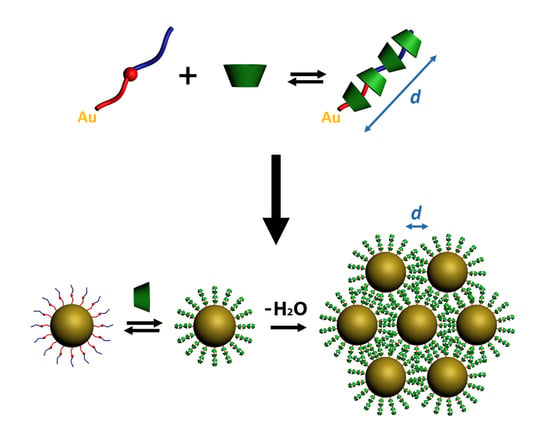
Supramolecular Control over the Interparticle Distance in Gold Nanoparticle Arrays by Cyclodextrin Polyrotaxanes
Abstract
:1. Introduction
2. Results and Discussion
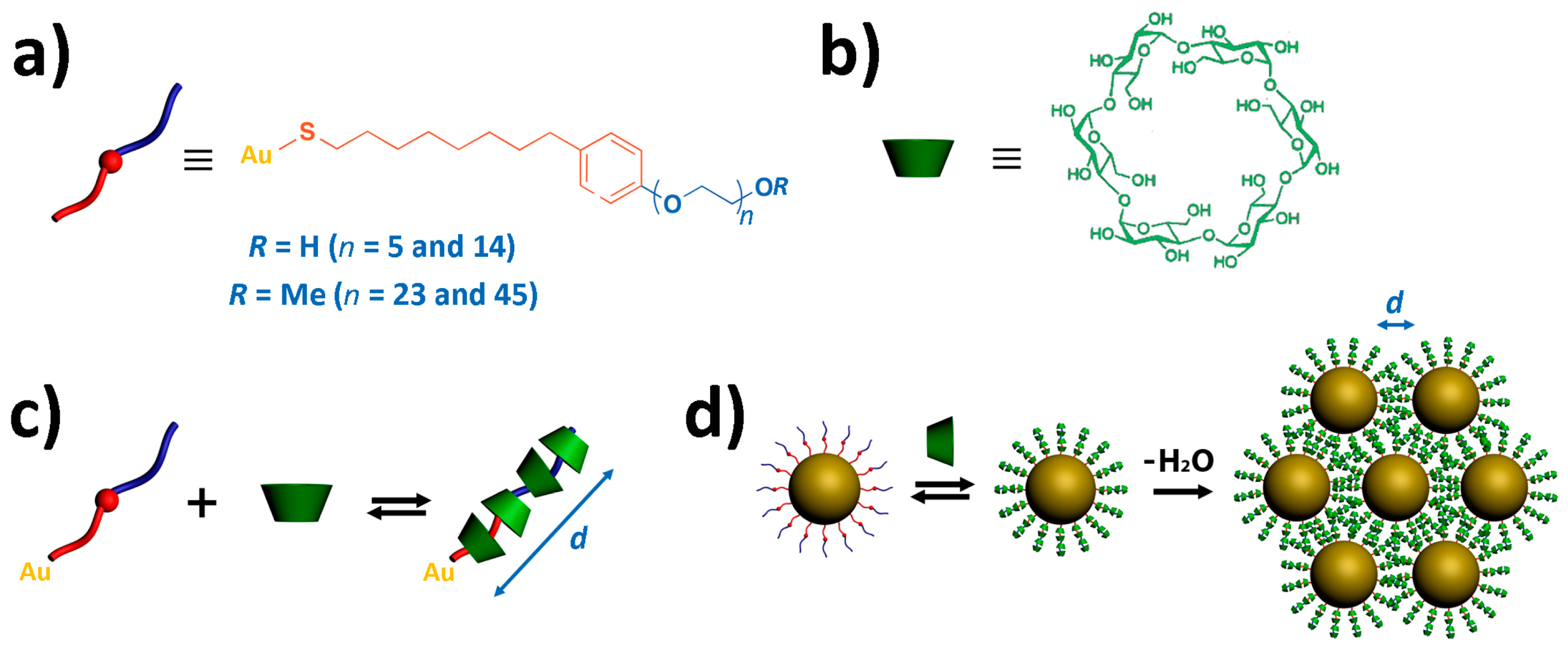
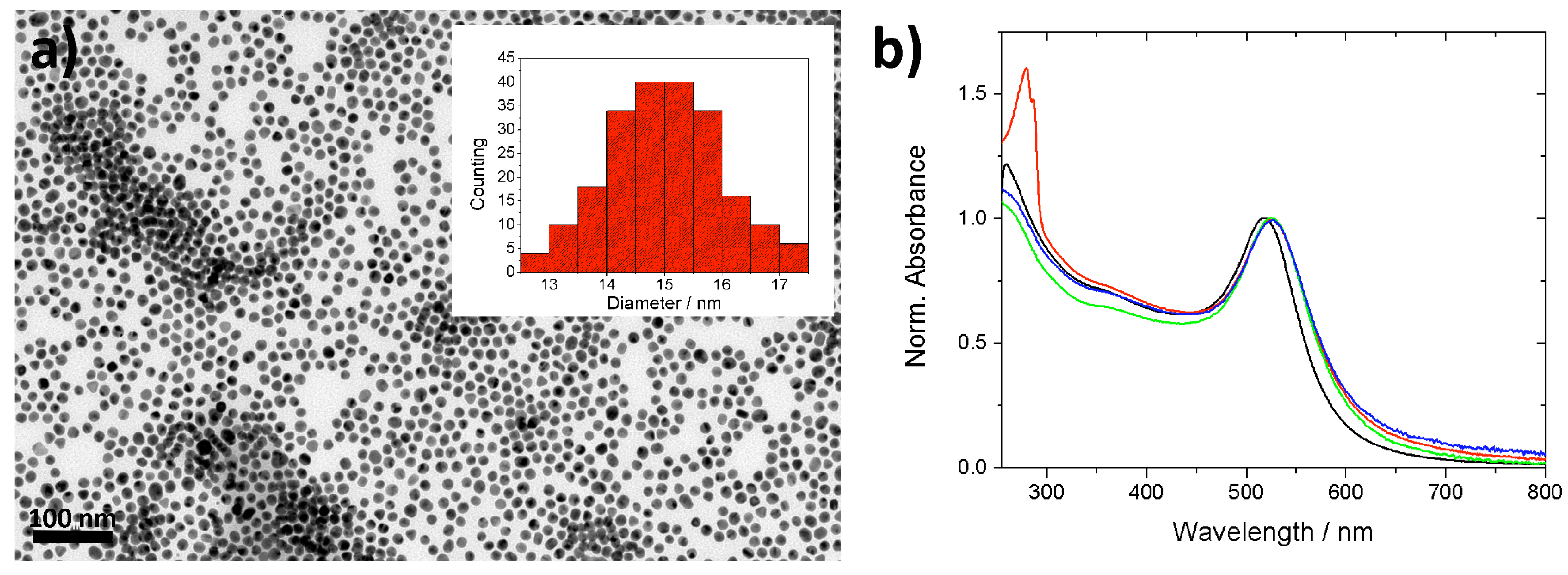
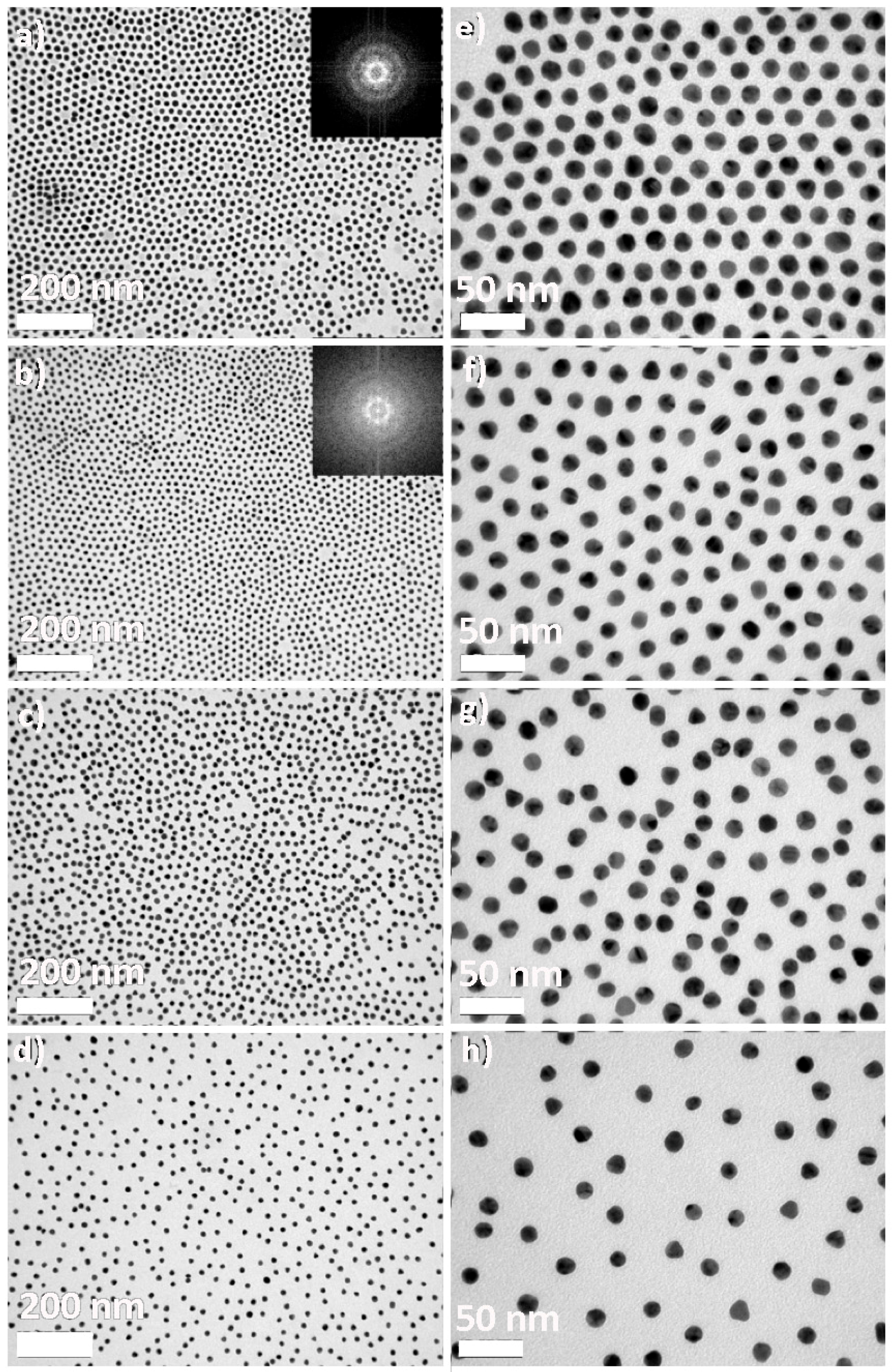
2.1. Synthesis and Functionalization of Gold Nanoparticles
2.2. Cyclodextrin Supramolecular Complexation at the Gold Nanoparticle Surface
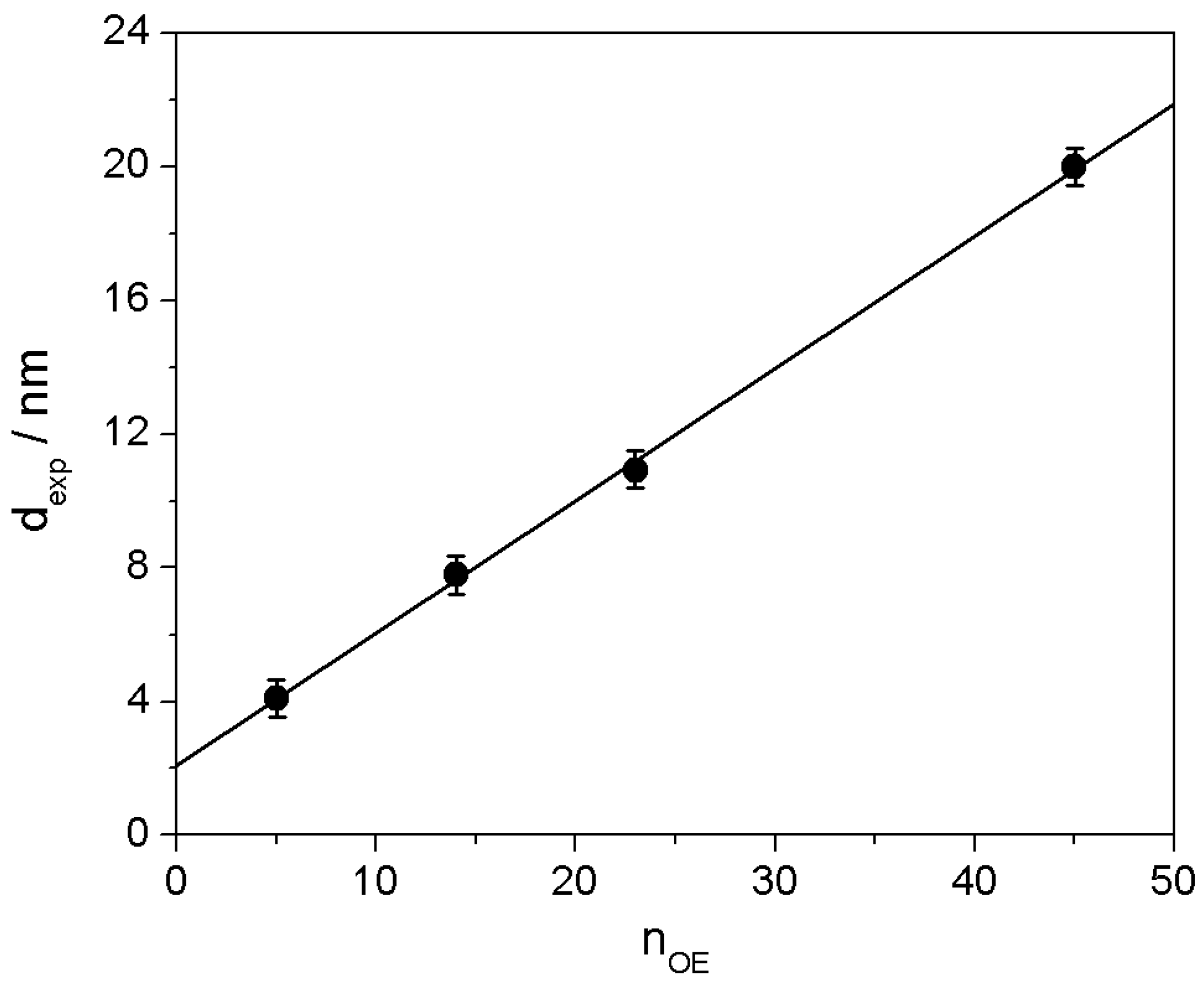
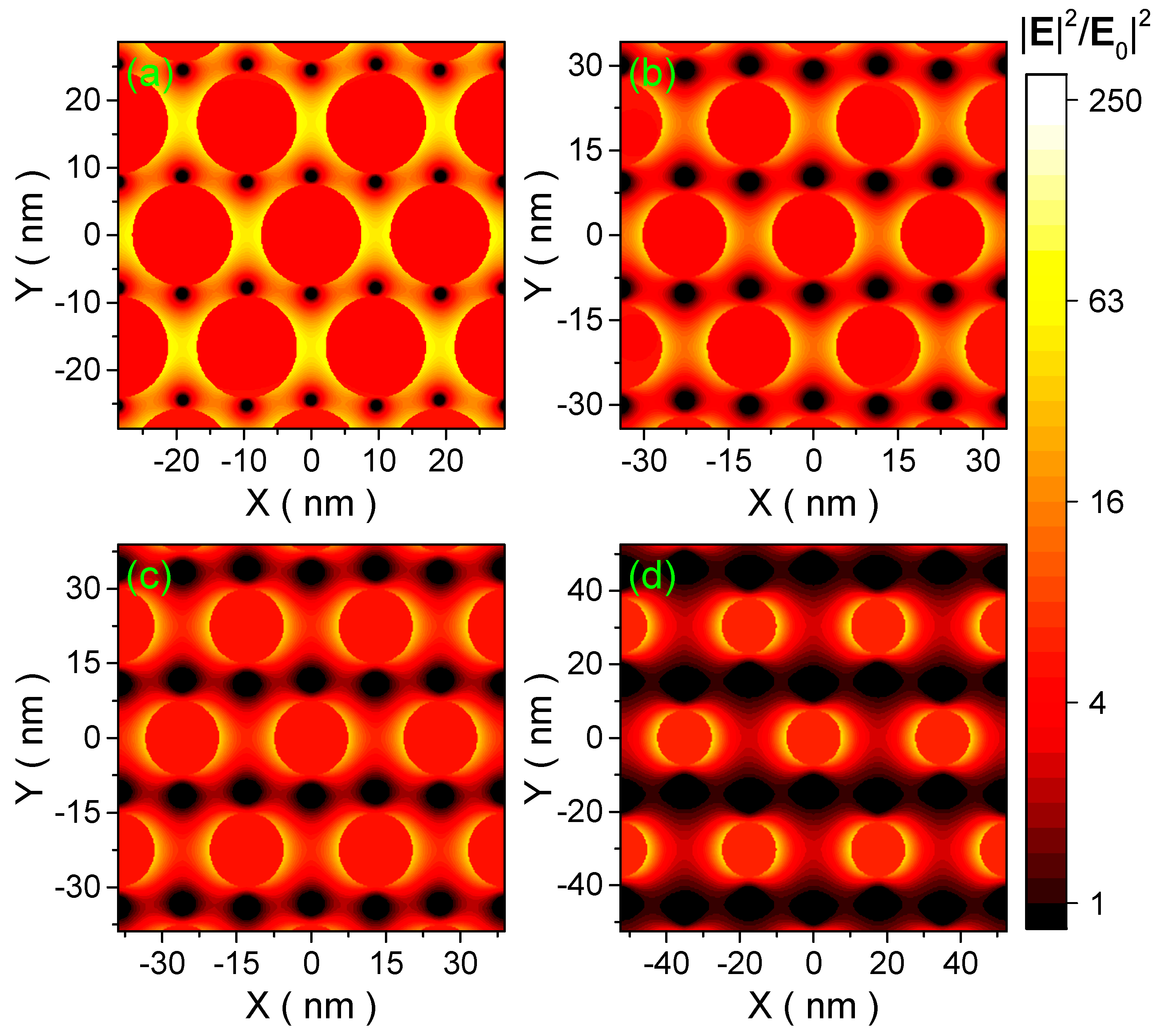
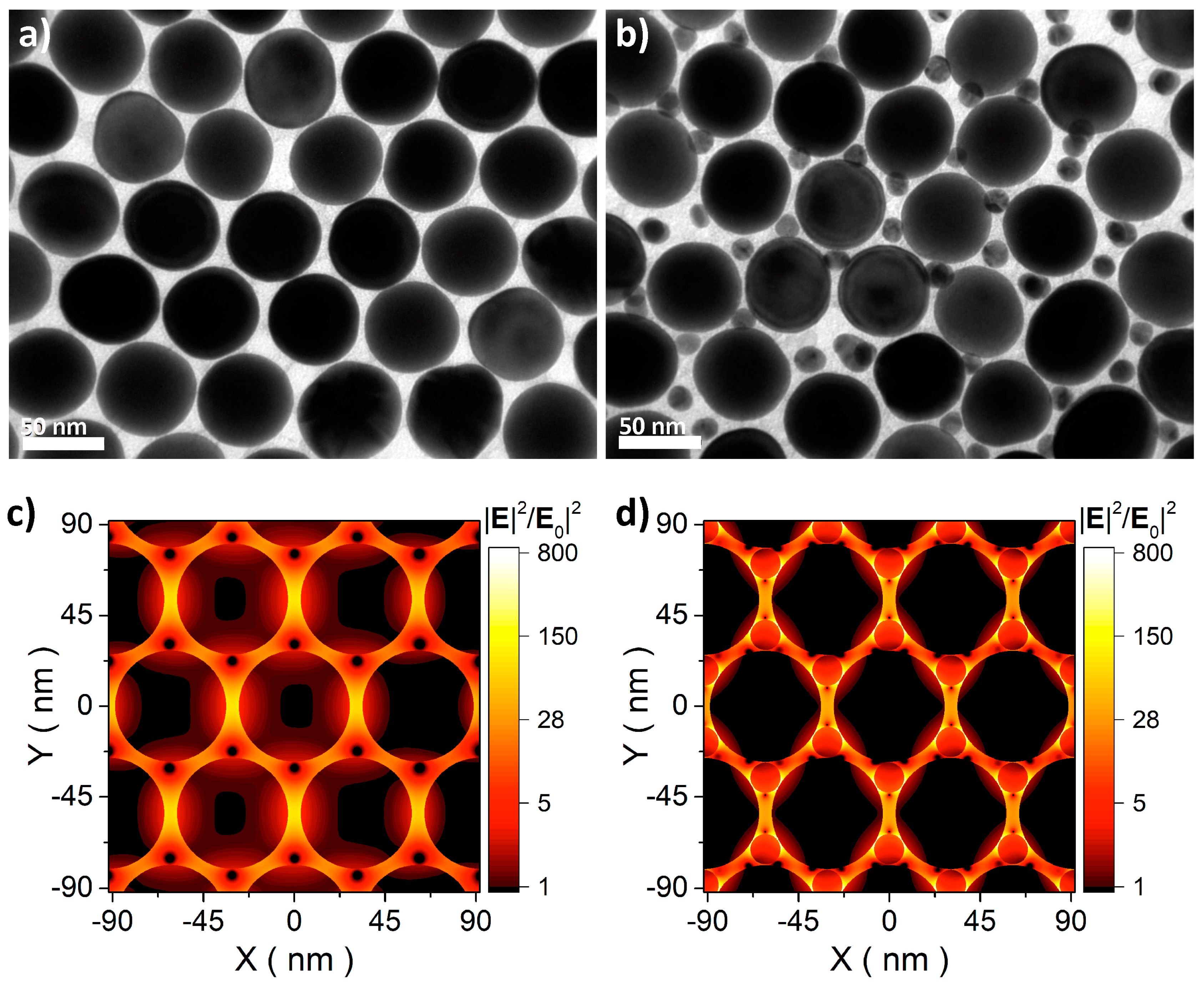
