Graphene-Supported Thin Metal Films for Nanophotonics and Optoelectronics
Abstract
:1. Introduction
2. Experimental Section
2.1. Sample Fabrication
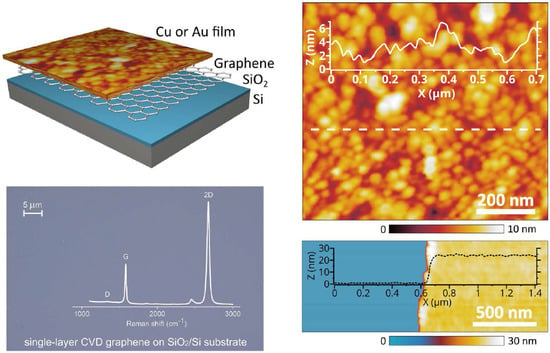
2.2. Structural Characterization
2.3. Optical and Electrical Characterization
3. Results and Discussion
3.1. Thin Gold Films
3.2. Thin Copper Films
3.3. Growth Mechanisms of Thin Metal Films on Graphene
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A



| t = 25 nm (rate = 5 A/s) | t = 50 nm (rate = 5 A/s) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| E (eV) | λ (nm) | ε′ | ε″ | n | k | ε′ | ε″ | n | k |
| 0.38 | 3298 | −517 | 94.9 | 2.08 | 22.8 | −528 | 91.3 | 1.98 | 23.1 |
| 0.45 | 2739 | −359 | 55.3 | 1.45 | 19.0 | −366 | 53.1 | 1.38 | 19.2 |
| 0.53 | 2343 | −262 | 34.9 | 1.08 | 16.2 | −267 | 33.5 | 1.02 | 16.4 |
| 0.61 | 2046 | −199 | 23.5 | 0.83 | 14.1 | −203 | 22.5 | 0.79 | 14.3 |
| 0.68 | 1816 | −155 | 16.6 | 0.66 | 12.5 | −158 | 15.8 | 0.63 | 12.6 |
| 0.76 | 1633 | −125 | 12.2 | 0.54 | 11.2 | −127 | 11.6 | 0.52 | 11.3 |
| 0.80 | 1550 | −111 | 10.6 | 0.49 | 10.5 | −113 | 10.0 | 0.47 | 10.7 |
| 0.84 | 1483 | −102 | 9.24 | 0.46 | 10.1 | −104 | 8.82 | 0.43 | 10.2 |
| 0.91 | 1359 | −84.0 | 7.23 | 0.39 | 9.17 | −85.7 | 6.88 | 0.37 | 9.27 |
| 0.95 | 1310 | −77.5 | 6.53 | 0.37 | 8.81 | −79.1 | 6.21 | 0.35 | 8.90 |
| 0.99 | 1253 | −70.3 | 5.79 | 0.34 | 8.39 | −71.8 | 5.50 | 0.32 | 8.48 |
| 1.07 | 1163 | −59.5 | 4.75 | 0.31 | 7.72 | −60.8 | 4.50 | 0.29 | 7.80 |
| 1.14 | 1085 | −50.7 | 3.98 | 0.28 | 7.13 | −51.9 | 3.76 | 0.26 | 7.21 |
| 1.16 | 1064 | −48.0 | 3.76 | 0.27 | 6.93 | −49.1 | 3.54 | 0.25 | 7.01 |
| 1.22 | 1017 | −43.5 | 3.41 | 0.26 | 6.60 | −44.6 | 3.21 | 0.24 | 6.68 |
| 1.30 | 956.7 | −37.6 | 2.98 | 0.24 | 6.13 | −38.5 | 2.79 | 0.22 | 6.21 |
| 1.37 | 903.3 | −32.5 | 2.65 | 0.23 | 5.71 | −33.4 | 2.47 | 0.21 | 5.79 |
| 1.45 | 855.5 | −28.3 | 2.41 | 0.23 | 5.32 | −29.1 | 2.23 | 0.21 | 5.40 |
| 1.53 | 812.5 | −24.6 | 2.23 | 0.22 | 4.97 | −25.4 | 2.05 | 0.20 | 5.04 |
| 1.59 | 780.0 | −22.0 | 2.12 | 0.23 | 4.69 | −22.7 | 1.94 | 0.20 | 4.77 |
| 1.60 | 773.7 | −21.4 | 2.11 | 0.23 | 4.64 | −22.2 | 1.92 | 0.20 | 4.71 |
| 1.68 | 738.3 | −18.7 | 2.03 | 0.23 | 4.33 | −19.4 | 1.83 | 0.21 | 4.41 |
| 1.76 | 706.1 | −16.2 | 1.99 | 0.25 | 4.04 | −16.9 | 1.78 | 0.22 | 4.12 |
| 1.83 | 676.6 | −14.1 | 1.98 | 0.26 | 3.76 | −14.8 | 1.76 | 0.23 | 3.85 |
| 1.91 | 649.4 | −12.2 | 2.01 | 0.29 | 3.50 | −12.8 | 1.77 | 0.25 | 3.59 |
| 1.96 | 633.0 | −10.8 | 2.06 | 0.31 | 3.30 | −11.5 | 1.76 | 0.26 | 3.39 |
| 1.99 | 624.3 | −10.4 | 2.08 | 0.32 | 3.25 | −11.1 | 1.81 | 0.27 | 3.34 |
| 2.06 | 601.1 | −8.87 | 2.18 | 0.36 | 3.00 | −9.49 | 1.88 | 0.30 | 3.10 |
| 2.14 | 579.6 | −7.44 | 2.32 | 0.42 | 2.76 | −8.05 | 1.99 | 0.35 | 2.86 |
| 2.22 | 559.5 | −6.14 | 2.52 | 0.50 | 2.53 | −6.73 | 2.14 | 0.41 | 2.63 |
| 2.29 | 540.8 | −4.95 | 2.76 | 0.60 | 2.30 | −5.52 | 2.35 | 0.49 | 2.40 |
| 2.33 | 532.0 | −4.27 | 2.95 | 0.68 | 2.18 | −4.83 | 2.50 | 0.55 | 2.27 |
| 2.37 | 523.3 | −3.87 | 3.08 | 0.73 | 2.10 | −4.42 | 2.62 | 0.60 | 2.19 |
