1. Introduction
The protein phosphorylation event is one of the key regulators of intracellular biological processes and may be one of the most studied post-translational modifications (PTM) [
1,
2]. Indeed, the phosphorylation of amino-acid residues allows the regulation of several biological processes such as metabolism, transcriptional and translational regulation, and the degradation of proteins. It is also involved in the phenomenon of homeostasis, in cellular signaling and in cell communication, proliferation, differentiation and survival [
3,
4]. These transient and reversible PTMs provoke changes in the conformation of a protein, and so have a strong influence on its activity and interactions with the environment within a very short time frame. Because phosphorylation is a reversible phenomenon, protein activities can be finely controlled by either phosphorylation (using kinase) or by dephosphorylation (using phosphatase) in response to cellular or environmental stimuli. Most of the phosphorylation events are mainly realized with serine, threonine, or tyrosine amino-acids residues. However, despite their ubiquitous and crucial roles, phosphoproteins are generally present in low abundance. Indeed, the phosphorylated forms of individual proteins are present at much lower levels compared to their native counterparts. Within the phosphoproteome, most of the phosphorylated proteins are transiently phosphorylated following pivotal cellular signals, metabolic disorders or diseases, whereas some others are constitutively modified [
5].
Since phosphorylation events help to maintain the living cell’s dynamics, the development of performant analytical methods for the detection/characterization of phosphorylated peptides is, obviously, crucial. Since phosphoproteins are present in relative low abundance with low phosphorylation stoichiometry, the need for sensitive and highly specific analytical strategies to characterize these PTMs is therefore crucial. In that context, phosphoproteins are mainly characterized, after proteolytic processing, through the use of mass spectrometry (MS).
However, phosphopeptide detection through MS is still challenging because of their lower ionization efficiency, which results in lower signal intensities, especially in the presence of non-phosphorylated peptide ions. Therefore, there is a clear need for efficient enrichment of the phosphorylated proteins or peptides, prior to MS analysis, in order to increase the sensitivity and obtain better phosphoproteome characterization. Many different phosphoproteomic enrichment strategies are currently used, and these depend heavily on the type of sample to be studied. Among them are: immunoprecipitation (immobilized anti-phosphoserine (pS), anti-phosphothreonine (pT) or anti-phosphotyrosine (pY) antibodies on magnetic beads) [
6,
7]; chemical derivatization (β-elimination, phosphoramidate-based chemistry) [
8]; and IMAC (immobilized metallic ion affinity chromatography) [
9,
10] or MOAC (metal oxide affinity chromatography) based chromatographies [
11,
12,
13]. IMAC adsorbents chelating Ga
3+, Ti
4+, Ni
2+, or Zr
4+ have been widely utilized for the enrichment of phosphopeptides in phosphoproteome analysis [
14]. However, the main drawback of IMAC is the non-specific interaction of acidic amino acid and histidine residues [
15]. MOAC is, therefore, preferred to IMAC, and metal oxides such as TiO
2 and ZrO
2 have been used for the enrichment of phosphopeptides due to the unique affinity between titania or zirconia and phosphopeptides [
14].
However, in all these cases, phosphopeptide enrichment is mainly achieved through an “off-plate” sample preparation procedure (capture and elution steps) prior to MS analysis. Nevertheless, “on-plate” enrichment was developed to perform in situ phosphopeptide enrichment followed by rinsing steps and organic matrix deposition (DHB) prior to matrix-assisted laser desorption ionization mass spectrometry (MALDI-MS) detection [
16]. This approach consists of modifying a MALDI target plate with spots of a material known to enrich phosphopeptides. In this case, the employed materials are similar to those used in IMAC e.g., polymer brushes made of poly(2-hydroxyethyl methacrylate) (pHEMA) derivatized with Fe(III)-nitriloacetate (NTA) or in MOAC e.g., a thin film of anatase titanium [
16,
17]. Enrichment is then achieved directly on the stainless steel MS target, and the non-specifically bounded peptides are removed using appropriate washing protocols.
