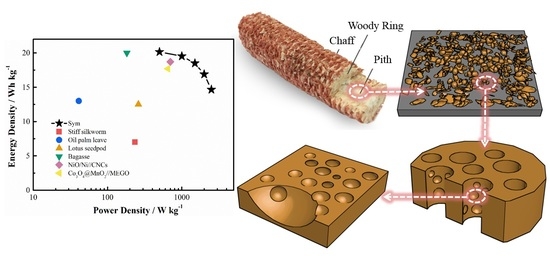
Converting Corncob to Activated Porous Carbon for Supercapacitor Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Pyrolyzed Carbon Materials
2.2. Preparation of Pyrolyzed-Activated Carbon Materials
2.3. Preparation of Carbon Electrodes and Supercapacitor Device
2.4. Characterization of Materials
2.5. Electrochemical Measurements
3. Results and Discussion
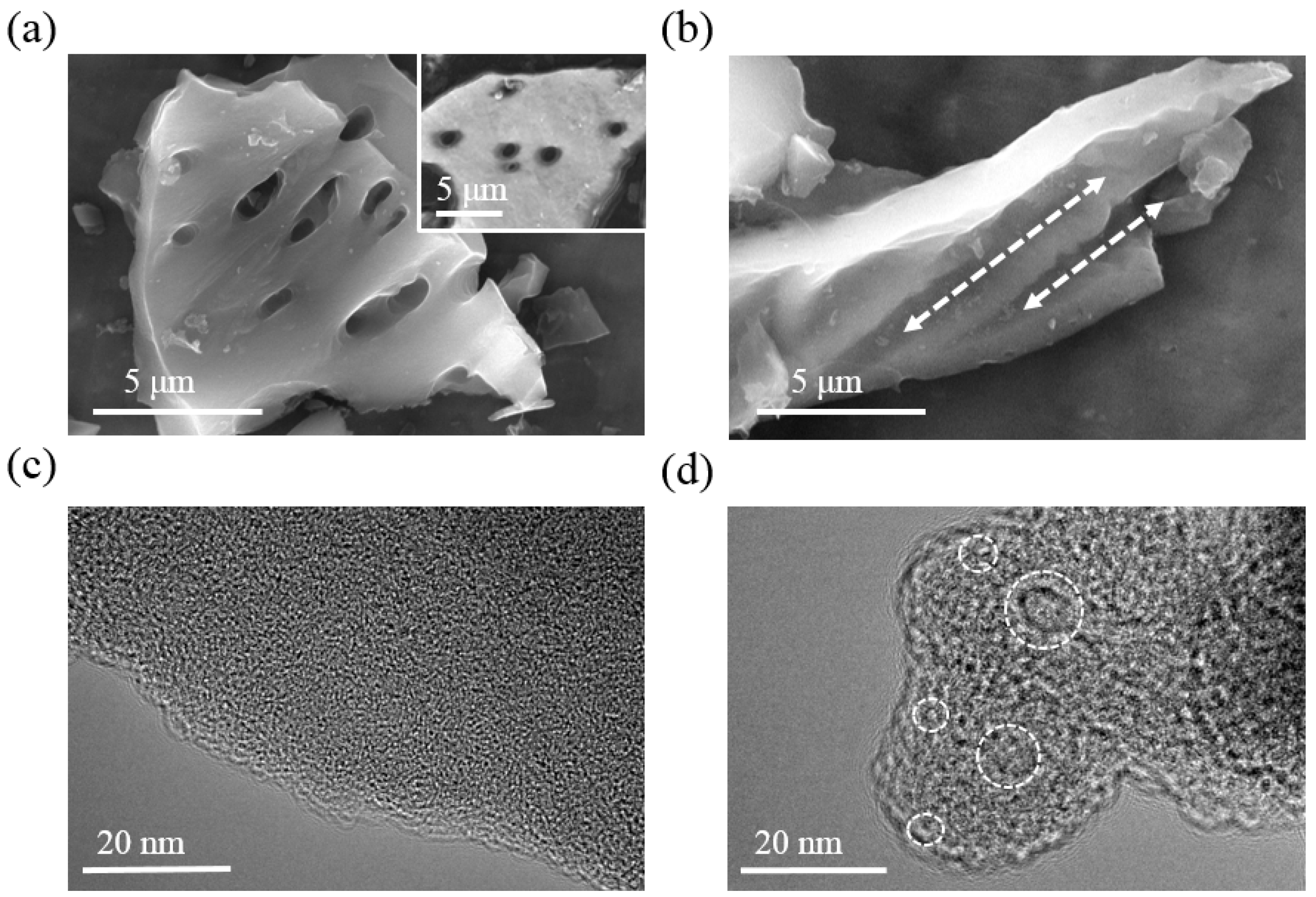
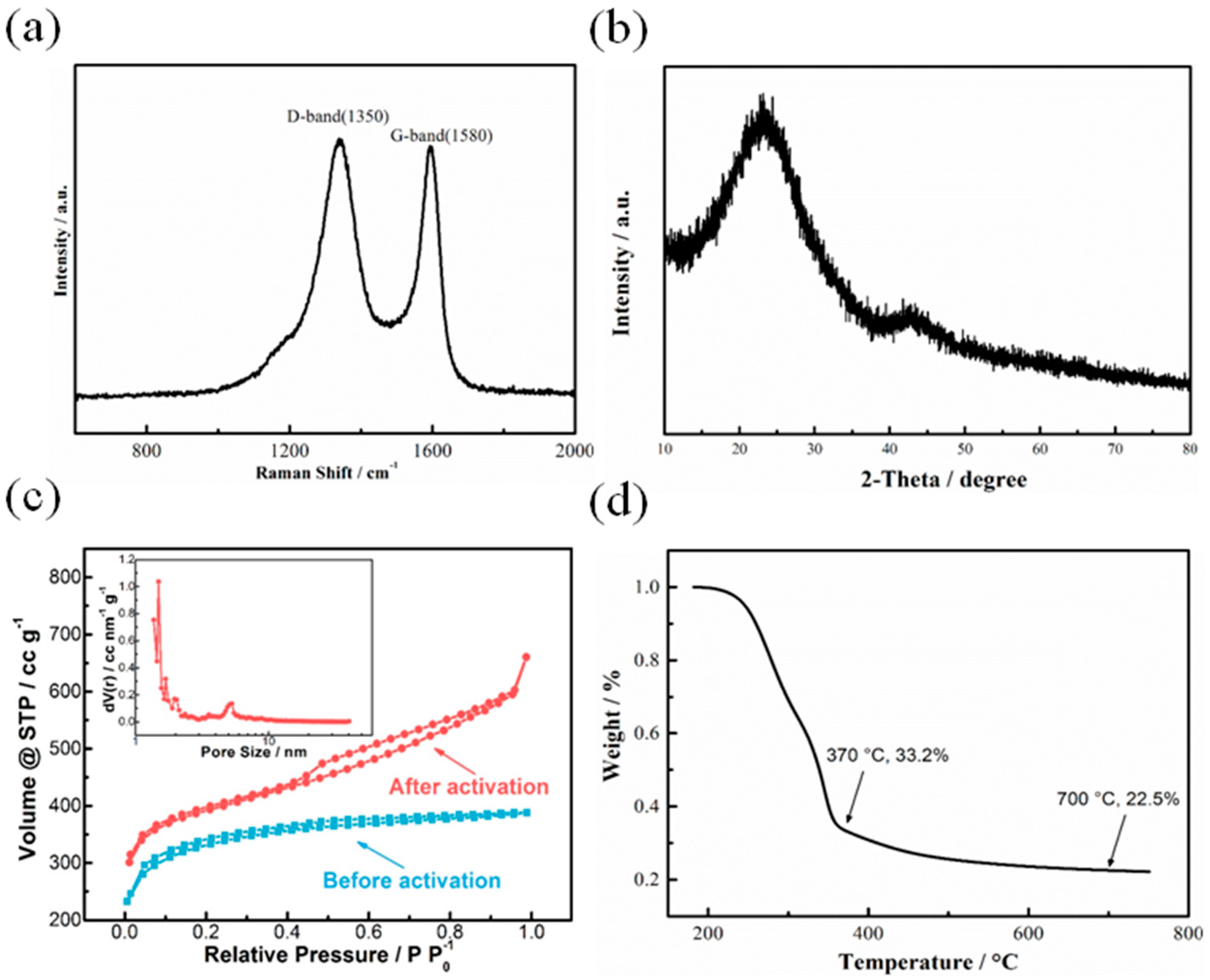
3.1. Structure Analysis
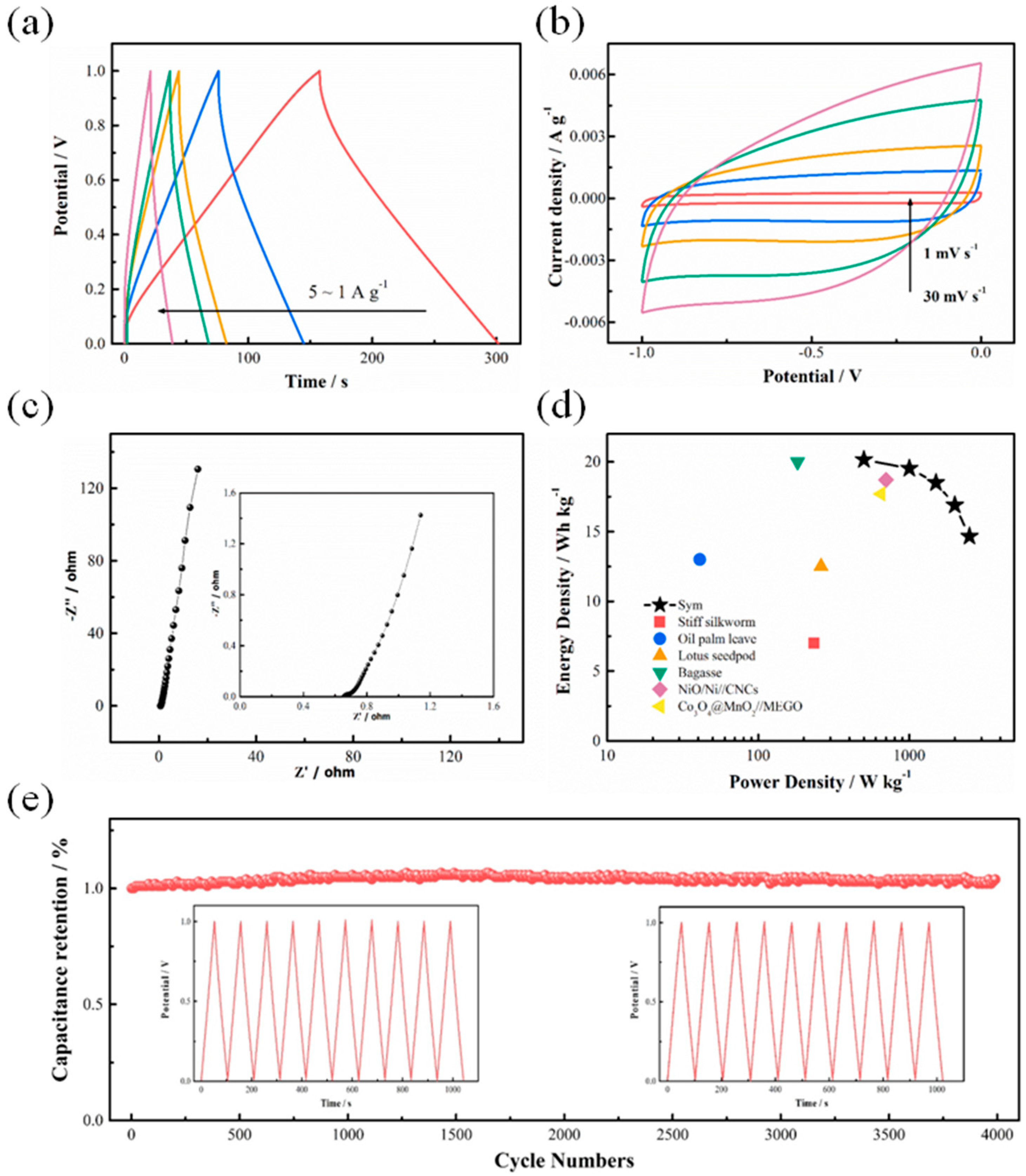
3.2. Electrochemical Behaviors
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gonzalez, A.; Goikolea, E.; Barrena, J.A.; Mysyk, R. Review on supercapacitors: Technologies and materials. Renew. Sust. Energy Rev. 2016, 58, 1189–1206. [Google Scholar] [CrossRef]
- Devillers, N.; Jemei, S.; Pera, M.C.; Bienaime, D.; Gustin, F. Review of characterization methods for supercapacitor modelling. J. Power Sources 2014, 246, 596–608. [Google Scholar] [CrossRef]
- Brousse, T.; Belanger, D.; Long, J.W. To be or not to be pseudocapacitive? J. Electrochem. Soc. 2015, 162, A5185–A5189. [Google Scholar] [CrossRef]
- Li, B.; Dai, F.; Xiao, Q.F.; Yang, L.; Shen, J.M.; Zhang, C.M.; Cai, M. Nitrogen-doped activated carbon for a high energy hybrid supercapacitor. Energy Environ. Sci. 2016, 9, 102–106. [Google Scholar] [CrossRef]
- Zhu, D.Z.; Wang, Y.W.; Gan, L.H.; Liu, M.X.; Cheng, K.; Zhao, Y.H.; Deng, X.X.; Sun, D.M. Nitrogen-containing carbon microspheres for supercapacitor electrodes. Electrochim. Acta 2015, 158, 166–174. [Google Scholar] [CrossRef]
