Synthesis, Optical, and Structural Studies of Iron Sulphide Nanoparticles and Iron Sulphide Hydroxyethyl Cellulose Nanocomposites from Bis-(Dithiocarbamato)Iron(II) Single-Source Precursors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Synthesis of Bis-(Dithiocarbamato)Iron(II) Complexes
2.3. Preparation of Iron Sulphide Nanoparticles
2.4. Preparation of Iron Sulphide Nanoparticles/hydroxyethylcellulose (HEC) Nanocomposites
2.5. Characterization Techniques
3. Results and Discussion
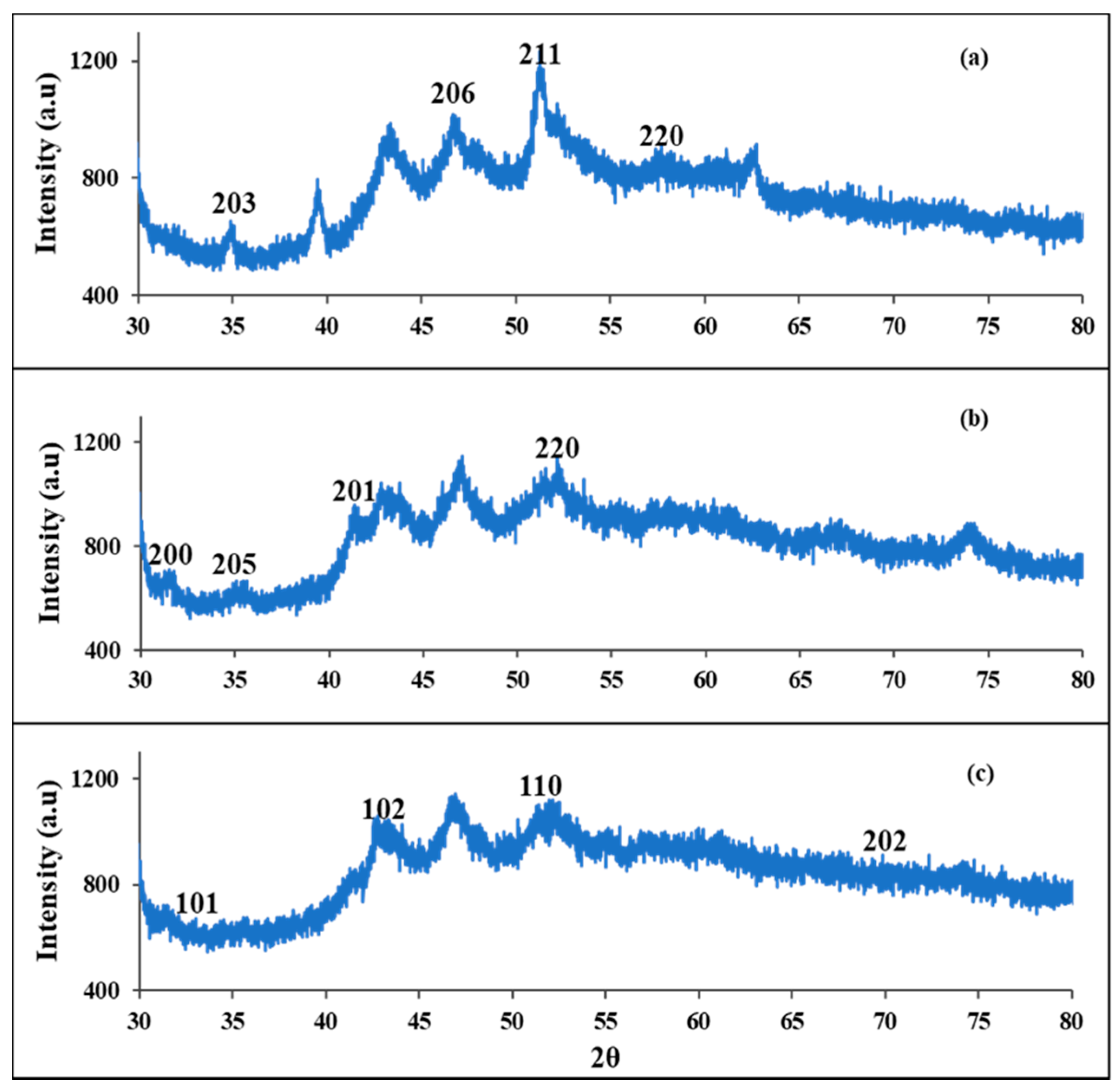
3.1. Powder X-ray Diffraction (pXRD) Studies of FeS1, FeS2, and FeS3 Iron Sulphide Nanoparticles
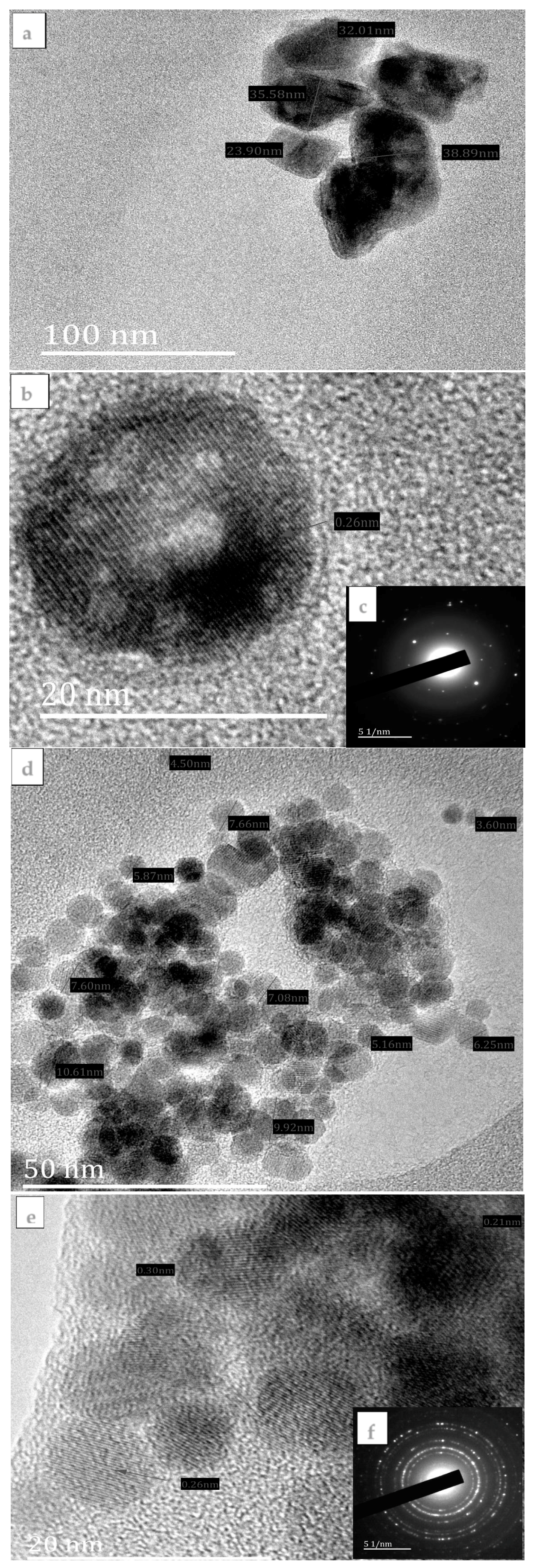
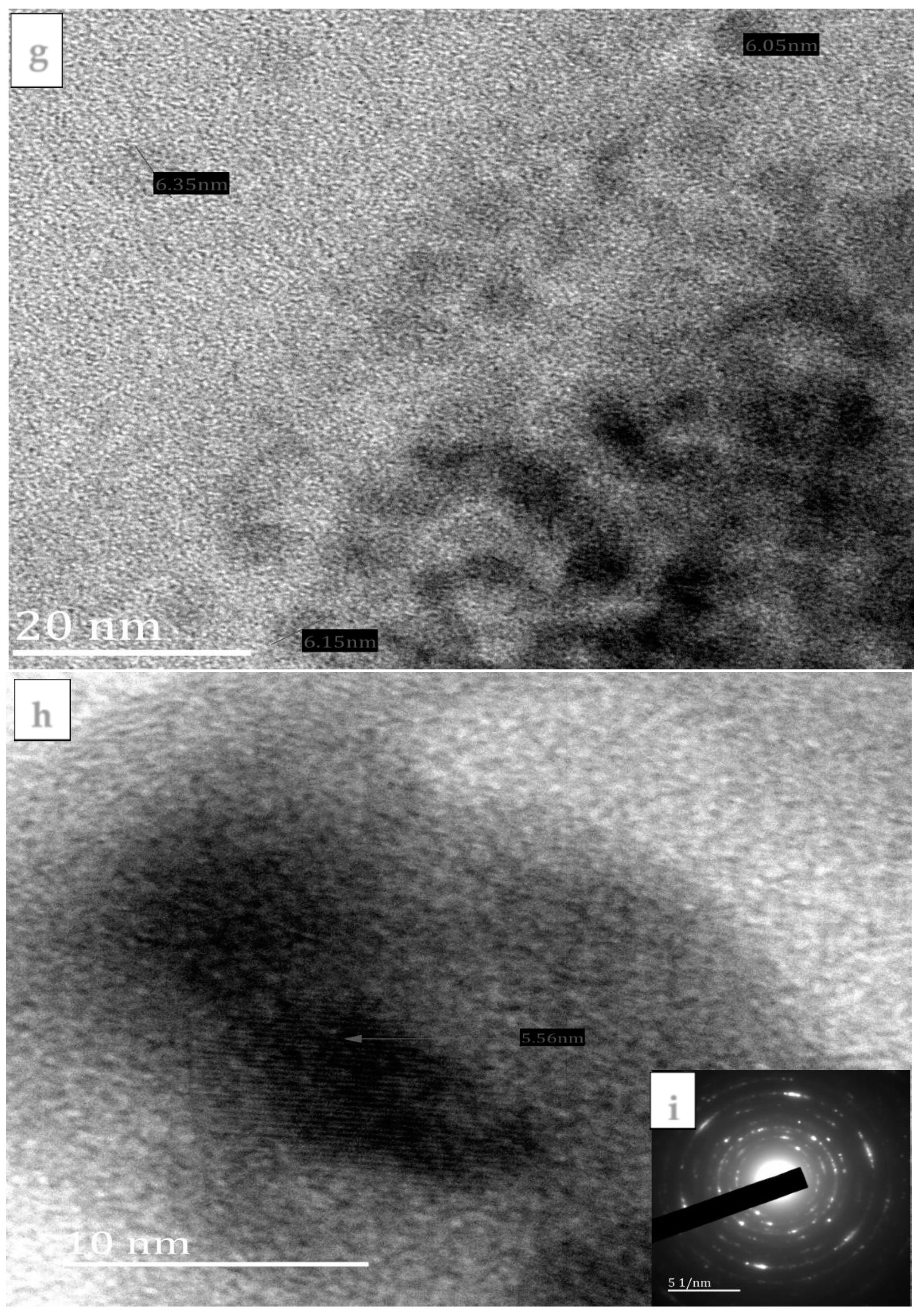
3.2. Morphological Studies of the FeS1, FeS2, and FeS3 Iron Sulphide Nanoparticles
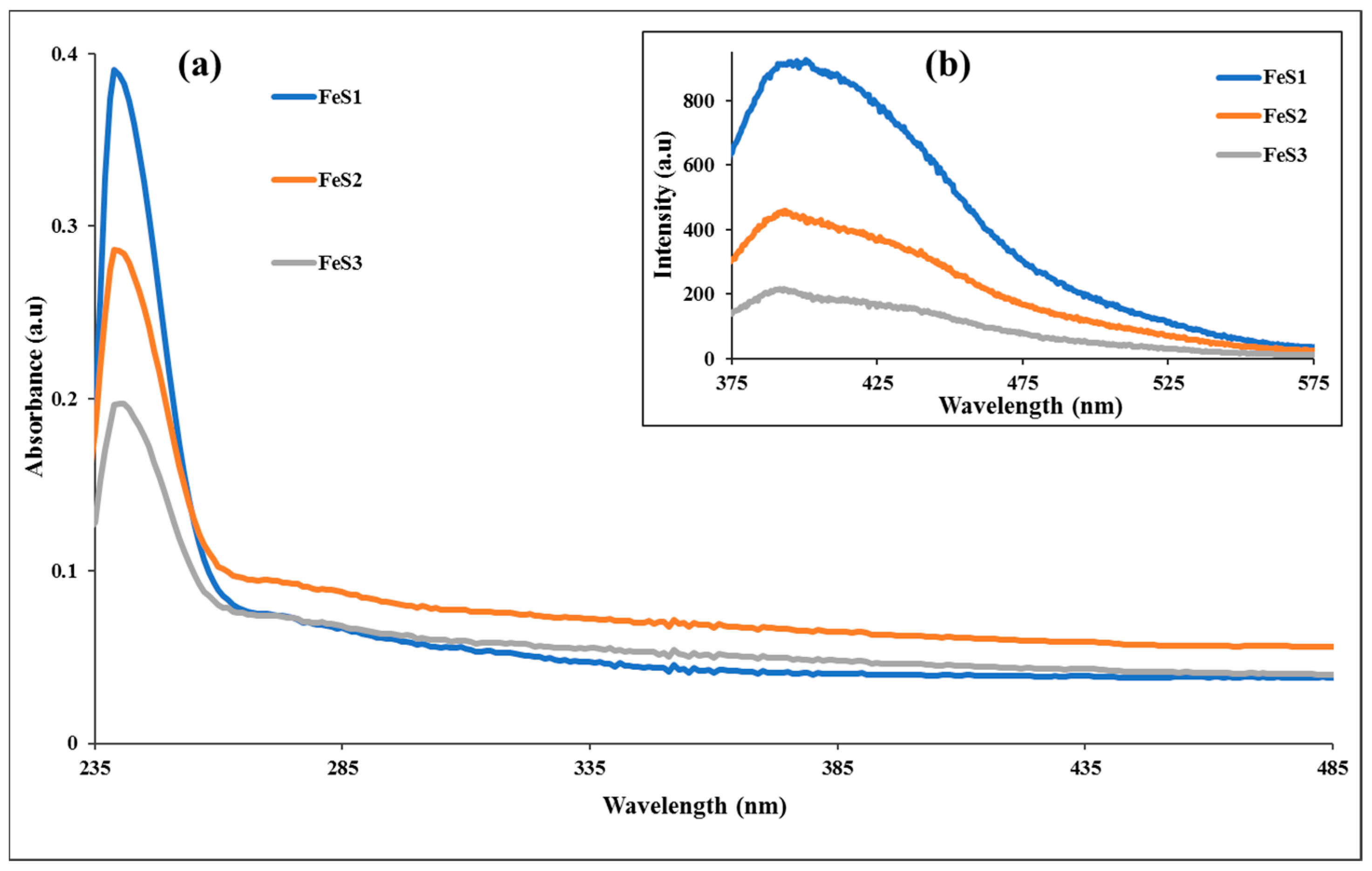
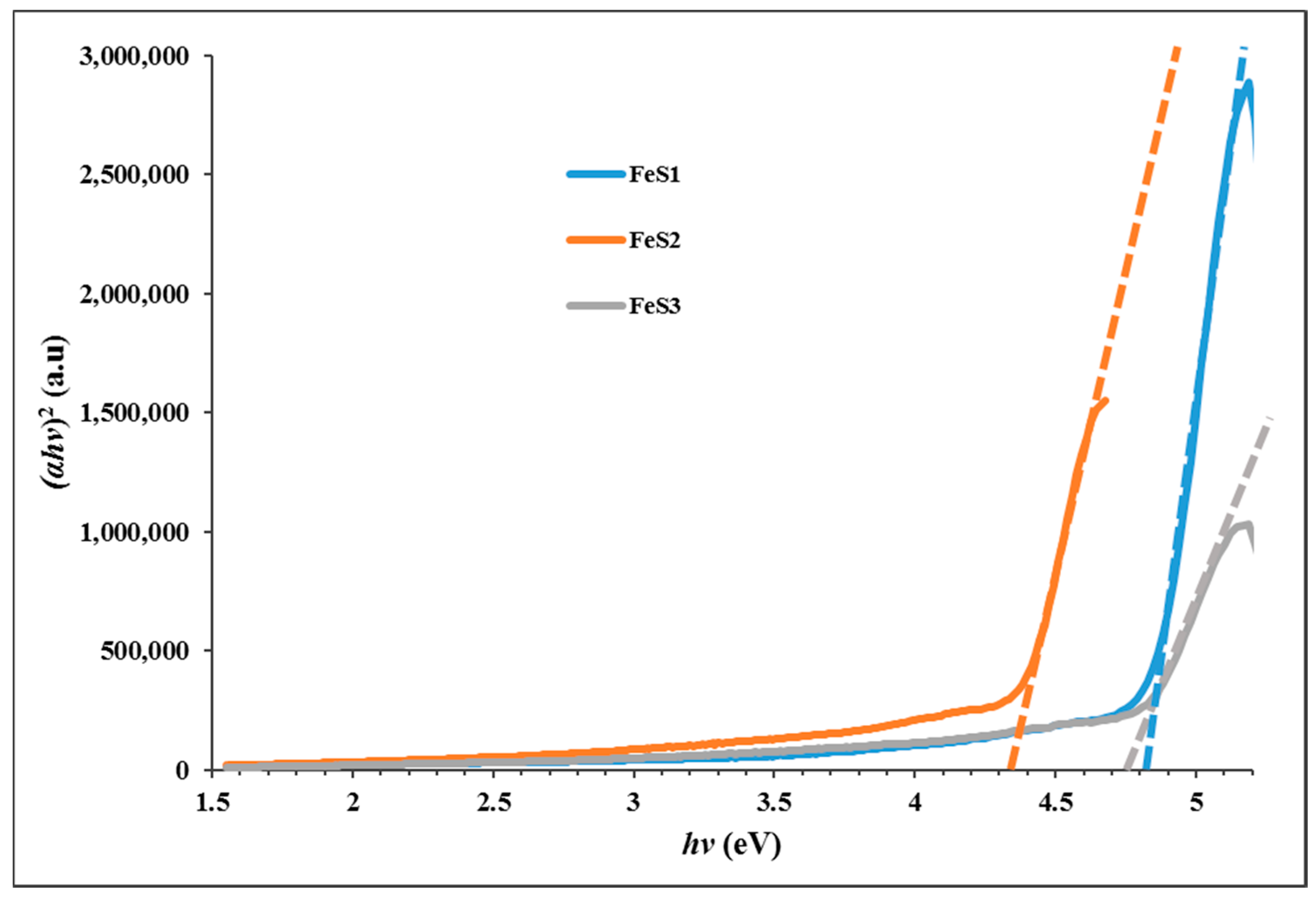
3.3. Optical Studies of FeS1, FeS2, and FeS3 Iron Sulphide Nanoparticles
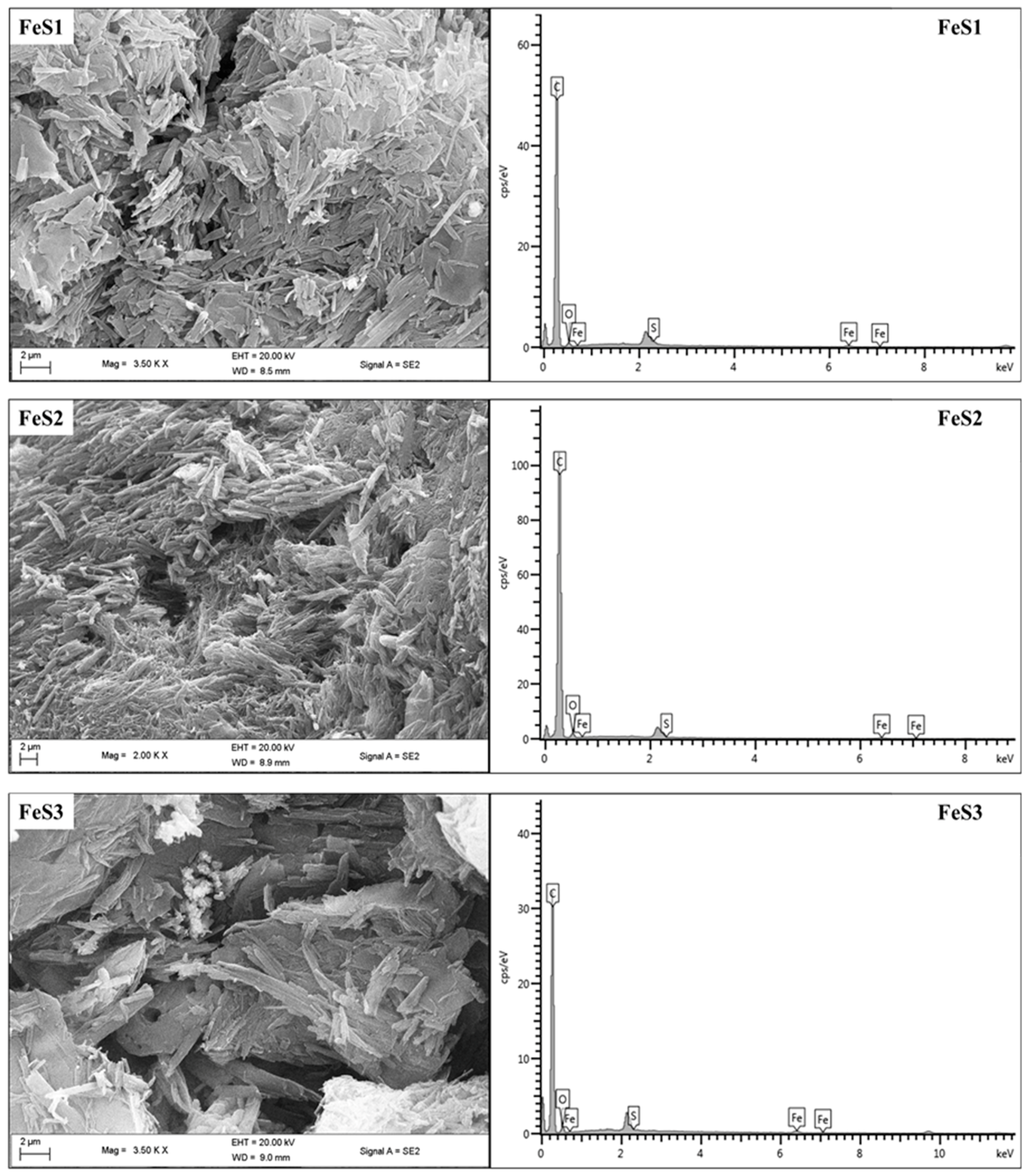
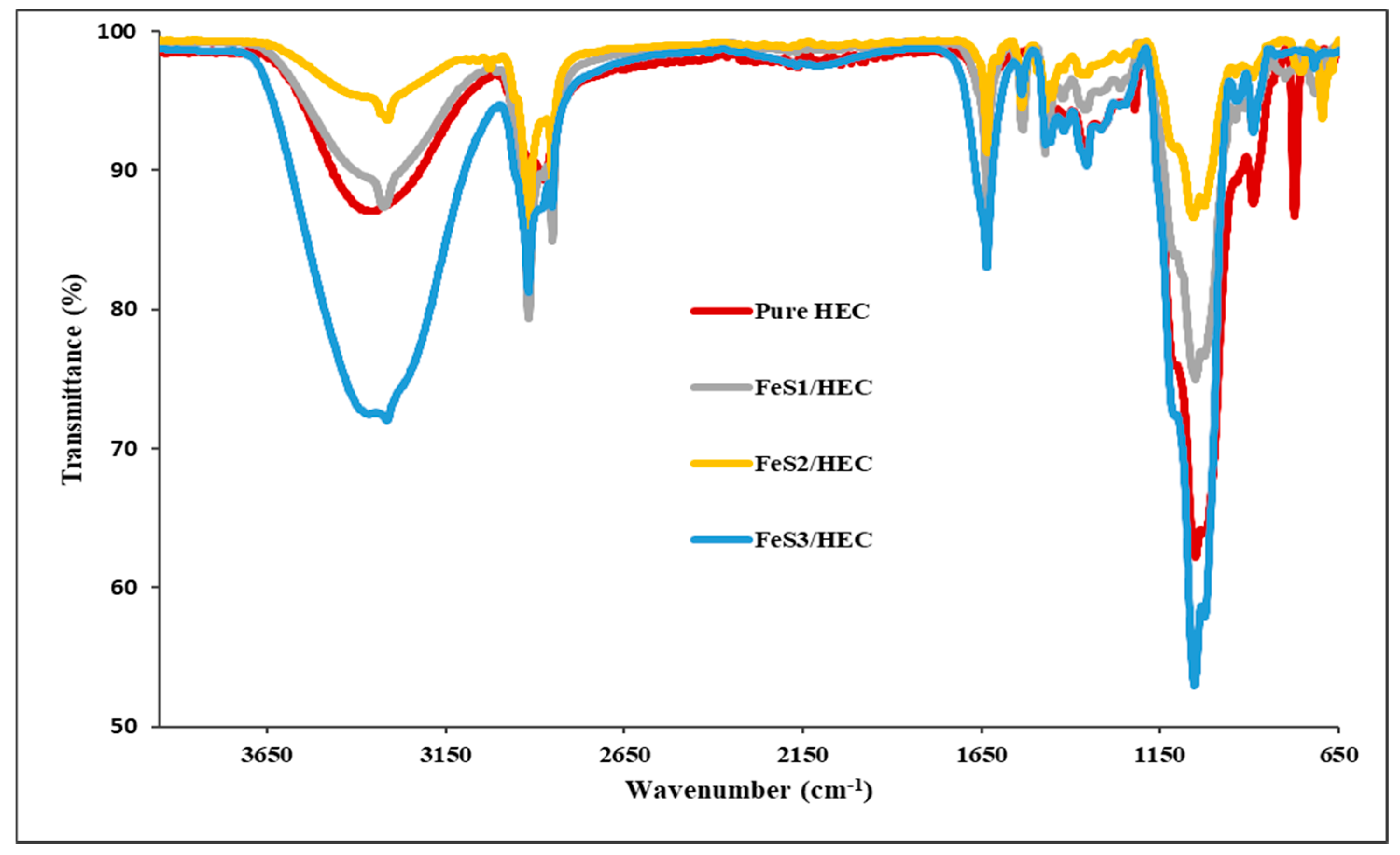
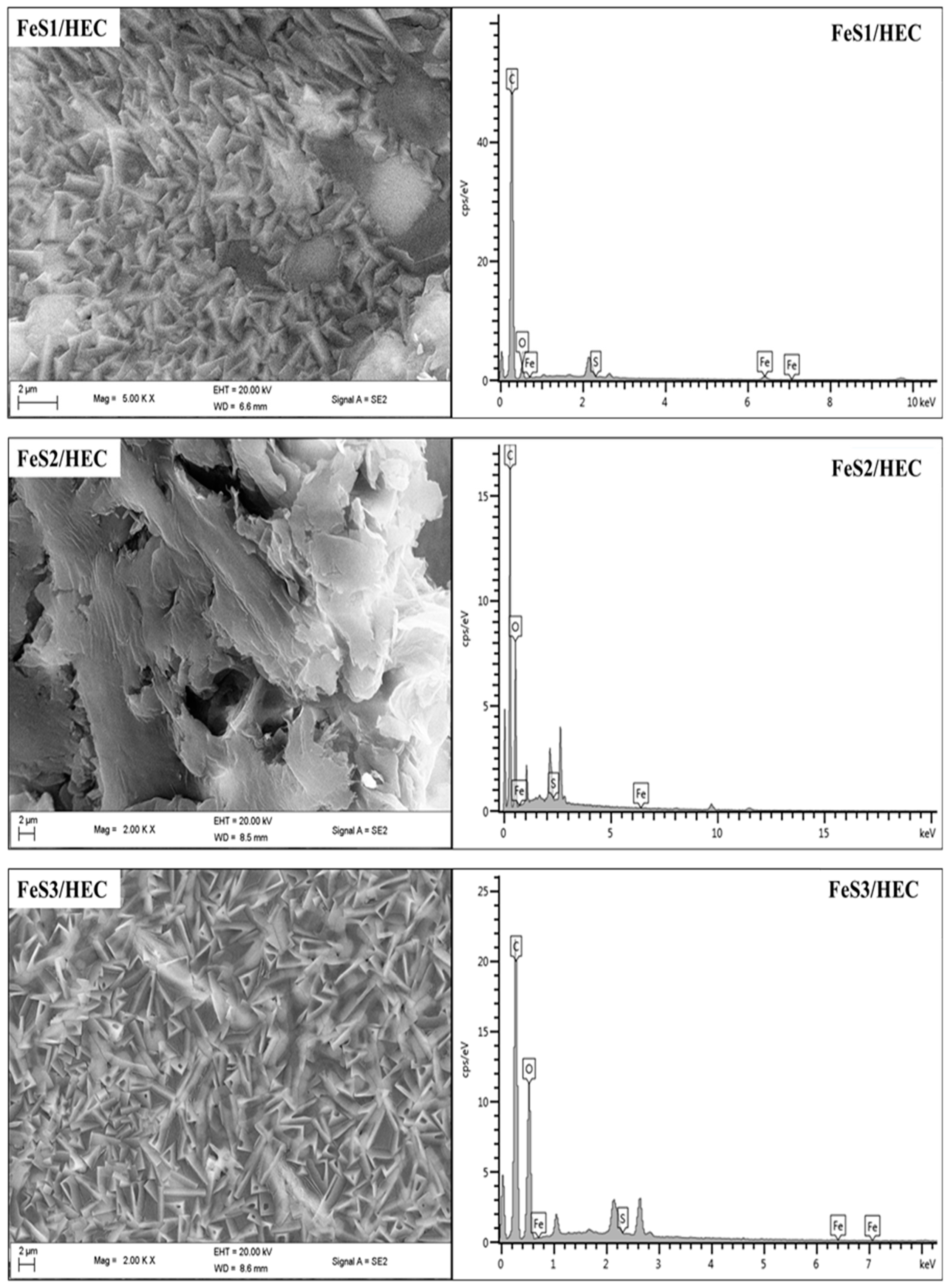
3.4. FTIR Spectra and FESEM Structural Studies of FeS1, FeS2, and FeS3 Iron Sulphide/HEC Nanocomposites
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pal, S.L.; Jana, U.; Manna, P.; Mohanta, G.; Manavalan, R. Nanoparticle: An overview of preparation and characterization. J. Appl. Pharm. Sci. 2011, 1, 228–234. [Google Scholar]
