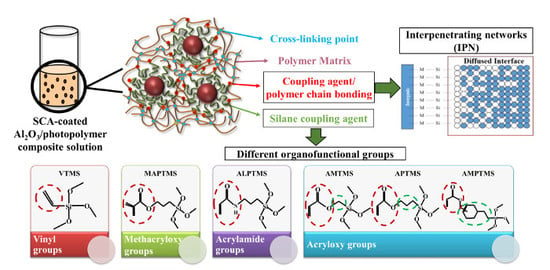
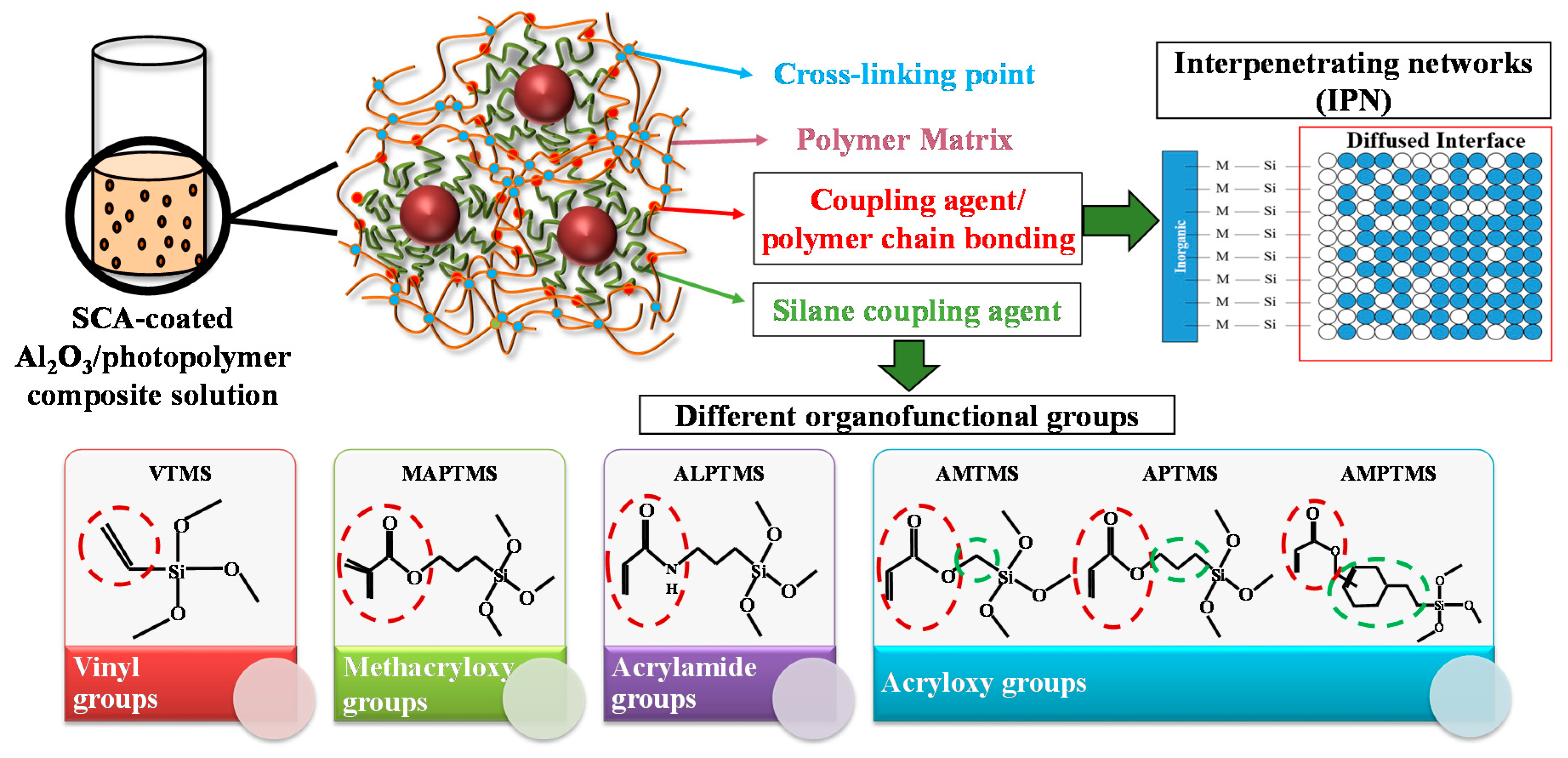
A Study on the Rheological and Mechanical Properties of Photo-Curable Ceramic/Polymer Composites with Different Silane Coupling Agents for SLA 3D Printing Technology
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Materials
2.2. Preparation of the SCA-Coated Al2O3/High-Temp Composite Solutions
2.3. Characterization of the SCA-Coated Al2O3 Particles and the SCA-Coated Al2O3/High-Temp Composite Solutions
3. Results and Discussion
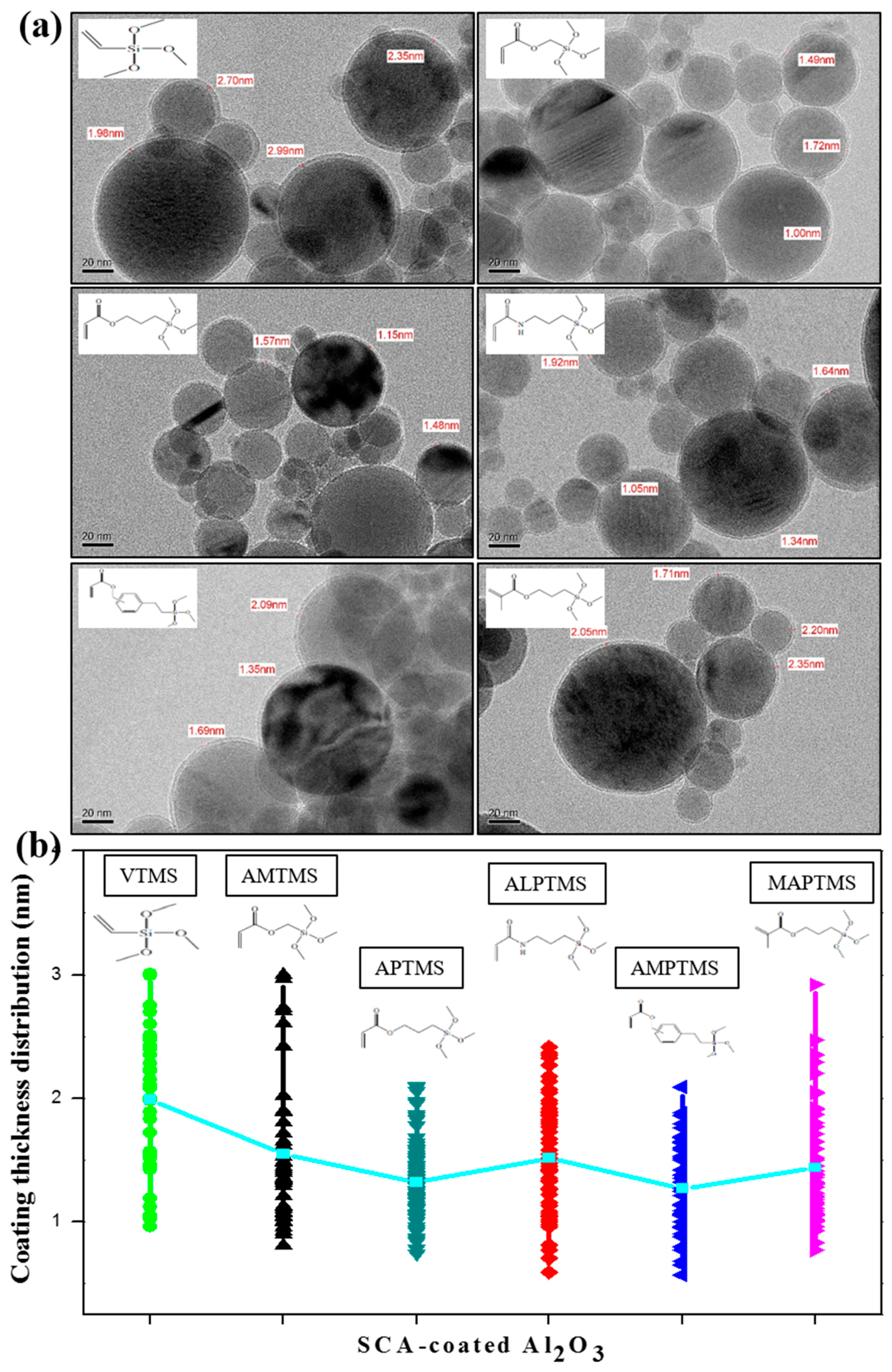
3.1. Characterization of the SCA-Coated Al2O3 Ceramic Particles
3.2. Initial Dispersibility and Dispersion Stability of the SCA-Coated Al2O3/High-Temp Composite Solutions
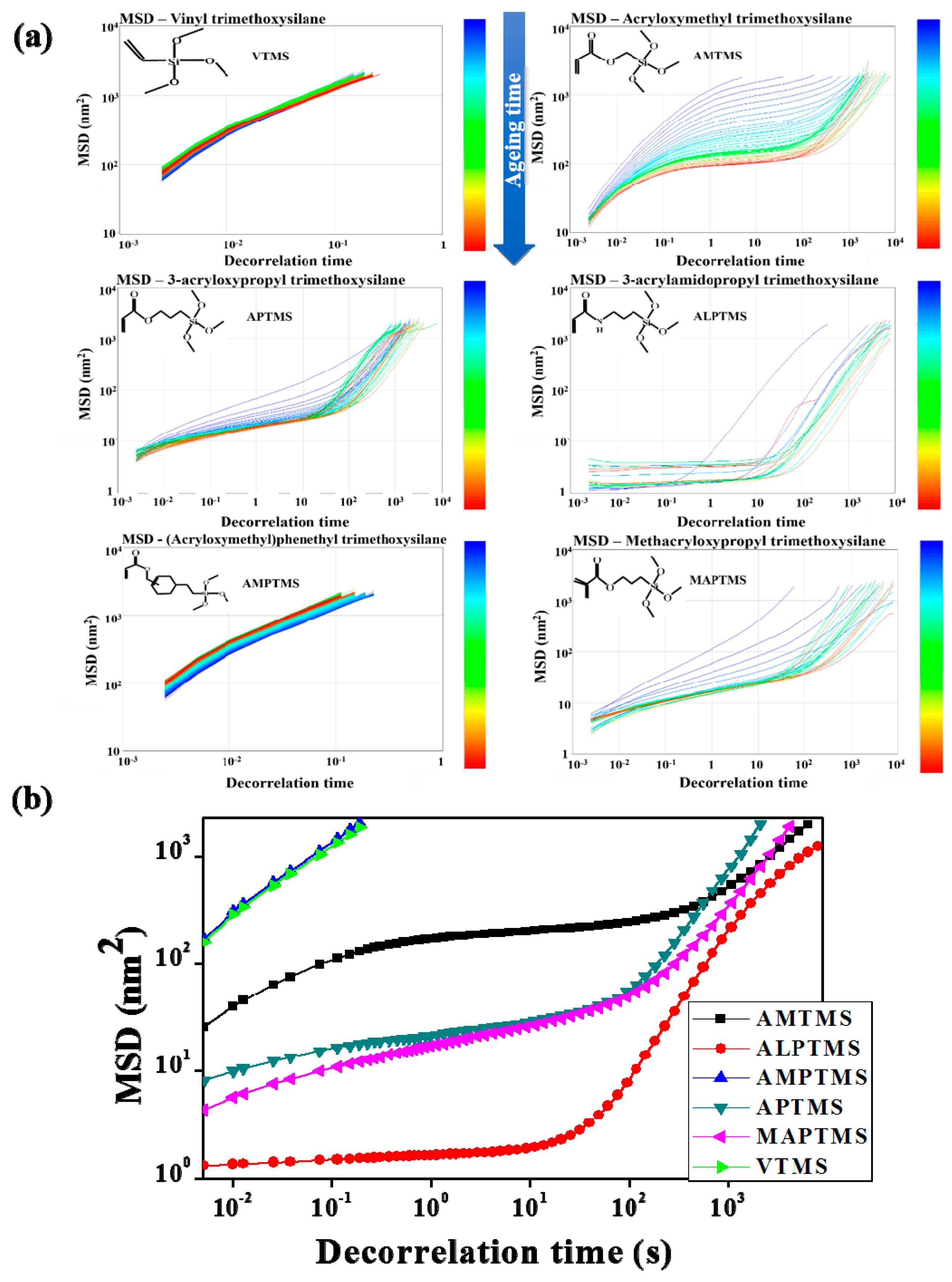
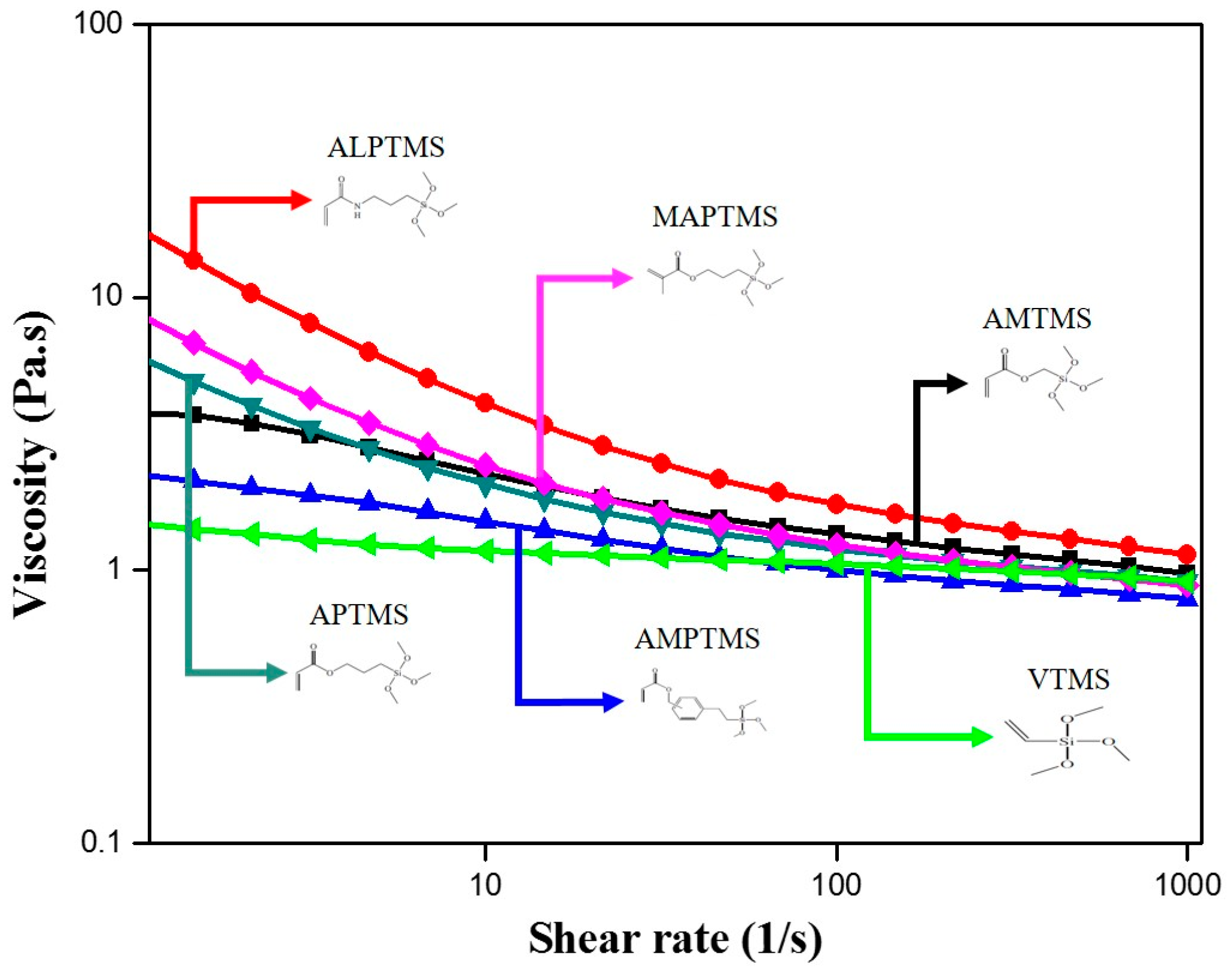
3.3. Rheological Behavior of the SCA-Coated Al2O3/High-Temp Composite Solutions
3.4. Mechanical Properties of the 3D-Printed Objects Printed Using the SCA-Coated Al2O3/High-Temp Composite Solutions
4. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Bertsch, A.; Jiguet, S.; Renaud, P. Microfabrication of ceramic components by microstereolithography. J. Micromech. Microeng. 2004, 14, 197–203. [Google Scholar] [CrossRef]
- Licciulli, A.; Corcione, C.E.; Greco, A.; Amicarelli, V.; Maffezzoli, A. Laser stereolithography of ZrO2 toughened Al2O3. J. Eur. Ceram. Soc. 2005, 25, 1581–1589. [Google Scholar] [CrossRef]
- Lasgorceix, M.; Champion, E.; Chartier, T. Shaping by microstereolithography and sintering of micro-porous silicon substituted hydroxyapatite. J. Eur. Ceram. Soc. 2016, 36, 1091–1101. [Google Scholar] [CrossRef]
- Dufaud, O.; Marchal, P.; Corbel, S. Rheological properties of PZT suspensions for stereolithography. J. Eur. Ceram. Soc. 2002, 22, 2081–2092. [Google Scholar] [CrossRef]
- Bae, C.H. Integrally Cored Ceramic Investment Casting Mold Fabricated by Ceramic Stereolithography. Ph.D. Thesis, Michigan University, Michigan, MI, USA, 2008. [Google Scholar]
- Sabzi, M.; Mirabedini, S.M.; Zohuriaan-Mehr, J.; Atai, M. Surface modification of TiO2 nano-particles with silane coupling agent and investigation of its effect on the properties of polyurethane composite coating. Prog. Org. Coat. 2009, 65, 222–228. [Google Scholar] [CrossRef]