- Frackowiak, E.; Beguin, F. Carbon materials for the electrochemical storage of energy in capacitors. Carbon 2001, 39, 937–950. [Google Scholar] [CrossRef]
- Gamby, J.; Taberna, P.L.; Simon, P.; Fauvarque, J.F.; Chesneau, M. Studies and characterisations of various activated carbons used for carbon/carbon supercapacitors. J. Power Sources 2001, 101, 109–116. [Google Scholar] [CrossRef]
- Oschatz, M.; Borchardt, L.; Thommes, M.; Cychosz, K.A.; Senkovska, I.; Klein, N.; Frind, R.; Leistner, M.; Presser, V.; Gogotsi, Y.; et al. Carbide-derived carbon monoliths with hierarchical pore architectures. Angew. Chem. 2012, 51, 7577–7580. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Kim, K.B.; Park, S.M.; Roh, K.C. Hierarchically structured activated carbon for ultracapacitors. Sci. Rep. 2016, 6, 21182. [Google Scholar] [CrossRef] [PubMed]
- Strubel, P.; Thieme, S.; Biemelt, T.; Helmer, A.; Oschatz, M.; Bruckner, J.; Althues, H.; Kaskel, S. ZnO hard templating for synthesis of hierarchical porous carbons with tailored porosity and high performance in lithium-sulfur battery. Adv. Funct. Mater. 2015, 25, 287–297. [Google Scholar] [CrossRef]
- Choudhury, S.; Agrawal, M.; Formanek, P.; Jehnichen, D.; Fischer, D.; Krause, B.; Albrecht, V.; Stamm, M.; Ionov, L. Nanoporous cathodes for high-energy Li-S Batteries from gyroid block copolymer templates. ACS Nano 2015, 9, 6147–6157. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Q.; Huang, X.D.; Liu, H.; Sun, B.; Yeoh, W.K.; Li, K.F.; Zhang, J.Q.; Wang, G.X. 3D hyperbranched hollow carbon nanorod architectures for high-performance lithium-sulfur batteries. Adv. Energy Mater. 2014, 4, 1301761. [Google Scholar] [CrossRef]