- Wang, X.; Wang, Y.; Chen, Z.G.; Shin, D.M. Advances of cancer therapy by nanotechnology. Cancer Res. Treat. 2009, 41, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, L.; Chen, Z.G.; Shin, D.M. Application of nanotechnology in cancer therapy and imaging. CA Cancer J. Clin. 2008, 58, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Abou El-Nour, K.M.M.; Eftaiha, A.A.; Al-Warthan, A.; Ammar, R.A.A. Synthesis and applications of silver nanoparticles. Arab. J. Chem. 2010, 3, 135–140. [Google Scholar] [CrossRef]
- Shi, X.; Sun, K.; Balogh, L.P.; Baker, J.R. Synthesis, characterization, and manipulation of dendrimer-stabilized iron sulfide nanoparticles. Nanotechnology 2006, 17, 4554–4560. [Google Scholar] [CrossRef]
- Vaseem, M.; Umar, A.; Hahn, Y.-B. ZnO nanoparticles: Growth, properties, and applications. In Metal Oxide. Nanostructures and Their Applications; American Scientific Publisher: New York, NY, USA, 2010; pp. 1–36, Chapter 4. [Google Scholar]
- Borm, P.J.; Robbins, D.; Haubold, S.; Kuhlbusch, T.; Fissan, H.; Donaldson, K.; Schins, R.; Stone, V.; Kreyling, W.; Lademann, J.; et al. The potential risks of nanomaterials: A review carried out for ECETOC. Part. Fibre Toxicol. 