| 2.45 | 506.9 | −2.90 | 3.48 | 0.90 | 1.93 | −3.41 | 2.98 | 0.75 | 1.99 |
| 2.52 | 491.5 | −2.06 | 3.96 | 1.10 | 1.81 | −2.53 | 3.43 | 0.93 | 1.84 |
| 2.60 | 477.0 | −1.39 | 4.53 | 1.29 | 1.75 | −1.81 | 3.99 | 1.13 | 1.76 |
| 2.68 | 463.3 | −0.94 | 5.14 | 1.46 | 1.76 | −1.34 | 4.61 | 1.32 | 1.75 |
| 2.75 | 450.4 | −0.71 | 5.73 | 1.59 | 1.80 | −1.13 | 5.21 | 1.45 | 1.80 |
| 2.83 | 438.2 | −0.71 | 6.18 | 1.66 | 1.86 | −1.20 | 5.60 | 1.50 | 1.86 |
| 2.91 | 426.7 | −0.82 | 6.42 | 1.68 | 1.91 | −1.32 | 5.71 | 1.51 | 1.89 |
| 2.98 | 415.7 | −0.90 | 6.47 | 1.68 | 1.93 | −1.33 | 5.62 | 1.49 | 1.88 |
| 3.06 | 405.3 | −0.87 | 6.43 | 1.68 | 1.92 | −1.17 | 5.49 | 1.49 | 1.84 |
| 3.14 | 395.4 | −0.73 | 6.39 | 1.69 | 1.89 | −0.90 | 5.43 | 1.52 | 1.79 |
| 3.21 | 385.9 | −0.53 | 6.43 | 1.72 | 1.87 | −0.60 | 5.49 | 1.57 | 1.75 |
| 3.29 | 376.9 | −0.34 | 6.57 | 1.77 | 1.86 | −0.34 | 5.66 | 1.63 | 1.73 |
| 3.37 | 368.4 | −0.20 | 6.80 | 1.82 | 1.87 | −0.16 | 5.92 | 1.70 | 1.74 |
| 3.44 | 360.2 | −0.17 | 7.10 | 1.86 | 1.91 | −0.10 | 6.22 | 1.75 | 1.78 |
| 3.52 | 352.3 | −0.30 | 7.42 | 1.89 | 1.97 | −0.20 | 6.53 | 1.78 | 1.84 |
| 3.60 | 344.8 | −0.58 | 7.70 | 1.89 | 2.04 | −0.44 | 6.79 | 1.78 | 1.90 |
| 3.67 | 337.6 | −0.97 | 7.90 | 1.87 | 2.11 | −0.77 | 6.96 | 1.77 | 1.97 |
| 3.75 | 330.7 | −1.46 | 7.98 | 1.82 | 2.19 | −1.19 | 7.02 | 1.72 | 2.04 |
| 3.83 | 324.1 | −2.01 | 7.85 | 1.75 | 2.25 | −1.64 | 6.89 | 1.65 | 2.09 |
| 3.90 | 317.7 | −2.53 | 7.60 | 1.65 | 2.30 | −2.07 | 6.65 | 1.57 | 2.13 |
| 3.98 | 311.6 | −2.99 | 7.19 | 1.55 | 2.32 | −2.43 | 6.29 | 1.47 | 2.14 |
| 4.06 | 305.7 | −3.32 | 6.68 | 1.44 | 2.32 | −2.69 | 5.84 | 1.37 | 2.14 |
| 4.13 | 300.0 | −3.59 | 6.13 | 1.33 | 2.31 | −2.89 | 5.37 | 1.27 | 2.12 |
| t = 25 nm (rate = 15 A/s) | t = 50 nm (rate = 15 A/s) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| E (eV) | λ (nm) | ε′ | ε″ | n | k | ε′ | ε″ | n | k |
| 0.38 | 3298 | −456 | 99.5 | 2.32 | 21.5 | −502 | 97.3 | 2.16 | 22.5 |
| 0.45 | 2739 | −318 | 58.4 | 1.63 | 17.9 | −349 | 56.9 | 1.52 | 18.7 |
| 0.53 | 2343 | −233 | 37.1 | 1.21 | 15.3 | −255 | 36.0 | 1.12 | 16.0 |
| 0.61 | 2046 | −177 | 25.1 | 0.94 | 13.4 | −194 | 24.3 | 0.87 | 14.0 |
| 0.68 | 1816 | −139 | 17.7 | 0.75 | 11.8 | −152 | 17.2 | 0.70 | 12.3 |
| 0.76 | 1633 | −111 | 13.1 | 0.62 | 10.6 | −122 | 12.7 | 0.57 | 11.0 |
| 0.80 | 1550 | −99.7 | 11.3 | 0.57 | 10.0 | −109 | 10.9 | 0.52 | 10.5 |
| 0.84 | 1483 | −90.8 | 10.0 | 0.52 | 9.54 | −99.3 | 9.66 | 0.48 | 9.98 |
| 0.91 | 1359 | −75.2 | 7.87 | 0.45 | 8.68 | −82.3 | 7.60 | 0.42 | 9.08 |
| 0.95 | 1310 | −69.4 | 7.14 | 0.43 | 8.34 | −76.0 | 6.89 | 0.39 | 8.73 |
| 0.99 | 1253 | −63.0 | 6.35 | 0.40 | 7.94 | −69.0 | 6.13 | 0.37 | 8.31 |
| 1.07 | 1163 | −53.3 | 5.26 | 0.36 | 7.31 | −58.4 | 5.08 | 0.33 | 7.65 |
| 1.14 | 1085 | −45.5 | 4.46 | 0.33 | 6.75 | −49.9 | 4.30 | 0.30 | 7.07 |
| 1.16 | 1064 | −43.0 | 4.23 | 0.32 | 6.57 | −47.3 | 4.08 | 0.30 | 6.88 |
| 1.22 | 1017 | −39.0 | 3.87 | 0.31 | 6.26 | −42.9 | 3.73 | 0.28 | 6.56 |
| 1.30 | 957 | −33.7 | 3.43 | 0.29 | 5.81 | −37.1 | 3.31 | 0.27 | 6.10 |