- Luo, T.; Wei, X.; Huang, X.; Huang, L.; Yang, F. Tribological properties of Al2O3 nanoparticles as lubricating oil additives. Ceram. Int. 2014, 40, 7143–7149. [Google Scholar] [CrossRef]
- Yun, J.S.; Park, T.-W.; Jeong, Y.H.; Cho, J.H. Development of ceramic-reinforced photopolymers for SLA 3D printing technology. Appl. Phys. A 2016, 112, 629. [Google Scholar] [CrossRef]
- Ahangaran, F.; Hassanzadeh, A.; Nouri, S. Surface modification of Fe3O4@SiO2 microsphere by silane coupling agent. Int. Nano Lett. 2013, 3, 23. [Google Scholar] [CrossRef]
- Dalle Vacche, S.; Oliveira, F.; Leterrier, Y.; Michaud, V.; Damjanovic, D.; Manson, J.-A.E. Effect of silane coupling agent on the morphology, structure, and properties of poly(vinylidene fluoride-trifluoroethylene)/BaTiO3 composites. J. Mater. Sci. 2014, 49, 4552–4564. [Google Scholar] [CrossRef]
- Zhao, J.; Milanova, M.; Warmoeskerken, M.M.C.G.; Dutschk, V. Surface modification of TiO2 nanoparticles with silane coupling agents. Colloids Surf. A 2012, 413, 273–279. [Google Scholar] [CrossRef]