2006, 3, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yaari, Z.; Da Silva, D.; Zinger, A.; Goldman, E.; Kajal, A.; Tshuva, R.; Barak, E.; Dahan, N.; Hershkovitz, D.; Goldfeder, M. Theranostic barcoded nanoparticles for personalized cancer medicine. Nat. Commun. 2016, 7, 13325. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Agarwal, P.; Zhao, S.; Yu, J.; Lu, X.; He, X. A biomimetic hybrid nanoplatform for encapsulation and precisely controlled delivery of theranostic agents. Nat. Commun. 2015, 6, 10081. [Google Scholar] [CrossRef] [PubMed]
- John, P. Green synthesis of silver nanoparticles using Ganoderma neo-japonicum Imazeki: A potential cytotoxic agent against breast cancer cells. Int. J. Nanomed. 2013, 8, 4399–4413. [Google Scholar]
- Chen, J.; Javaheri, H.; Al-Chikh Sulaiman, B.; Dahman, Y. Synthesis, characterization and applications of nanoparticles. Appl. Nanobiomater. 2016, 1, 1–27. [Google Scholar]
- Andrew, F.P.; Ajibade, P.A. Metal complexes of alkyl-aryl dithiocarbamates: Structural studies, anticancer potentials and applications as precursors for semiconductor nanocrystals. J. Mol. Struct. 2018, 1155, 843–855. [Google Scholar] [CrossRef]
- Mbese, J.Z.; Ajibade, P.A. Synthesis, spectroscopic, structural and optical studies of Ru2S3 nanoparticles prepared from single-source molecular precursors. J. Mol. Struct. 2017, 1143, 274–281. [Google Scholar] [CrossRef]