| 1.37 | 903 | −29.2 | 3.10 | 0.29 | 5.41 | −32.2 | 3.00 | 0.26 | 5.68 |
| 1.45 | 856 | −25.4 | 2.87 | 0.28 | 5.04 | −28.1 | 2.77 | 0.26 | 5.31 |
| 1.53 | 813 | −22.1 | 2.71 | 0.29 | 4.71 | −24.5 | 2.61 | 0.26 | 4.96 |
| 1.59 | 780 | −19.7 | 2.62 | 0.29 | 4.45 | −21.9 | 2.53 | 0.27 | 4.69 |
| 1.60 | 774 | −19.2 | 2.61 | 0.30 | 4.39 | −21.4 | 2.52 | 0.27 | 4.64 |
| 1.68 | 738 | −16.8 | 2.56 | 0.31 | 4.11 | −18.8 | 2.47 | 0.28 | 4.34 |
| 1.76 | 706 | −14.6 | 2.56 | 0.33 | 3.83 | −16.4 | 2.48 | 0.31 | 4.06 |
| 1.83 | 677 | −12.6 | 2.62 | 0.37 | 3.58 | −14.3 | 2.52 | 0.33 | 3.80 |
| 1.91 | 649 | −10.9 | 2.72 | 0.41 | 3.33 | −12.4 | 2.62 | 0.37 | 3.55 |
| 1.96 | 633 | −9.71 | 2.84 | 0.45 | 3.15 | −11.1 | 2.74 | 0.41 | 3.36 |
| 1.99 | 624 | −9.37 | 2.89 | 0.47 | 3.10 | −10.8 | 2.78 | 0.42 | 3.31 |
| 2.06 | 601 | −8.00 | 3.13 | 0.54 | 2.88 | −9.26 | 3.00 | 0.49 | 3.08 |
| 2.14 | 580 | −6.78 | 3.45 | 0.64 | 2.68 | −7.92 | 3.31 | 0.58 | 2.87 |
| 2.22 | 560 | −5.74 | 3.88 | 0.77 | 2.52 | −6.76 | 3.71 | 0.69 | 2.69 |
| 2.29 | 541 | −4.95 | 4.39 | 0.91 | 2.40 | −5.81 | 4.21 | 0.83 | 2.55 |
| 2.33 | 532 | −4.60 | 4.71 | 1.00 | 2.36 | −5.35 | 4.56 | 0.92 | 2.49 |
| 2.37 | 523 | −4.46 | 4.91 | 1.04 | 2.35 | −5.14 | 4.79 | 0.97 | 2.47 |
| 2.45 | 507 | −4.25 | 5.31 | 1.13 | 2.35 | −4.80 | 5.33 | 1.09 | 2.45 |
| 2.52 | 492 | −4.23 | 5.45 | 1.16 | 2.36 | −4.74 | 5.66 | 1.15 | 2.46 |
| 2.60 | 477 | −4.17 | 5.32 | 1.14 | 2.34 | −4.79 | 5.67 | 1.15 | 2.47 |
| 2.68 | 463 | −3.96 | 5.08 | 1.11 | 2.28 | −4.73 | 5.44 | 1.11 | 2.44 |
| 2.75 | 450 | −3.63 | 4.84 | 1.10 | 2.20 | −4.50 | 5.13 | 1.08 | 2.38 |
| 2.83 | 438 | −3.22 | 4.70 | 1.11 | 2.11 | −4.14 | 4.87 | 1.06 | 2.29 |
| 2.91 | 427 | −2.82 | 4.64 | 1.14 | 2.03 | −3.73 | 4.70 | 1.07 | 2.21 |
| 2.98 | 416 | −2.45 | 4.65 | 1.19 | 1.96 | −3.32 | 4.62 | 1.09 | 2.12 |
| 3.06 | 405 | −2.14 | 4.72 | 1.23 | 1.91 | −2.96 | 4.61 | 1.12 | 2.05 |
| 3.14 | 395 | −1.89 | 4.82 | 1.28 | 1.88 | −2.65 | 4.65 | 1.16 | 2.00 |
| 3.21 | 386 | −1.70 | 4.94 | 1.33 | 1.86 | −2.40 | 4.71 | 1.20 | 1.96 |
| 3.29 | 377 | −1.58 | 5.06 | 1.36 | 1.85 | −2.22 | 4.79 | 1.24 | 1.94 |
| 3.37 | 368 | −1.52 | 5.17 | 1.39 | 1.86 | −2.09 | 4.87 | 1.27 | 1.92 |
| 3.44 | 360 | −1.52 | 5.26 | 1.41 | 1.87 | −2.02 | 4.93 | 1.29 | 1.92 |
| 3.52 | 352 | −1.57 | 5.31 | 1.41 | 1.88 | −2.00 | 4.96 | 1.29 | 1.92 |
| 3.60 | 345 | −1.65 | 5.32 | 1.40 | 1.90 | −2.02 | 4.97 | 1.29 | 1.92 |
| 3.67 | 338 | −1.75 | 5.30 | 1.38 | 1.92 | −2.06 | 4.94 | 1.28 | 1.93 |
| 3.75 | 331 | −1.87 | 5.23 | 1.36 | 1.93 | −2.12 | 4.88 | 1.26 | 1.93 |
| 3.83 | 324 | −1.99 | 5.11 | 1.32 | 1.93 | −2.18 | 4.76 | 1.24 | 1.93 |
| 3.90 | 318 | −2.10 | 4.95 | 1.28 | 1.93 | −2.24 | 4.63 | 1.20 | 1.92 |
| 3.98 | 312 | −2.20 | 4.77 | 1.24 | 1.93 | −2.29 | 4.46 | 1.17 | 1.91 |
| 4.06 | 306 | −2.26 | 4.55 | 1.19 | 1.92 | −2.32 | 4.27 | 1.13 | 1.90 |
| 4.13 | 300 | −2.32 | 4.33 | 1.14 | 1.90 | −2.34 | 4.07 | 1.08 | 1.88 |
References
- Grigorenko, A.N.; Polini, M.; Novoselov, K.S. Graphene plasmonics. Nat. Photonics 2012, 6, 749–758. [Google Scholar] [CrossRef]