- Tomovska, R.; Daniloska, V.; Asua, J.M. Surface modification of TiO2 nanoparticles via photocataliticaly induced reaction: Influence of functionality of silane coupling agent. Appl. Surf. Sci. 2013, 264, 670–673. [Google Scholar] [CrossRef]
- Pardo, S.G.; Bernal, C.; Ares, A.; Abad, M.J.; Cano, J. Rheological, Thermal, and Mechanical Characterization of Fly Ash-Thermoplastic Composites With Different Coupling Agents. Polym. Compos. 2010, 31, 1722–1730. [Google Scholar] [CrossRef]
- Kaewsakul, W.; Sahakaro, K.; Dierkes, W.K.; Noordermeer, J.W.M. Mechanistic Aspects of Silane Coupling Agents with Different Functionalities on Reinforcement of Silica-Filled Natural Rubber Compounds. Polym. Eng. Sci. 2015, 55, 836–842. [Google Scholar] [CrossRef]
- Siriwong, C.; Sae-Oui, P.; Sirisinha, C. Comparison of coupling effectiveness among amino-, chloro-, and mercapto silanes in chloroprene rubber. Polym. Test. 2014, 38, 64–72. [Google Scholar] [CrossRef]
- Mengual, O.; Meunier, G.; Cayre, I.; Puech, K.; Snabre, P. TURBISCAN MA 2000: Multiple light scattering measurement for concentrated emulsion and suspension instability analysis. Talanta 1999, 50, 445–456. [Google Scholar] [CrossRef]
- Pérez-Mosqueda, L.M.; Trujillo-Cayado, L.A.; Carrillo, F.; Ramírez, P.; Muñoz, J. Formulation and optimization by experimental design of eco-friendly emulsions based on d-limonene. Colloids Suf. B 2015, 128, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-Q.; Chou, P.-L.; Cheng, C.-Y.; Chiang, C.-C.; Wei, M.-T.; Chuang, C.-T.; Chen, Y.-L.S.; Chiou, A. Microrheology of human synovial fluid of arthritis patients studied by diffusing wave spectroscopy. J. Biophotonics 2012, 5, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Dou, P.; Liu, Z.; Cao, Z.; Zheng, J.; Wang, C. Rapid synthesis of hierarchical nanostructured Polyaniline hydrogel for high power density energy storage application and three-dimensional multilayers printing. J. Mater. Sci. 2016, 51, 4274–4282. [Google Scholar] [CrossRef]