- Ajibade, P.A.; Mbese, J.Z.; Omondi, B. Group 12 dithiocarbamate complexes: Synthesis, characterization and X-ray crystal structures of Zn(II) and Hg(II) complexes and their use as precursors for metal sulphide nanoparticles. Synth. React. Inorg. Met.-Org. Nano-Met. Chem. 2017, 47, 202–212. [Google Scholar] [CrossRef]
- Ajibade, P.A.; Botha, N.L. Synthesis, optical and structural properties of copper sulphide nanocrystals from single molecule precursors. Nanomaterials 2017, 7, 32. [Google Scholar] [CrossRef] [PubMed]
- Barrelet, C.J.; Wu, Y.; Bell, D.C.; Lieber, C.M. Synthesis of CdS and ZnS nanowires using single-source molecular precursors. J. Am. Chem. Soc. 2003, 125, 11498–11499. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Brennan, J.G.; Siegrist, T.; Carroll, P.; Stuczynski, S.; Brus, L.; Steigerwald, M. The preparation of large semiconductor clusters via the pyrolysis of a molecular precursor. J. Am. Chem. Soc. 1989, 111, 4141–4143. [Google Scholar] [CrossRef]
- Cumberland, S.L.; Hanif, K.M.; Javier, A.; Khitrov, G.A.; Strouse, G.F.; Woessner, S.M.; Yun, C.S. Inorganic clusters as single-source precursors for preparation of CdSe, ZnSe, and CdSe/ZnS nanomaterials. Chem. Mater. 2002, 14, 1576–1584. [Google Scholar] [CrossRef]