- Li, X.; Zhu, J.; Wei, B. Hybrid nanostructures of metal/two-dimensional nanomaterials for plasmon-enhanced applications. Chem. Soc. Rev. 2016, 45, 3145–3187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giubileo, F.; Di Bartolomeo, A. The role of contact resistance in graphene field-effect devices. Prog. Surf. Sci. 2017, 92, 143–175. [Google Scholar] [CrossRef] [Green Version]
- Nair, R.R.; Blake, P.; Grigorenko, A.N.; Novoselov, K.S.; Booth, T.J.; Stauber, T.; Peres, N.M.R.; Geim, A.K. Fine structure constant defines visual transparency of graphene. Science 2008, 320, 1308. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Q.; Henriksen, E.A.; Jiang, Z.; Hao, Z.; Martin, M.C.; Kim, P.; Stormer, H.L.; Basov, D.N. Dirac charge dynamics in graphene by infrared spectroscopy. Nat. Phys. 2008, 4, 532–535. [Google Scholar] [CrossRef] [Green Version]
- Dawlaty, J.M.; Shivaraman, S.; Strait, J.; George, P.; Chandrashekhar, M.; Rana, F.; Spencer, M.G.; Veksler, D.; Chen, Y. Measurement of the optical absorption spectra of epitaxial graphene from terahertz to visible. Appl. Phys. Lett. 2008, 93, 131905. [Google Scholar] [CrossRef] [Green Version]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Bolotin, K.I.; Sikes, K.J.; Jiang, Z.; Klima, M.; Fudenberg, G.; Hone, J.; Kim, P.; Stormer, H.L. Ultrahigh electron mobility in suspended graphene. Solid State Commun. 2008, 146, 351–355. [Google Scholar] [CrossRef] [Green Version]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef]
- Balandin, A.A. Thermal properties of graphene and nanostructured carbon materials. Nat. Mater. 2011, 10, 569–581. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Leenaerts, O.; Partoens, B.; Peeters, F.M. Graphene: A perfect nanoballoon. Appl. Phys. Lett. 2008, 93, 193107. [Google Scholar] [CrossRef] [Green Version]
- Bunch, J.S.; Verbridge, S.S.; Alden, J.S.; van der Zande, A.M.; Parpia, J.M.; Craighead, H.G.; McEuen, P.L. Impermeable atomic membranes from graphene sheets. Nano Lett. 2008, 8, 2458–2462. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Lee, S.; Lee, S.; Han, H.; Mahata, C.; Yeon, H.-W.; Koo, B.; Kim, S.-I.; Nam, T.; Byun, K.; et al. Graphene as an atomically thin barrier to Cu diffusion into Si. Nanoscale 2014, 6, 7503–7511. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Regan, W.; Geng, B.; Alemán, B.; Kessler, B.M.; Wang, F.; Crommie, M.F.; Zettl, A. High-temperature stability of suspended single-layer graphene. Phys. Status Solidi RRL 2010, 4, 302–304. [Google Scholar] [CrossRef]
- Nan, H.Y.; Ni, Z.H.; Wang, J.; Zafar, Z.; Shi, Z.X.; Wang, Y.Y. The thermal stability of graphene in air investigated by Raman spectroscopy. J. Raman Spectrosc. 2013, 44, 1018–1021. [Google Scholar] [CrossRef]
- Stebunov, Y.V.; Arsenin, A.V.; Volkov, V.S. Functionalization of chemically derived graphene for surface plasmon resonance (SPR) biosensors. In Chemically Derived Graphene: Functionalization, Properties and Applications; The Royal Society of Chemistry: London, UK, 2018; pp. 328–353. [Google Scholar]