2.3. Polyrotaxane-Mediated Self-Assembly of Gold Nanoparticles into 2D Arrays
2.4. 2D Arrays Based on the Cyclodextrin Complexation of Polyoxyethylene Glycol-Functionalized Gold Nanoparticles
3. Materials and Methods
3.1. Characterization Techniques
- 1H and 13C NMR measurements: 1H and 13C NMR spectra (see Supplementary Materials) were recorded on a Bruker Avance DPX-300 spectrometer (300 MHz, 7.05 T, Bruker, Silberstreifen, Germany). Chemical shifts are given in ppm relative to TMS (1H, 0.0 ppm) and CDCl3 (13C, 77.0 ppm). Coupling constants are given in Hertz.
- UV-vis measurements: UV-vis absorption spectra were recorded on a UVICON XL spectrophotometer (Bio-Tex Instruments, Varanasi, India). All experiments were carried out at 298 K, using quartz cuvettes with an optical path of 1 cm.
- Steady-state fluorescence measurements: Fluorescence spectra were recorded at 298 K using an AMINCO Bowman Series 2 spectrofluorimeter (Aminco Bowman, Silver Spring, MD, USA) with 4.0-nm bandwidth for excitation and emission and quartz cuvettes with optical paths of 1 cm. The excitation wavelength was fixed at 280 nm.
- Time-resolved fluorescence measurements: Fluorescence decays were measured using the time-correlated single-photon-counting method on an FL-900 spectrofluorimeter from Edinburgh Analytical Instruments (Edinburgh, UK). The excitation source was a hydrogen nanosecond flash lamp with a repetition rate of 40 kHz, an excitation pulse width shorter than 1 ns, and a temporal resolution of 100 ps.
- Transmission electron microscopy: TEM images were obtained with a JEOL JEM 2100 transmission electron microscope (JEOL, Peabody, MA, USA) operating at an acceleration voltage of 200 kV.