- Malik, M.A.; Revaprasadu, N.; O’Brien, P. Air-stable single-source precursors for the synthesis of chalcogenide semiconductor nanoparticles. Chem. Mater. 2001, 13, 913–920. [Google Scholar] [CrossRef]
- Trindade, T.; O’Brien, P.; Zhang, X.-M. Synthesis of CdS and CdSe nanocrystallites using a novel single-molecule precursors approach. Chem. Mater. 1997, 9, 523–530. [Google Scholar] [CrossRef] [Green Version]
- Ajibade, P.A.; Ejelonu, B.C. Group 12 dithiocarbamate complexes: Synthesis, spectral studies and their use as precursors for metal sulfides nanoparticles and nanocomposites. Spectrochim. Acta Part A 2013, 113, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Ajibade, P.A.; Onwudiwe, D.C.; Moloto, M.J. Synthesis of hexadecylamine capped nanoparticles using group 12 complexes of N-alkyl-N-phenyl dithiocarbamate as single-source precursors. Polyhedron 2011, 30, 246–252. [Google Scholar] [CrossRef]
- Paca, A.M.; Ajibade, P.A. Synthesis and structural studies of iron sulphide nanocomposites prepared from Fe(III) dithiocarbamates single source precursors. Mater. Chem. Phys. 2017, 202, 143–150. [Google Scholar] [CrossRef]
- Buchmaier, C.; Glänzer, M.; Torvisco, A.; Poelt, P.; Wewerka, K.; Kunert, B.; Gatterer, K.; Trimmel, G.; Rath, T. Nickel sulfide thin films and nanocrystals synthesized from nickel xanthate precursors. J. Mater. Sci. 2017, 52, 10898–10914. [Google Scholar] [CrossRef]