- Stebunov, Y.V.; Aftenieva, O.A.; Arsenin, A.V.; Volkov, V.S. Highly sensitive and selective sensor chips with graphene-oxide linking layer. ACS Appl. Mater. Interfaces 2015, 7, 21727–21734. [Google Scholar] [CrossRef] [PubMed]
- Echtermeyer, T.J.; Britnell, L.; Jasnos, P.K.; Lombardo, A.; Gorbachev, R.V.; Grigorenko, A.N.; Geim, A.K.; Ferrari, A.C.; Novoselov, K.S. Strong plasmonic enhancement of photovoltage in graphene. Nat. Commun. 2011, 2, 458. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Cheng, R.; Liao, L.; Zhou, H.; Bai, J.; Liu, G.; Liu, L.; Huang, Y.; Duan, X. Plasmon resonance enhanced multicolour photodetection by graphene. Nat. Commun. 2011, 2, 579. [Google Scholar] [CrossRef] [Green Version]
- Fang, Z.; Liu, Z.; Wang, Y.; Ajayan, P.M.; Nordlander, P.; Halas, N.J. Graphene-antenna sandwich photodetector. Nano Lett. 2012, 12, 3808–3813. [Google Scholar] [CrossRef]
- Kim, J.; Son, H.; Cho, D.J.; Geng, B.; Regan, W.; Shi, S.; Kim, K.; Zettl, A.; Shen, Y.-R.; Wang, F. Electrical control of optical plasmon resonance with graphene. Nano Lett. 2012, 12, 5598–5602. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Martinez, A.; Wang, F. Optical modulators with 2D layered materials. Nat. Photonics 2016, 10, 227–238. [Google Scholar] [CrossRef] [Green Version]
- Fang, J.; Wang, D.; DeVault, C.T.; Chung, T.-F.; Chen, Y.P.; Boltasseva, A.; Shalaev, V.M.; Kildishev, A.V. Enhanced graphene photodetector with fractal metasurface. Nano Lett. 2017, 17, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lee, J.; Yeom, M.S.; Shin, J.W.; Kim, H.; Cui, Y.; Kysar, J.W.; Hone, J.; Jung, Y.; Jeon, S.; et al. Strengthening effect of single-atomic-layer graphene in metal–graphene nanolayered composites. Nat. Commun. 2013, 4, 2114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, B.; Kim, W.; Kim, J.; Lee, S.; Lim, S.; Kim, S.; Oh, S.H.; Ryu, S.; Han, S.M. Role of graphene in reducing fatigue damage in Cu/Gr nanolayered composite. Nano Lett. 2017, 17, 4740–4745. [Google Scholar] [CrossRef]
- Kim, M.; Kang, P.; Leem, J.; Nam, S. A stretchable crumpled graphene photodetector with plasmonically enhanced photoresponsivity. Nanoscale 2017, 9, 4058–4065. [Google Scholar] [CrossRef] [PubMed]
- Kholmanov, I.N.; Magnuson, C.W.; Aliev, A.E.; Li, H.; Zhang, B.; Suk, J.W.; Zhang, L.L.; Peng, E.; Mousavi, S.H.; Khanikaev, A.B.; et al. Improved electrical conductivity of graphene films integrated with metal nanowires. Nano Lett. 2012, 12, 5679–5683. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-T.; Kim, H.; Kenney, M.; Park, H.S.; Kim, H.-D.; Min, B.; Zhang, S. Amplitude modulation of anomalously refracted terahertz waves with gated-graphene metasurfaces. Adv. Opt. Mater. 2018, 6, 1700507. [Google Scholar] [CrossRef]
- Lu, C.; Hu, X.; Shi, K.; Hu, Q.; Zhu, R.; Yang, H.; Gong, Q. An actively ultrafast tunable giant slow-light effect in ultrathin nonlinear metasurfaces. Light Sci. Appl. 2015, 4, e302. [Google Scholar] [CrossRef]
