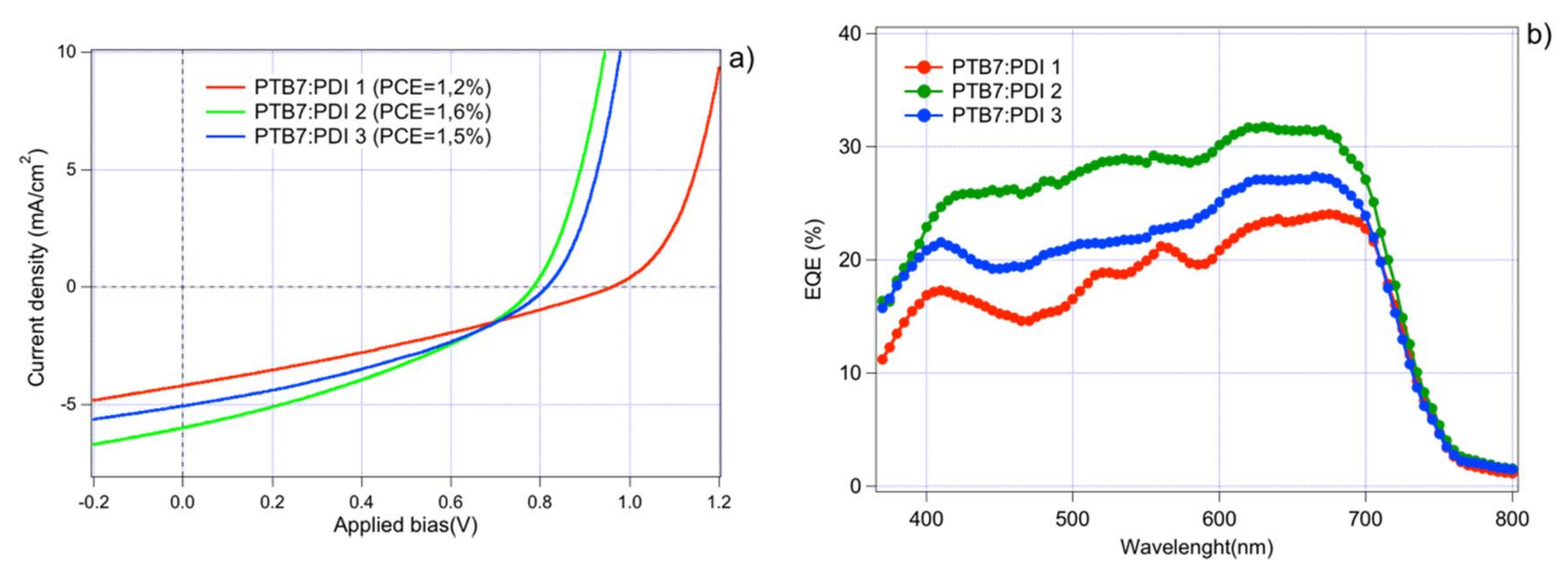


| Acceptor | AL Thickness (nm) | JSC (mA/cm2) | VOC (mV) | FF | PCE (%) |
|---|---|---|---|---|---|
| PDI 1 | 100 | 4.20 | 0.960 | 0.29 | 1.20 |
| PDI 2 | 90 | 5.98 | 0.784 | 0.35 | 1.61 |
| PDI 3 | 85 | 5.04 | 0.814 | 0.36 | 1.54 |
| Acceptor | n0 | γ | β | φ | μh (cm2/V) | μe (cm2/Vs) | RS (Ω/cm2) | Rshunt (Ω/cm2) |
|---|---|---|---|---|---|---|---|---|
| PDI 1 | 5.7 × 1015 | 1.47 | 25.7 | 16.4 | 3.4 × 10−4 | 2.75 × 10−7 | 28.3 | 311.6 |
| PDI 2 | 3.0 × 1015 | 2.21 | 13.7 | 8.8 | 4.3 × 10−4 | 6.02 × 10−7 | 7.5 | 255.7 |
| PDI 3 | 3.8 × 1014 | 2.14 | 5.2 | 4.0 | 4.1 × 10−4 | 9.53 × 10−7 | 7.05 | 327.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stenta, C.; Molina, D.; Viterisi, A.; Montero-Rama, M.P.; Pla, S.; Cambarau, W.; Fernández-Lázaro, F.; Palomares, E.; Marsal, L.F.; Sastre-Santos, Á. Diphenylphenoxy-Thiophene-PDI Dimers as Acceptors for OPV Applications with Open Circuit Voltage Approaching 1 Volt. Nanomaterials 2018, 8, 211. https://doi.org/10.3390/nano8040211
Stenta C, Molina D, Viterisi A, Montero-Rama MP, Pla S, Cambarau W, Fernández-Lázaro F, Palomares E, Marsal LF, Sastre-Santos Á. Diphenylphenoxy-Thiophene-PDI Dimers as Acceptors for OPV Applications with Open Circuit Voltage Approaching 1 Volt. Nanomaterials. 2018; 8(4):211. https://doi.org/10.3390/nano8040211
Chicago/Turabian StyleStenta, Caterina, Desiré Molina, Aurélien Viterisi, María Pilar Montero-Rama, Sara Pla, Werther Cambarau, Fernando Fernández-Lázaro, Emilio Palomares, Lluis F. Marsal, and Ángela Sastre-Santos. 2018. "Diphenylphenoxy-Thiophene-PDI Dimers as Acceptors for OPV Applications with Open Circuit Voltage Approaching 1 Volt" Nanomaterials 8, no. 4: 211. https://doi.org/10.3390/nano8040211