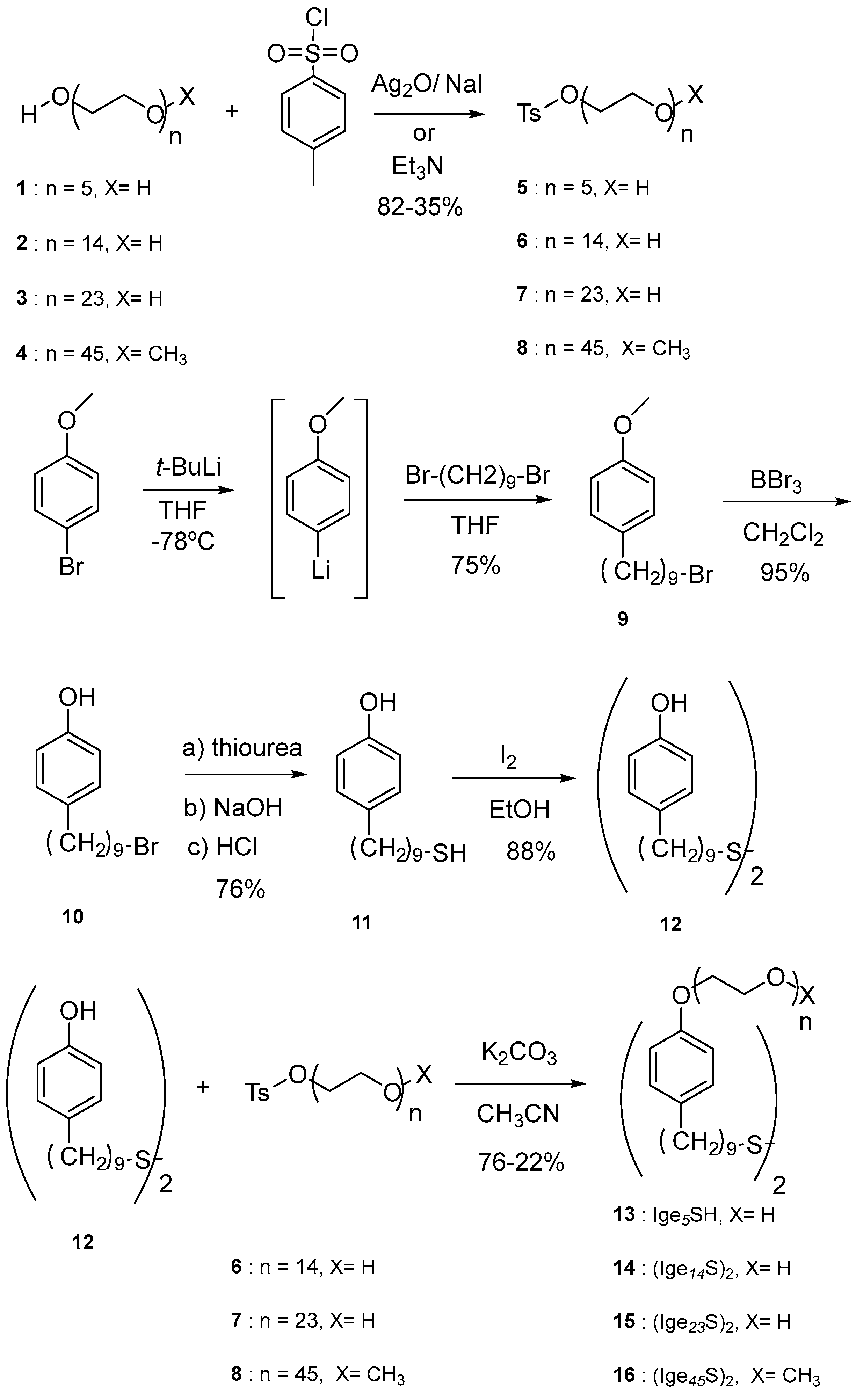
3.2. Synthesis of Nonionic Ligands IgenS-
3.3. Synthesis of Spherical Gold Nanoparticles
3.4. Calculations
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ghosh, S.K.; Pal, T. Interparticle coupling effect on the surface plasmon resonance of gold nanoparticles: From theory to applications. Chem. Rev. 2007, 107, 4797–4862. [Google Scholar] [CrossRef] [PubMed]
- Myroshnychenko, V.; Rodríguez-Fernández, J.; Pastoriza-Santos, I.; Funston, A.M.; Novo, C.; Mulvaney, P.; Liz-Marzán, L.M.; de Abajo, F.J.G. Modelling the optical response of gold nanoparticles. Chem. Soc. Rev. 2008, 37, 1792–1805. [Google Scholar] [CrossRef] [PubMed]
- Liz-Marzán, L.M. Nanometals: Formation and color. Mater. Today 2004, 7, 26–31. [Google Scholar] [CrossRef]
- Álvarez-Puebla, R.; Liz-Marzán, L.M.; de Abajo, F.J.G. Light concentration at the nanometer scale. J. Phys. Chem. Lett. 2010, 1, 2428–2434. [Google Scholar] [CrossRef]
- Moskovits, M. Imaging: Spot the hotspot. Nature 2011, 469, 307–308. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Andrade, G.F.S.; Ahmed, A.; Souza, M.L.; Coombs, N.; Tumarkin, E.; Liu, K.; Gordon, R.; Brolo, A.G.; Kumacheva, E. Probing dynamic generation of hot-spots in self-assembled chains of gold nanorods by surface-enhanced Raman scattering. J. Am. Chem. Soc. 2011, 133, 7563–7570. [Google Scholar] [CrossRef] [PubMed]
- Moskovits, M. Surface enhanced spectroscopy. Rev. Mod. Phys. 1985, 57, 738–826. [Google Scholar] [CrossRef]
- Lakowicz, J.R.; Ray, K.; Chowdhury, M.; Szmacinski, H.; Fu, Y.; Zhang, J.; Nowaczyk, K. Plasmon controlled fluorescence: A new paradigm in fluorescence spectroscopy. Analyst 2008, 133, 1308–1346. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.R.; Macfarlane, R.J.; Lee, B.; Zhang, J.; Young, K.L.; Senesi, A.J.; Mirkin, C.A. DNA-nanoparticle superlattices formed from anisotropic building blocks. Nat. Mater. 2010, 9, 913–917. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Campolongo, M.J.; Cha, J.J.; Tan, S.J.; Umbach, C.C.; Muller, D.A.; Luo, D. Free-standing nanoparticle superlattice sheets controlled by DNA. Nat. Mater. 2009, 8, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Liu, X.; Tang, Q.; Hill, J.P.; Ariga, K.; Quingmin, J. Morphology Adjustable Silica Nanosheets for Immobilization of Gold Nanoparticles. ChemistrySelect 2017, 2, 5793–5799. [Google Scholar] [CrossRef]
- González-Rubio, G.; Díaz-Núñez, P.; Rivera, A.; Prada, A.; Tardajos, G.; González-Izquierdo, J.; Bañares, L.; Llombart, P.; Macdowell, L.G.; Alcolea Palafox, M.; et al. Femtosecond laser reshaping yields gold nanorods with ultranarrow surface plasmon resonances. Science 2017, 358, 640–644. [Google Scholar] [CrossRef] [PubMed]
- González-Rubio, G.; González-Izquierdo, J.; Bañares, L.; Tardajos, G.; Rivera, A.; Altantzis, T.; Bals, S.; Peña-Rodríguez, O.; Guerrero-Martínez, A.; Liz-Marzán, L.M. Femtosecond laser-controlled tip-to-tip assembly and welding of gold nanorods. Nano Lett. 2015, 15, 8282–8288. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Martínez, A.; Grzelczak, M.; Liz-Marzán, L.M. Molecular thinking for nanoplasmonic design. ACS Nano 2012, 6, 3655–3662. [Google Scholar] [CrossRef] [PubMed]
- Young, K.L.; Jones, M.R.; Zhang, J.; Macfarlane, R.J.; Esquivel-Sirvent, R.; Nap, R.J.; Wu, J.; Schatz, G.C.; Lee, B.; Mirkin, C.A. Assembly of reconfigurable one-dimensional colloidal superlattices due to a synergy of fundamental nanoscale forces. Proc. Natl. Acad. Sci. USA 2012, 109, 2240–2245. [Google Scholar] [CrossRef] [PubMed]
- Grzelczak, M.; Vermant, J.; Furst, E.M.; Liz-Marzán, L.M. Directed self-assembly of nanoparticles. ACS Nano 2010, 4, 3591–3605. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Martínez, A.; Pérez-Juste, J.; Carbó-Argibay, E.; Tardajos, G.; Liz-Marzán, L.M. Gemini surfactant-directed self-assembly of monodisperse gold nanorods into standing superlattices. Angew. Chem. Int. Ed. 2009, 48, 9484–9488. [Google Scholar] [CrossRef] [PubMed]