- Aygar, A.M.; Balci, O.; Cakmakyapan, S.; Kocabas, C.; Caglayan, H.; Ozbay, E. Comparison of back and top gating schemes with tunable graphene fractal metasurfaces. ACS Photonics 2016, 3, 2303–2307. [Google Scholar] [CrossRef]
- Zhao, Y.T.; Wu, B.; Huang, B.J.; Cheng, Q. Switchable broadband terahertz absorber/reflector enabled by hybrid graphene-gold metasurface. Opt. Express 2017, 25, 7161–7169. [Google Scholar] [CrossRef] [PubMed]
- Marin, B.C.; Ramirez, J.; Root, S.E.; Aklile, E.; Lipomi, D.J. Metallic nanoislands on graphene: A metamaterial for chemical, mechanical, optical, and biological applications. Nanoscale Horiz. 2017, 2, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Turcheniuk, K.; Boukherroub, R.; Szunerits, S. Gold–graphene nanocomposites for sensing and biomedical applications. J. Mater. Chem. B 2015, 3, 4301–4324. [Google Scholar] [CrossRef]
- Leem, J.; Wang, M.C.; Kang, P.; Nam, S. Mechanically self-assembled, three-dimensional graphene–gold hybrid nanostructures for advanced nanoplasmonic sensors. Nano Lett. 2015, 15, 7684–7690. [Google Scholar] [CrossRef] [PubMed]
- Park, D.-W.; Schendel, A.A.; Mikael, S.; Brodnick, S.K.; Richner, T.J.; Ness, J.P.; Hayat, M.R.; Atry, F.; Frye, S.T.; Pashaie, R.; et al. Graphene-based carbon-layered electrode array technology for neural imaging and optogenetic applications. Nat. Commun. 2014, 5, 5258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuzum, D.; Takano, H.; Shim, E.; Reed, J.C.; Juul, H.; Richardson, A.G.; de Vries, J.; Bink, H.; Dichter, M.A.; Lucas, T.H.; et al. Transparent, flexible, low noise graphene electrodes for simultaneous electrophysiology and neuroimaging. Nat. Commun. 2014, 5, 5259. [Google Scholar] [CrossRef] [PubMed]
- Kostarelos, K.; Vincent, M.; Hebert, C.; Garrido, J.A. Graphene in the design and engineering of next-generation neural interfaces. Adv. Mater. 2017, 29, 1700909. [Google Scholar] [CrossRef]
- Wong, J.; Jariwala, D.; Tagliabue, G.; Tat, K.; Davoyan, A.R.; Sherrott, M.C.; Atwater, H.A. High photovoltaic quantum efficiency in Ultrathin van der Waals Heterostructures. ACS Nano 2017, 11, 7230–7240. [Google Scholar] [CrossRef]
- Iranzo, D.A.; Nanot, S.; Dias, E.J.C.; Epstein, I.; Peng, C.; Efetov, D.K.; Lundeberg, M.B.; Parret, R.; Osmond, J.; Hong, J.; et al. Probing the ultimate plasmon confinement limits with a van der Waals heterostructure. Science 2018, 360, 291–295. [Google Scholar] [CrossRef] [Green Version]
- Parzefall, M.; Szabo, A.; Taniguchi, T.; Watanabe, K.; Luisier, M.; Novotny, L. Light from Van der Waals quantum tunneling devices. arXiv, 2018; arXiv:1804.06163. [Google Scholar]
- Tantiwanichapan, K.; Wang, X.; Durmaz, H.; Li, Y.; Swan, A.K.; Paiella, R. Graphene terahertz plasmons: A combined transmission spectroscopy and Raman microscopy study. ACS Photonics 2017, 4, 2011–2017. [Google Scholar] [CrossRef]
- Johnson, P.B.; Christy, R.W. Optical constants of the noble metals. Phys. Rev. B 1972, 6, 4370–4379. [Google Scholar] [CrossRef]