- Tang, C.; Li, B.-Q.; Zhang, Q.; Zhu, L.; Wang, H.-F.; Shi, J.-L.; Wei, F. CaO-templated growth of hierarchical porous graphene for high-power lithium-sulfur battery applications. Adv. Funct. Mater. 2016, 26, 577–585. [Google Scholar] [CrossRef]
- Xia, K.S.; Gao, Q.M.; Jiang, J.H.; Hu, J. Hierarchical porous carbons with controlled micropores and mesopores for supercapacitor electrode materials. Carbon 2008, 46, 1718–1726. [Google Scholar] [CrossRef]
- Zhang, H.; Cao, G.; Wang, Z.; Yang, Y.; Shi, Z.; Gu, Z. Growth of manganese oxide nanoflowers on vertically-aligned carbon nanotube arrays for high-rate electrochemical capacitive energy storage. Nano Lett. 2008, 8, 2664–2668. [Google Scholar] [CrossRef] [PubMed]
- Qie, L.; Chen, W.M.; Xu, H.H.; Xiong, X.Q.; Jiang, Y.; Zou, F.; Hu, X.L.; Xin, Y.; Zhang, Z.L.; Huang, Y.H. Synthesis of functionalized 3D hierarchical porous carbon for high-performance supercapacitors. Energy Environ. Sci. 2013, 6, 2497–2504. [Google Scholar] [CrossRef]
- Wang, D.W.; Li, F.; Liu, M.; Lu, G.Q.; Cheng, H.M. 3D aperiodic hierarchical porous graphitic carbon material for high-rate electrochemical capacitive energy storage. Angew. Chem. 2008, 47, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.N.; Li, D.L.; Luo, C.Z.; Fu, Q.; Pan, C.X. Highly porous graphitic biomass carbon as advanced electrode materials for supercapacitors. Green Chem. 2017, 19, 4132–4140. [Google Scholar] [CrossRef]