In parallel, the development of matrix-free laser desorption/ionization mass spectrometry (LDI-MS) methods, based on micro- and nanostructured laser desorption ionization (LDI) targets, were proposed to eliminate the need for co-crystallization of the sample with an organic MALDI matrix. Among the main recognized advantages of a surface laser desorption/ionization (SALDI) matrix-free soft ionization method is the avoidance of intense matrix peaks present at low mass range of the spectra, increasing the background while lowering the sensitivity of detection of small molecules [
18]. One of the most notable developments in this domain has been the development of desorption/ionization on porous silicon (DIOS), realized by the Siuzdak group, which uses an etched
p-type Si target [
19]. For certain classes of analytes at least, SALDI-MS equaled, or even exceeded, the sensitivity of classical MALDI-MS.
We have recently demonstrated that silicon nanowires (SiNWs) decorated with Cu/CuO particles can be used for “on-plate” His-tag peptide enrichment from serum spiked with His-tagged (
m/
z 1770) and untagged (
m/
z 905) peptides. In this case, Cu
2+, contained in copper oxide, can interact with unprotonated (pH 7) nitrogen from imidazole groups (His*6-tag). The matrix-free LDI-MS detection was of a sensitivity down to 10 fmol/µL [
20]. Concerning the phosphopeptides’ enrichment and their subsequent detection by SALDI-MS, TiO
2 nanoparticles spots on an aluminum foil-based surface, thereby combining specific capture and mass spectrometry analysis, was used. The TiO
2 was used as a specific affinity for in situ enrichment of phosphopeptides and for SALDI-MS analysis with lower sensitivity (few pmole) [
21]. In the same vein, we proposed performing phosphorylated peptide enrichment and subsequent SALDI-MS detection. To do so, SiNWs made using metal-assisted chemical etching (MACE) were covered by TiO
2 layers through thermal evaporation or atomic layer deposition techniques (ALD), respectively. Then, an equimolar mixture composed of non-phosphorylated peptides and mono- and diphosphorylated peptides was used to assess the performance of our interfaces as an efficient “on-plate” enrichment method and sensitive matrix-free LDI-MS technique.
2. Results and Discussion
The SiNWs investigated in this work were prepared using the MACE technique following a protocol already described in previous studies [
22,
23]. By adjusting reaction parameters such as time, temperature and reagent concentrations, various silicon surface morphologies can be achieved that present different nanostructure length, diameter, and overall porosity [