- Yakubovsky, D.I.; Arsenin, A.V.; Stebunov, Y.V.; Fedyanin, D.Y.; Volkov, V.S. Optical constants and structural properties of thin gold films. Opt. Express 2017, 25, 25574–25587. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Han, Y.; Evans, J.W.; Engstfeld, A.K.; Behm, R.J.; Tringides, M.C.; Hupalo, M.; Lin, H.-Q.; Huang, L.; Ho, K.-M.; et al. Growth morphology and properties of metals on graphene. Progr. Surf. Sci. 2015, 90, 397–443. [Google Scholar] [CrossRef] [Green Version]
- Ruffino, F.; Giannazzo, F. A Review on metal nanoparticles nucleation and growth on/in graphene. Crystals 2017, 7, 219. [Google Scholar] [CrossRef]
- Hodas, M.; Siffalovic, P.; Jergel, M.; Pelletta, M.; Halahovets, Y.; Vegso, K.; Kotlar, M.; Majkova, E. Kinetics of copper growth on graphene revealed by time-resolved small-angle X-ray scattering. Phys. Rev. B 2017, 95, 035424. [Google Scholar] [CrossRef]
- Liu, X.; Wang, C.Z.; Hupalo, M.; Lu, W.C.; Tringides, M.C.; Yao, Y.X.; Ho, K.M. Metals on graphene: Correlation between adatom adsorption behavior and growth morphology. Phys. Chem. Chem. Phys. 2012, 14, 9157–9166. [Google Scholar] [CrossRef]
- Liu, X.; Wang, C.Z.; Hupalo, M.; Lin, H.Q.; Ho, K.M.; Tringides, M.C. Metals on graphene: Interactions, growth morphology, and thermal stability. Crystals 2013, 3, 79–111. [Google Scholar] [CrossRef]
- Malureanu, R.; Lavrinenko, A. Ultra-thin films for plasmonics: A technology overview. Nanotechnol. Rev. 2015, 4, 1–17. [Google Scholar] [CrossRef]
- McPeak, K.M.; Jayanti, S.V.; Kress, S.J.P.; Meyer, S.; Iotti, S.; Rossinelli, A.; Norris, D.J. Plasmonic films can easily be better: Rules and recipes. ACS Photonics 2015, 2, 326–333. [Google Scholar] [CrossRef]
- Fedyanin, D.Y.; Yakubovsky, D.I.; Kirtaev, R.V.; Volkov, V.S. Ultralow-loss CMOS copper plasmonic waveguides. Nano Lett. 2016, 16, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Stebunov, Y.V.; Yakubovsky, D.I.; Fedyanin, D.Y.; Arsenin, A.V.; Volkov, V.S. Superior sensitivity of copper-based plasmonic biosensors. Langmuir 2018, 34, 4681–4687. [Google Scholar] [CrossRef] [PubMed]
- Hu, E.T.; Cai, Q.Y.; Zhang, R.J.; Wei, Y.F.; Zhou, W.C.; Wang, S.Y.; Zheng, Y.X.; Wei, W.; Chen, L.Y. Effective method to study the thickness-dependent dielectric functions of nanometal thin film. Opt. Lett. 2016, 41, 4907–4910. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Y.; Wang, Z.Y.; Zhang, T.N.; Zhang, Y.; Zhang, R.J.; Chen, X.; Sun, Y.; Zheng, Y.X.; Wang, S.Y.; Chen, L.Y. Thickness-dependent free-electron relaxation time of Au thin films in near-infrared region. J. Nanophoton. 2016, 10, 033009. [Google Scholar] [CrossRef]
- Yakubovsky, D.I.; Fedyanin, D.Y.; Arsenin, A.V.; Volkov, V.S. Optical constant of thin gold films: Structural morphology determined optical response. AIP Conf. Proc. 2017, 1874, 040057. [Google Scholar]
- Park, J.H.; Nagpal, P.; Oh, S.-H.; Norris, D.J. Improved dielectric functions in metallic films obtained via template stripping. Appl. Phys. Lett. 2012, 100, 081105. [Google Scholar] [CrossRef]