- Zhang, S.; Guo, M.; Chen, Z.; Liu, Q.H.; Liu, X. Grafting photosensitive polyurethane onto colloidal silica for use in UV-curing polyurethane nanocomposites. Colloids Surf. A 2014, 443, 525–534. [Google Scholar] [CrossRef]
- Ouyang, L.; Highley, C.B.; Rodell, C.B.; Sun, W.; Burdick, J.A. 3D printing of shear-thinning hyaluronic acid hydrogels with secondary crosslinking. ACS Biomater. Sci. Eng. 2016, 2, 1743–1751. [Google Scholar] [CrossRef]
- Ament, K.A.; Kessler, M.R.; Akine, M. Shear thinning behavior of aqueous alumina nanoparticle suspensions with saccharides. Ceram. Int. 2014, 40, 3533–3542. [Google Scholar] [CrossRef]
- Lahijania, Y.Z.K.; Mohseni, M.; Bastani, S. Characterization of mechanical behavior of UV-cured urethane acrylate nanocomposite films loaded with silane treated nanosilica by the aid of nanoindentation and nanoscratch experiments. Tribol. Int. 2014, 69, 10–18. [Google Scholar] [CrossRef]



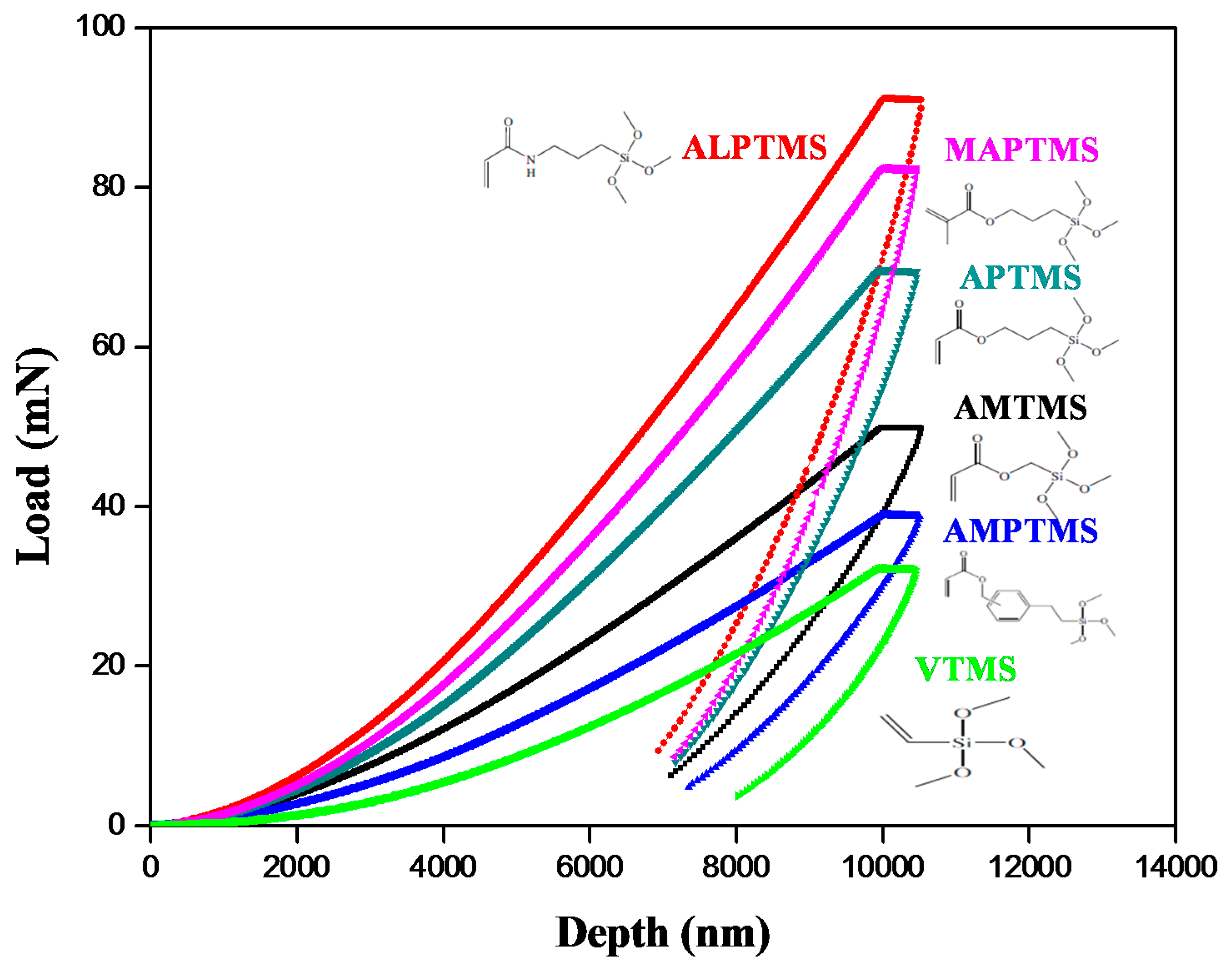
| Sample ID | Nano Indentation Hardness (Gpa) | Nano Indentation Elastic Modulus (Gpa) | Microhardness (Gpa) |
|---|---|---|---|
| VTMS | 0.014 | 0.7 | 0.020 |