22,
24,
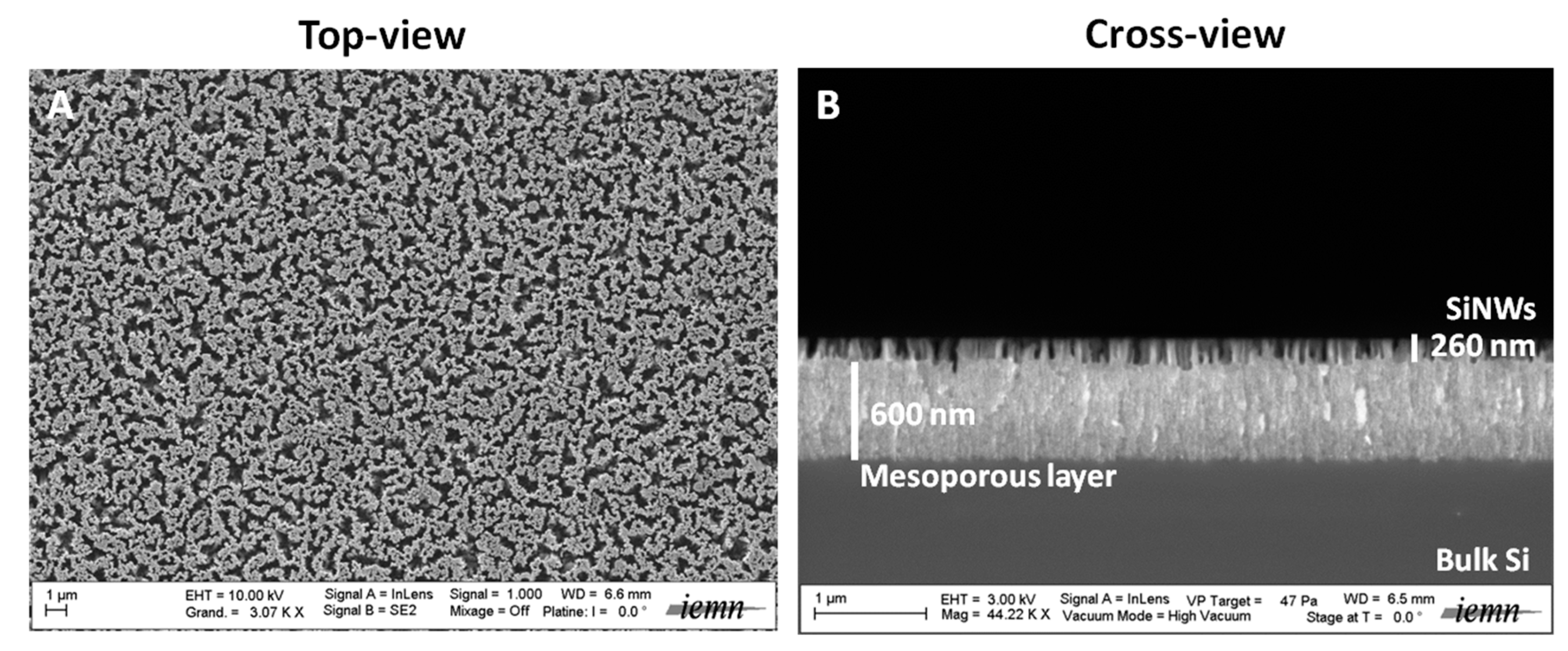
25]. This method displays several advantages over others, as it takes place at room temperature and does not require any specific and expensive equipment. This straightforward preparation technique allows the generation of nanostructured surfaces presenting reproducible structural properties. MACE is also cheap and easy to undertake and scale up. The morphology of the SiNWs studied here consisted of a mesoporous film (600 nm thick) surmounted by porous silicon nanostructures (260 nm high), as seen in the SEM images in
Figure 1 [
22]. These SiNWs had already been used for the realization of LDI of peptides and various other small compounds and their subsequent mass spectrometry detection [
22,
23,
26]. The LDI-MS mechanism involved on such silicon nanostructures is, in essence, based on the thermal mechanism [
27]. Indeed, the silicon nanostructures (band gap: 1.1 eV) will absorb photons from the UV laser (λ = 355 nm, 3.68 eV). Then, the energy is transferred to the deposited analytes for desorption and ionization, allowing their MS detection.
Here, we introduced Ti4+ ions on SiNWs through the deposition of O2 films for specific enrichment of phosphopeptides. To do so, two strategies were used. The first was the use of metallization (by thermal evaporation) of titanium films (5 and 10 nm) with atmospheric oxidation to form a TiO2 overlayer (called oxidized Ti-SiNWs). The second was the direct deposition of three different thicknesses of TiO2 (2 nm, 5 nm and 10 nm) on silicon nanostructures by atomic layer deposition (ALD).
Freshly prepared SiNWs and TiO
2-SiNWs were chemically modified by silanization using octadecyltrichlorosilane (OTS). After the reaction, all SiNWs presented superhydrophobic behavior, as already shown in previous studies [
24]. Then, photolithography was used to either metalize SiNWs on specific locations (oxidized Ti deposition) or to locally degrade the hydrophobic layer (OTS), thus creating specific hydrophilic apertures making TiO
2 layers accessible to interaction with phosphate groups. Wetting contrast allowed liquid droplet confinement, increasing the sensitivity of detection.
Figure 2 represents the two approaches used in this study to obtain accessible TiO
2 coatings on SiNWs.
To confirm the presence of TiO
2 on both surfaces, we performed energy dispersive X-ray spectroscopy (EDX) analyses on the obtained interfaces.