- Reddy, H.; Guler, U.; Kildishev, A.V.; Boltasseva, A.; Shalaev, V.M. Temperature-dependent optical properties of gold thin films. Opt. Mater. Express 2016, 6, 2776–2802. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef]
- Zaporojtchenko, V.; Zekonyte, J.; Biswas, A.; Faupel, F. Controlled growth of nano-size metal clusters on polymers by using VPD method. Surf. Sci. 2003, 532–535, 300–305. [Google Scholar] [CrossRef]
- Schwartzkopf, M.; Hinz, A.; Polonskyi, O.; Strunskus, T.; Löhrer, F.C.; Körstgens, V.; Müller-Buschbaum, P.; Faupel, F.; Roth, S.V. Role of sputter deposition rate in tailoring nanogranular gold structures on polymer surfaces. ACS Appl. Mater. Interfaces 2017, 9, 5629–5637. [Google Scholar] [CrossRef]
- Mahmoodi, N.; Rushdi, A.I.; Bowen, J.; Sabouri, A.; Anthony, C.J.; Mendes, P.M.; Preece, J.A. Room temperature thermally evaporated thin Au film on Si suitable for application of thiol self-assembled monolayers in micro/nano-electro-mechanical-systems sensors. J. Vac. Sci. Technol. A 2017, 35, 041514. [Google Scholar] [CrossRef] [Green Version]
- Marconi, D.; Colniţă, A.; Turcu, I. The influence of deposition rate on the structure and morphology of gold/silicon(111) growth by molecular beam epitaxy. Anal. Lett. 2016, 49, 400–410. [Google Scholar] [CrossRef]
- Venables, J.A.; Spiller, G.D.T.; Hanbucken, M. Nucleation and growth of thin films. Rep. Prog. Phys. 1984, 47, 399–459. [Google Scholar] [CrossRef]
- Müller, B.; Nedelmann, L.; Fischer, B.; Brune, H.; Kern, K. Initial stages of Cu epitaxy on Ni(100): Postnucleation and a well-defined transition in critical island size. Phys. Rev. B 1996, 54, 17858. [Google Scholar] [CrossRef]
- Fan, X.F.; Zheng, W.T.; Chihaia, V.; Shen, Z.X.; Kuo, J.L. Interaction between graphene and the surface of SiO2. J. Phys.: Condens. Matter 2012, 24, 305004. [Google Scholar] [CrossRef] [PubMed]
- Volmer, M.; Weber, A. Nucleus formation in supersaturated systems. Z. Phys. Chem. 1926, 119, 277–301. [Google Scholar]
- Taylor, J.B.; Langmuir, I. The evaporation of atoms, ions and electrons from caesium films on tungsten. Phys. Rev. 1933, 44, 423–458. [Google Scholar] [CrossRef]
- Antczak, G.; Ehrlich, G. Surface Diffusion: Metals, Metal Atoms, and Clusters; Cambridge University Press: New York, NY, USA, 2010. [Google Scholar]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yakubovsky, D.I.; Stebunov, Y.V.; Kirtaev, R.V.; Voronin, K.V.; Voronov, A.A.; Arsenin, A.V.; Volkov, V.S. Graphene-Supported Thin Metal Films for Nanophotonics and Optoelectronics. Nanomaterials 2018, 8, 1058. https://doi.org/10.3390/nano8121058
Yakubovsky DI, Stebunov YV, Kirtaev RV, Voronin KV, Voronov AA, Arsenin AV, Volkov VS. Graphene-Supported Thin Metal Films for Nanophotonics and Optoelectronics. Nanomaterials. 2018; 8(12):1058. https://doi.org/10.3390/nano8121058
Chicago/Turabian StyleYakubovsky, Dmitry I., Yury V. Stebunov, Roman V. Kirtaev, Kirill V. Voronin, Artem A. Voronov, Aleksey V. Arsenin, and Valentyn S. Volkov. 2018. "Graphene-Supported Thin Metal Films for Nanophotonics and Optoelectronics" Nanomaterials 8, no. 12: 1058. https://doi.org/10.3390/nano8121058