- Akhtar, M.; Malik, M.A.; Tuna, F.; O’Brien, P. The synthesis of iron sulfide nanocrystals from tris (O-alkylxanthato)iron(III) complexes. J. Mater. Chem. A 2013, 1, 8766–8774. [Google Scholar] [CrossRef]
- Matthews, P.D.; Akhtar, M.; Malik, M.A.; Revaprasadu, N.; O’Brien, P. Synthetic routes to iron chalcogenide nanoparticles and thin films. Dalton Trans. 2016, 45, 18803–18812. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, N.; Efrima, S. Single-precursor, one-pot versatile synthesis under near ambient conditions of tunable, single and dual band fluorescing metal sulfide nanoparticles. J. Am. Chem. Soc. 2003, 125, 2050–2051. [Google Scholar] [CrossRef] [PubMed]
- Kadajji, V.G.; Betageri, G.V. Water Soluble Polymers for Pharmaceutical Applications. Polymers 2011, 3, 1972–2009. [Google Scholar] [CrossRef]
- Singhal, S.; Garg, A.; Chandra, K. Spin crossover studies in tris(N,N′-dialkyldithiocarbamato)iron(III) complexes. Trans. Met. Chem. 2005, 30, 44–52. [Google Scholar] [CrossRef]
- Onwudiwe, D.C.; Arfin, T.; Strydom, C.A. Fe(II) and Fe(III) complexes of N-ethyl-N-phenyl dithiocarbamate: Electrical conductivity studies and Thermal properties. Electrochim. Acta 2014, 127, 283–289. [Google Scholar] [CrossRef]