| AMTMS | 0.045 | 1.3 | 0.035 |
| APTMS | 0.053 | 1.6 | 0.038 |
| AMPTMS | 0.030 | 1.0 | 0.029 |
| ALPTMS | 0.074 | 2.7 | 0.047 |
| MAPTMS | 0.061 | 1.9 | 0.041 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, S.Y.; Park, M.S.; Lee, J.W.; Yun, J.S. A Study on the Rheological and Mechanical Properties of Photo-Curable Ceramic/Polymer Composites with Different Silane Coupling Agents for SLA 3D Printing Technology. Nanomaterials 2018, 8, 93. https://doi.org/10.3390/nano8020093
Song SY, Park MS, Lee JW, Yun JS. A Study on the Rheological and Mechanical Properties of Photo-Curable Ceramic/Polymer Composites with Different Silane Coupling Agents for SLA 3D Printing Technology. Nanomaterials. 2018; 8(2):93. https://doi.org/10.3390/nano8020093
Chicago/Turabian StyleSong, Se Yeon, Min Soo Park, Jung Woo Lee, and Ji Sun Yun. 2018. "A Study on the Rheological and Mechanical Properties of Photo-Curable Ceramic/Polymer Composites with Different Silane Coupling Agents for SLA 3D Printing Technology" Nanomaterials 8, no. 2: 93. https://doi.org/10.3390/nano8020093