- Senthilkumar, S.T.; Selvan, R.K. Flexible fiber supercapacitor using biowaste-derived porous carbon. Chemelectrochem 2015, 2, 1111–1116. [Google Scholar] [CrossRef]
- Hegde, G.; Manaf, S.A.A.; Kumar, A.; Ali, G.A.M.; Chong, K.F.; Ngaini, Z.; Sharma, K.V. Biowaste sago bark based catalyst free carbon nanospheres: Waste to wealth approach. ACS Sustain. Chem. Eng. 2015, 3, 2247–2253. [Google Scholar] [CrossRef]
- Genovese, M.; Jiang, J.H.; Lian, K.; Holm, N. High capacitive performance of exfoliated biochar nanosheets from biomass waste corn cob. J. Mater. Chem. A 2015, 3, 2903–2913. [Google Scholar] [CrossRef]
- Collins, J.; Zheng, D.; Ngo, T.; Qu, D.Y.; Foster, M. Partial graphitization of activated carbon by surface acidification. Carbon 2014, 79, 500–517. [Google Scholar] [CrossRef]
- Chen, C.J.; Zhang, Y.; Li, Y.J.; Dai, J.Q.; Song, J.W.; Yao, Y.G.; Gong, Y.H.; Kierzewski, I.; Xie, J.; Hu, L.B. All-wood, low tortuosity, aqueous, biodegradable supercapacitors with ultra-high capacitance. Energy Environ. Sci. 2017, 10, 538–545. [Google Scholar] [CrossRef]
- Gong, C.C.; Wang, X.Z.; Ma, D.H.; Chen, H.F.; Zhang, S.S.; Liao, Z.X. Microporous carbon from a biological waste-stiff silkworm for capacitive energy storage. Electrochim. Acta 2016, 220, 331–339. [Google Scholar] [CrossRef]
- Ali, G.A.M.; Manaf, S.A.A.; Divyashree, A.; Chong, K.F.; Hegde, G. Superior supercapacitive performance in porous nanocarbons. J. Energy Chem. 2016, 25, 734–739. [Google Scholar] [CrossRef]