- Ramalingam, K.; Srinivasan, S. Synthesis, spectral, single crystal X-ray structural, CShM and BVS characterization of iron(III) cyclohexyl dithiocarbamates and their solvothermal decomposition to nano iron(II) sulphide. J. Mol. Struct. 2015, 1100, 290–298. [Google Scholar] [CrossRef]
- Kharadi, G. Effect of the thermal decomposition and in vitro antimicrobial activity of mixed ligand copper(II) complexes. Synth. React. Inorg. Met.-Org. Nano-Met. Chem. 2012, 42, 424–431. [Google Scholar] [CrossRef]
- Ma, X.-X.; Su, Y.; He, X.-Q. Fe9S10-decorated N, S co-doped graphene as a new and efficient electrocatalyst for oxygen reduction and oxygen evolution reactions. Catal. Sci. Technol. 2017, 7, 1181–1192. [Google Scholar] [CrossRef]
- Cummins, D.R.; Russell, H.B.; Jasinski, J.B.; Menon, M.; Sunkara, M.K. Iron sulfide (FeS) nanotubes using sulfurization of hematite nanowires. Nano Lett. 2013, 13, 2423–2430. [Google Scholar] [CrossRef] [PubMed]
- Dunne, P.W.; Starkey, C.L.; Gimeno-Fabra, M.; Lester, E.H. The rapid size-and shape-controlled continuous hydrothermal synthesis of metal sulphide nanomaterials. Nanoscale 2014, 6, 2406–2418. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lim, W.P.; Wong, C.T.; Xu, H.; Yin, F.; Chin, W.S. From metal thiobenzoates to metal sulfide nanocrystals: An experimental and theoretical investigation. Nanomaterials 2012, 2, 113–133. [Google Scholar] [CrossRef] [PubMed]