- Kuzyk, A.; Schreiber, R.; Fan, Z.; Pardatscher, G.; Rolle, E.-M.; Högele, A.; Simmel, F.C.; Govorov, A.O.; Liedl, T. DNA-Based Self-assembly of chiral plasmonic nanostructures with tailored optical response. Nature 2012, 483, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Van Herrikhuyzen, J.; Janssen, R.A.J.; Meijer, E.W.; Meskers, S.C.J.; Schenning, A.P.H.J. Fractal-like Self-Assembly of Oligo(p-phenylene vinylene) capped gold nanoparticles. J. Am. Chem. Soc. 2006, 128, 686–687. [Google Scholar] [CrossRef] [PubMed]
- Nie, Z.; Fava, D.; Kumacheva, E.; Zou, S.; Walker, G.C.; Rubinstein, M. Self-assembly of metal-polymer analogues of amphiphilic triblock copolymers. Nat. Mater. 2007, 6, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Lei, D.Y.; Amin, F.; Martmann, R.; Acuna, G.P.; Guerrero-Martínez, A.; Maier, S.A.; Tinnefeld, P.; Carregal-Romero, S.; Parak, W.J. Distance control in-between plasmonic nanoparticles via biological and polymeric spacers. Nano Today 2013, 8, 480–493. [Google Scholar] [CrossRef]
- Sánchez-Iglesias, A.; Grzelczak, M.; Altantzis, T.; Goris, B.; Perez-Juste, J.; Bals, S.; Van Tendeloo, G.; Donaldson, S.H., Jr.; Chmelka, B.F.; Israelachvili, J.N.; et al. Hydrophobic interactions modulate self-assembly of nanoparticles. ACS Nano 2012, 6, 11059–11065. [Google Scholar] [CrossRef] [PubMed]
- Coelho, J.P.; Tardajos, G.; Stepanenko, V.; Roedle, A.; Fernández, G.; Guerrero-Martínez, A. Cooperative self-assembly transfer from hierarchical supramolecular polymers to gold nanoparticles. ACS Nano 2015, 9, 11241–11248. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Graña, S.; Pérez-Juste, R.; Álvarez-Puebla, R.A.; Guerrero-Martínez, A.; Liz-Marzán, L.M. Self-Assembly of Au@Ag nanorods mediated by gemini surfactants for highly efficient SERS-active supercrystals. Adv. Opt. Mater. 2013, 1, 477–481. [Google Scholar] [CrossRef]
- Ahijado-Guzmán, R.; González-Rubio, G.; Izquierdo, J.G.; Bañares, L.; López-Montero, I.; Calzado-Martín, A.; Calleja, M.; Tardajos, G.; Guerrero-Martínez, A. Intracellular pH-induced tip-to-tip assembly of gold nanorods for enhanced plasmonic photothermal therapy. ACS Omega 2016, 1, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Sashuk, V.; Winkler, K.; Żywociński, A.; Wojciechowski, T.; Górecka, E.; Fiałkowski, M. Nanoparticles in a capillary Trap: Dynamic self-assembly at fluid interfaces. ACS Nano 2013, 7, 8833–8839. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Graña, S.; Fernández-López, C.; Polavarapu, L.; Salmon, J.-B.; Leng, J.; Pastoriza-Santos, I.; Pérez-Juste, J. Gold Nanooctahedra with tunable size and microfluidic-induced 3D assembly for highly uniform SERS-active supercrystals. Chem. Mater. 2015, 27, 8310–8317. [Google Scholar] [CrossRef]
- Montes-García, V.; Pérez-Juste, J.; Patoriza-Santos, I.; Liz-Marzán, L.M. Metal nanoparticles and supramolecular macrocycles: A tale of synergy. Chem. Eur. J. 2014, 20, 10874–10883. [Google Scholar] [CrossRef] [PubMed]
- Coelho, J.P.; Mayoral, M.J.; Camacho, L.; Martín-Romero, M.T.; Tardajos, G.; López-Montero, I.; Sanz, E.; Ávila-Brande, D.; Giner-Casares, J.J.; Fernández, G.; et al. Mechanosensitive gold colloidal membranes mediated by supramolecular interfacial self-assembly. J. Am. Chem. Soc. 2017, 113, 1120–1128. [Google Scholar] [CrossRef] [PubMed]
- Ye, T.; Chen, X.; Fan, X.; Shen, Z. Ordered gold nanoparticle arrays obtained with supramolecular block copolymers. Soft Matter 2013, 9, 4715–4724. [Google Scholar] [CrossRef]
- Coelho, J.P.; González-Rubio, G.; Delices, A.; Osío Barcina, J.; Salgado, C.; Ávila, D.; Peña-Rodríguez, O.; Tardajos, G.; Guerrero-Martínez, A. Polyrotaxane-mediated self-assembly of gold nanospheres into fully reversible supercrystals. Angew. Chem. Int. Ed. 2014, 53, 12751–12755. [Google Scholar] [CrossRef] [PubMed]
- Atwood, J.L.; Davies, J.E.D.; Macnicol, D.D.; Vögtle, F.C. Volume 3: Cyclodextrins. In Comprehensive Supramolecular Chemistry; Szejtli, J., Osa, T., Eds.; Pergamon: Tarrytown, NY, USA, 1996. [Google Scholar]
- Miyabe, K.; Suzuki, N.; Shimazaki, Y. Determination of Association and Dissociation Rate Constants in an Inclusion Complex System between Thymol and Sulfated-β-cyclodextrin by Moment Analysis—Affinity Capillary Electrophoresis. Chem. Soc. Jpn. 2016, 89, 1219–1224. [Google Scholar] [CrossRef]