- Liu, B.; Zhou, X.H.; Chen, H.B.; Liu, Y.J.; Li, H.M. Promising porous carbons derived from lotus seedpods with outstanding supercapacitance performance. Electrochim. Acta 2016, 208, 55–63. [Google Scholar] [CrossRef]
- Feng, H.B.; Hu, H.; Dong, H.W.; Xiao, Y.; Cai, Y.J.; Lei, B.F.; Liu, Y.L.; Zheng, M.T. Hierarchical structured carbon derived from bagasse wastes: A simple and efficient synthesis route and its improved electrochemical properties for high-performance supercapacitors. J. Power Sources 2016, 302, 164–173. [Google Scholar] [CrossRef]
- Huang, M.; Zhang, Y.X.; Li, F.; Zhang, L.L.; Wen, Z.Y.; Liu, Q. Facile synthesis of hierarchical Co3O4@MnO2 core-shell arrays on Ni foam for asymmetric supercapacitors. J. Power Sources 2014, 252, 98–106. [Google Scholar] [CrossRef]
- Peng, H.; Ma, G.F.; Sun, K.J.; Zhang, Z.G.; Li, J.D.; Zhou, X.Z.; Lei, Z.Q. A novel aqueous asymmetric supercapacitor based on petal-like cobalt selenide nanosheets and nitrogen-doped porous carbon networks electrodes. J. Power Sources 2015, 297, 351–358. [Google Scholar] [CrossRef]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Zhang, K. Converting Corncob to Activated Porous Carbon for Supercapacitor Application. Nanomaterials 2018, 8, 181. https://doi.org/10.3390/nano8040181
Yang S, Zhang K. Converting Corncob to Activated Porous Carbon for Supercapacitor Application. Nanomaterials. 2018; 8(4):181. https://doi.org/10.3390/nano8040181
Chicago/Turabian StyleYang, Shaoran, and Kaili Zhang. 2018. "Converting Corncob to Activated Porous Carbon for Supercapacitor Application" Nanomaterials 8, no. 4: 181. https://doi.org/10.3390/nano8040181