- Mlowe, S.; Lewis, D.J.; Malik, M.A.; Raftery, J.; Mubofu, E.B.; O’Brien, P.; Revaprasadu, N. Heterocyclic dithiocarbamato-iron(III) complexes: Single-source precursors for aerosol-assisted chemical vapour deposition (AACVD) of iron sulfide thin films. Dalton Trans. 2016, 45, 2647–2655. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paca, A.M.; Ajibade, P.A. Synthesis, Optical, and Structural Studies of Iron Sulphide Nanoparticles and Iron Sulphide Hydroxyethyl Cellulose Nanocomposites from Bis-(Dithiocarbamato)Iron(II) Single-Source Precursors. Nanomaterials 2018, 8, 187. https://doi.org/10.3390/nano8040187
Paca AM, Ajibade PA. Synthesis, Optical, and Structural Studies of Iron Sulphide Nanoparticles and Iron Sulphide Hydroxyethyl Cellulose Nanocomposites from Bis-(Dithiocarbamato)Iron(II) Single-Source Precursors. Nanomaterials. 2018; 8(4):187. https://doi.org/10.3390/nano8040187
Chicago/Turabian StylePaca, Athandwe M., and Peter A. Ajibade. 2018. "Synthesis, Optical, and Structural Studies of Iron Sulphide Nanoparticles and Iron Sulphide Hydroxyethyl Cellulose Nanocomposites from Bis-(Dithiocarbamato)Iron(II) Single-Source Precursors" Nanomaterials 8, no. 4: 187. https://doi.org/10.3390/nano8040187