- González-Gaitano, G.; Isasi, J.R.; Velz, I.; Zornoza, A. Drug Carrier Systems Based on Cyclodextrin Supramolecular Assemblies and Polymers: Present and Perspectives. Curr. Pharm. Des. 2017, 23, 411–432. [Google Scholar] [CrossRef] [PubMed]
- Saenger, W.; Jacob, J.; Gessler, K.; Steiner, T.; Hoffmann, D.; Sanbe, H.; Loizumi, K.; Smith, S.M.; Takaha, T. Structures of the common cyclodextrins and their larger analogues—Beyond the doughnut. Chem. Rev. 1998, 98, 1787–1802. [Google Scholar] [CrossRef] [PubMed]
- Rekharsky, M.V.; Inoue, Y. Complexation Thermodynamics of cyclodextrins. Chem. Rev. 1998, 98, 1875–1918. [Google Scholar] [CrossRef] [PubMed]
- Her, S.; Jaffray, D.A.; Allen, C. Gold nanoparticles for applications in cancer radiotherapy: Mechanisms and recent advancements. Adv. Drug Deliv. Rev. 2017, 109, 84–101. [Google Scholar] [CrossRef] [PubMed]
- Wenz, G.; Han, B.H.; Muller, A. Cyclodextrin rotaxanes and polyrotaxanes. Chem. Rev. 2006, 106, 782–817. [Google Scholar] [CrossRef] [PubMed]
- Harada, A.; Li, J.; Kamachi, M. The molecular necklace: A rotaxane containing many threaded α-cyclodextrins. Nature 1992, 356, 325–327. [Google Scholar] [CrossRef]
- Guerrero-Martínez, A.; Montoro, T.; Viñas, M.H.; González-Gaitano, G.; Tardajos, G. Study of the interaction between a nonyl phenyl ether and β-cyclodextrin: Declouding nonionic surfactant solutions by complexation. J. Phys. Chem. B 2007, 111, 1368–1376. [Google Scholar] [CrossRef] [PubMed]
- Airga, K.; Naito, M.; Ji, Q.; Payra, D. Molecular cavity nanoarchitectonics for biomedical application and mechanical cavity manipulation. CrystEngComm 2016, 18, 4890–4899. [Google Scholar] [CrossRef]
- Guerrero-Martínez, A.; Ávila, D.; Martínez-Casado, F.J.; Ripmeester, J.A.; Enright, G.D.; De Cola, L.; Tardajos, G. Solid crystal network of self-assembled cyclodextrin and noionic surfactant pseudorotaxanes. J. Phys. Chem. B 2010, 114, 11489–11495. [Google Scholar] [CrossRef] [PubMed]
- Lo Nostro, P.; Giustini, L.; Fratini, E.; Ninham, B.W.; Ridi, F.; Baglioni, P. Threading, growth and aggregation of pseudopolyrotaxanes. J. Phys. Chem. B 2008, 112, 1071–1081. [Google Scholar] [CrossRef] [PubMed]
- Akita, I.; Ishida, Y.; Yonezawa, T. Ligand effect on the formation of gold nanoparticles via sputtering deposition over a liquid matrix. Bull. Chem. Soc. Jpn. 2016, 89, 1054–1056. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hilier, J. The formation of colloidal gold. J. Phys. Chem. 1953, 57, 670–673. [Google Scholar] [CrossRef]
- Zhao, J.; Jensen, L.; Sung, J.; Zou, S.; Schatz, G.C.; Van Duyne, R.P. Interaction of plasmon and molecular resonances of Rhodamine 6G adsorbed on silver nanoparticles. J. Am. Chem. Soc. 2007, 129, 7647–7656. [Google Scholar] [CrossRef] [PubMed]
- Connors, K.A. Binding Constants: The Measurement of Molecular Stability; Chapter 4; John Wiley & Sons: New York, NY, USA, 1987. [Google Scholar]
- Buschmann, H.-J.; Cleve, E.; Schollmeyer, E. The interaction between noionic surfactants and cyclodextrins studied by fluorescence measurements. J. Incl. Phenom. Macrocycl. Chem. 1999, 33, 233–241. [Google Scholar] [CrossRef]
- Udachin, K.A.; Wilson, L.D.; Ripmeester, J.A. Solid polyrotaxanes of polyethylene glicol and cyclodextrins: The single crystal X-ray structure of PEG-β-cyclodextrin. J. Am. Chem. Soc. 2000, 122, 12375–12376. [Google Scholar] [CrossRef]
- Mackowski, D.W.; Mishchenko, M.I. Calculation of the T matrix and the scattering matrix for ensembles of spheres. J. Opt. Soc. Am. A 1996, 13, 2266–2278. [Google Scholar] [CrossRef]
- Mackowski, D.W.; Mishchenko, M.I. A multiple sphere T-matrix Fortran code for use on parallel computer clusters. J. Quant. Spectrosc. Radiat. Transf. 2011, 112, 2182–2192. [Google Scholar] [CrossRef]
- Shevchenko, E.V.; Talapin, D.V.; Kotov, N.A.; O’Brien, S.; Murray, C.B. Structural diversity in binary nanoparticle superlattices. Nature 2006, 439, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Del Pino, P.; Yang, F.; Pelaz, B.; Zhang, Q.; Kantner, K.; Hartmann, R.; de Baroja, N.M.; Gallego, M.; Möller, M.; Manshian, B.B.; et al. Basic physicochemical properties of polyethylene glycol coated gold nanoparticles that determine their interaction with cells. Angew. Chem. Int. Ed. 2016, 55, 5483–5487. [Google Scholar] [CrossRef] [PubMed]
- Fernández, C.; González-Rubio, G.; Langer, L.; Tardajos, G.; Liz-Marzán, L.M.; Giraldo, R.; Guerrero-Martínez, A. Nucleation of Amyloid Oligomers by RepA-WH1-Prionoid-Functionalized Gold Nanorods. Angew. Chem. Int. Ed. 2016, 55, 11237–11241. [Google Scholar] [CrossRef] [PubMed]
- Badwaik, V.D.; Aicart, E.; Mondjinou, Y.A.; Johnson, M.A.; Bowman, V.D.; Thompson, D.H. Structure-property relationship for in vitro siRNA delivery performance of cationic 2-hydroxypropyl-β-cyclodextrin: PEG-PPG-PEG polyrotaxane vectors. Biomaterials 2016, 84, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zuo, F.; Zheng, Z.; Ding, X.; Peng, Y. A Novel Approach to molecular recognition surface of magnetic nanoparticles based on host–guest effect. Nanoscale Res. Lett. 2009, 4, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Wahl, F.; Jäschke, A. PEG-tethered guanosine acetal conjugates for the enzymatic synthesis of modified RNA. Biochem. Biophys. Res. Commun. 2012, 417, 1224–1226. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Wang, X.; He, Y. Synthesis of side-on liquid crystalline diblock copolymers through macromolecular azo coupling reaction. Eur. Polym. J. 2015, 69, 584–591. [Google Scholar] [CrossRef]
- Spencer, T.A.; Onofrey, T.J.; Cann, R.O.; Russel, J.S.; Lee, L.E.; Blanchard, D.E.; Castro, A.; Gu, P.; Jiang, G.; Shechter, I. Zwitterionic sulfobetaine inhibitors of squalene synthase. J. Org. Chem. 1999, 64, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhong, X.; Li, Z.; Xia, Y. Successive, Seed–Mediated Growth for the Synthesis of Single–Crystal Gold Nanospheres with Uniform Diameters Controlled in the Range of 5–150 nm. Part. Part. Syst. Charact. 2014, 31, 266–273. [Google Scholar] [CrossRef]
- Waterman, P.C. Symmetry, Unitarity, and Geometry in Electromagnetic Scattering. Phys. Rev. D 1971, 3, 825–839. [Google Scholar] [CrossRef]
- Mishchenko, M.I.; Travis, L.D.; Lacis, A.A. Scattering, Absorption, and Emission of Light by Small Particles, 1st ed.; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]


| AuNP 1 | λIgenS- 2/nm | λLSPR 3/nm | λem 4/nm | τIgenS- 5/ns | τα-CD 6/ns | Kb 7 × 10−4/M−1 | a 8 |
|---|---|---|---|---|---|---|---|
| Ige5S- | 280 | 524 | 300 | 4.0 ± 0.1 | 6.4 ± 0.1 | 3.7 ± 0.4 | 1.52 ± 0.01 |
| Ige14S- | 280 | 524 | 300 | 4.3 ± 0.1 | 6.2 ± 0.1 | 1.7 ± 0.1 | 1.53 ± 0.01 |
| Ige23S- | 280 | 524 | 300 | 4.4 ± 0.1 | 6.3 ± 0.1 | 1.6 ± 0.2 | 1.50 ± 0.01 |
| Ige45S- | 280 | 524 | 300 | 4.5 ± 0.1 | 6.7 ± 0.1 | 1.3 ± 0.2 | 1.48 ± 0.02 |
| AuNPs 1 | nOE 2 | nα-CD 3 | dth 4 | dexp 5 |
|---|---|---|---|---|
| Ige5S- | 5 | 5 | 4.0 ± 0.5 | 4.1 ± 0.5 |
| Ige14S- | 14 | 9 | 7.1 ± 0.5 | 7.8 ± 0.5 |
| Ige23S- | 23 | 14 | 11.1 ± 0.5 | 10.9 ± 0.5 |
| Ige45S- | 45 | 25 | 19.8 ± 0.5 | 20.0 ± 0.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paulo Coelho, J.; Osío Barcina, J.; Junquera, E.; Aicart, E.; Tardajos, G.; Gómez-Graña, S.; Cruz-Gil, P.; Salgado, C.; Díaz-Núñez, P.; Peña-Rodríguez, O.; et al. Supramolecular Control over the Interparticle Distance in Gold Nanoparticle Arrays by Cyclodextrin Polyrotaxanes. Nanomaterials 2018, 8, 168. https://doi.org/10.3390/nano8030168
Paulo Coelho J, Osío Barcina J, Junquera E, Aicart E, Tardajos G, Gómez-Graña S, Cruz-Gil P, Salgado C, Díaz-Núñez P, Peña-Rodríguez O, et al. Supramolecular Control over the Interparticle Distance in Gold Nanoparticle Arrays by Cyclodextrin Polyrotaxanes. Nanomaterials. 2018; 8(3):168. https://doi.org/10.3390/nano8030168
Chicago/Turabian StylePaulo Coelho, Joao, José Osío Barcina, Elena Junquera, Emilio Aicart, Gloria Tardajos, Sergio Gómez-Graña, Pablo Cruz-Gil, Cástor Salgado, Pablo Díaz-Núñez, Ovidio Peña-Rodríguez, and et al. 2018. "Supramolecular Control over the Interparticle Distance in Gold Nanoparticle Arrays by Cyclodextrin Polyrotaxanes" Nanomaterials 8, no. 3: 168. https://doi.org/10.3390/nano8030168