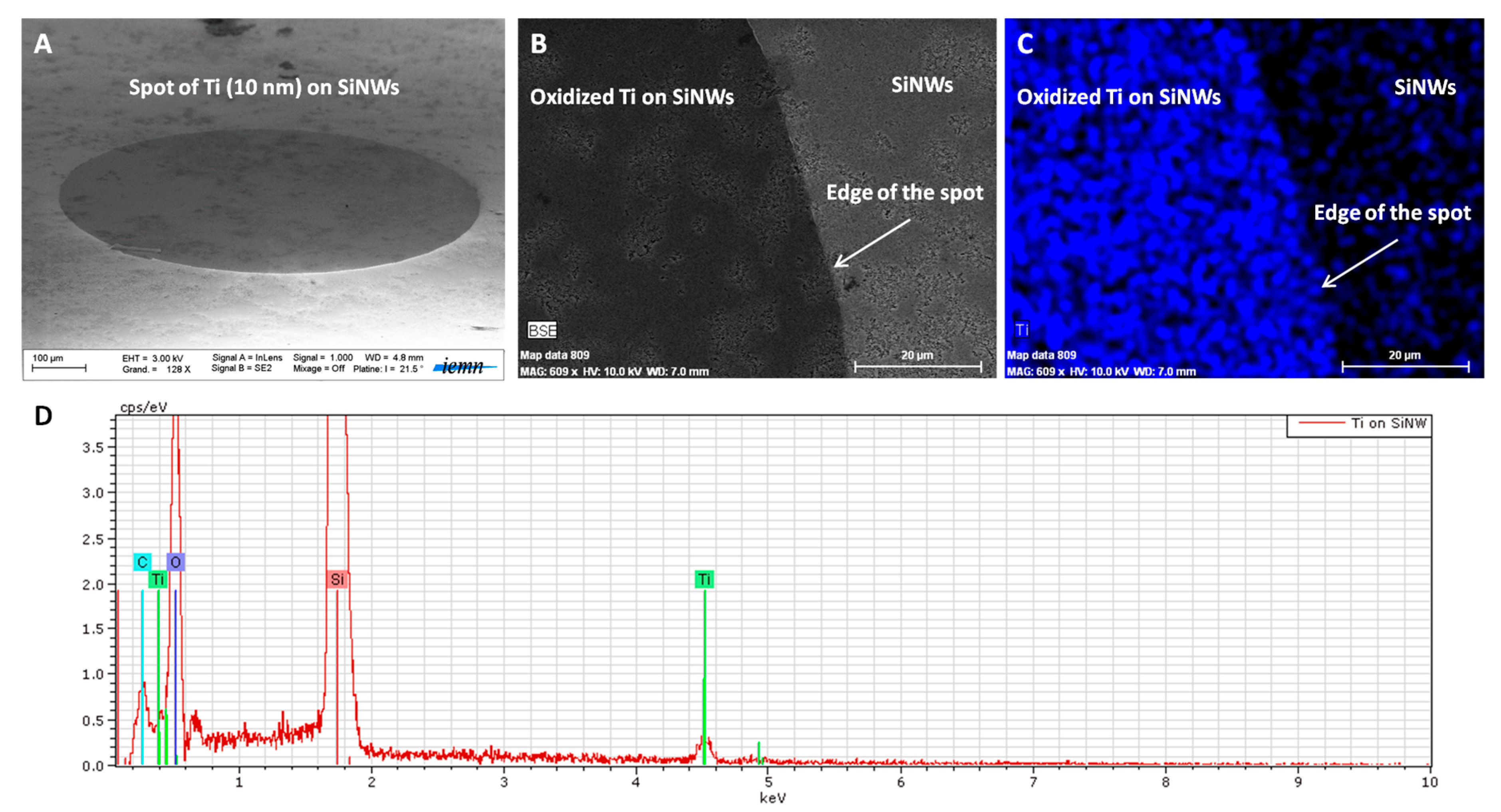
Figure 3 shows how EDX was performed on SiNWs presenting oxidized Ti spots (800 µm in diameter). On
Figure 3A, we can clearly identify the oxidized Ti spot on the SiNWs’ surface.
Figure 3B,C shows the SEM image of the edge of the oxidized Ti-SiNWs’ spot (dark area) and the corresponding EDX mapping, respectively. The blue color corresponds to the Ti element. The EDX spectrum, displayed in
Figure 3D, confirmed the presence of the Ti, O, and Si elements.
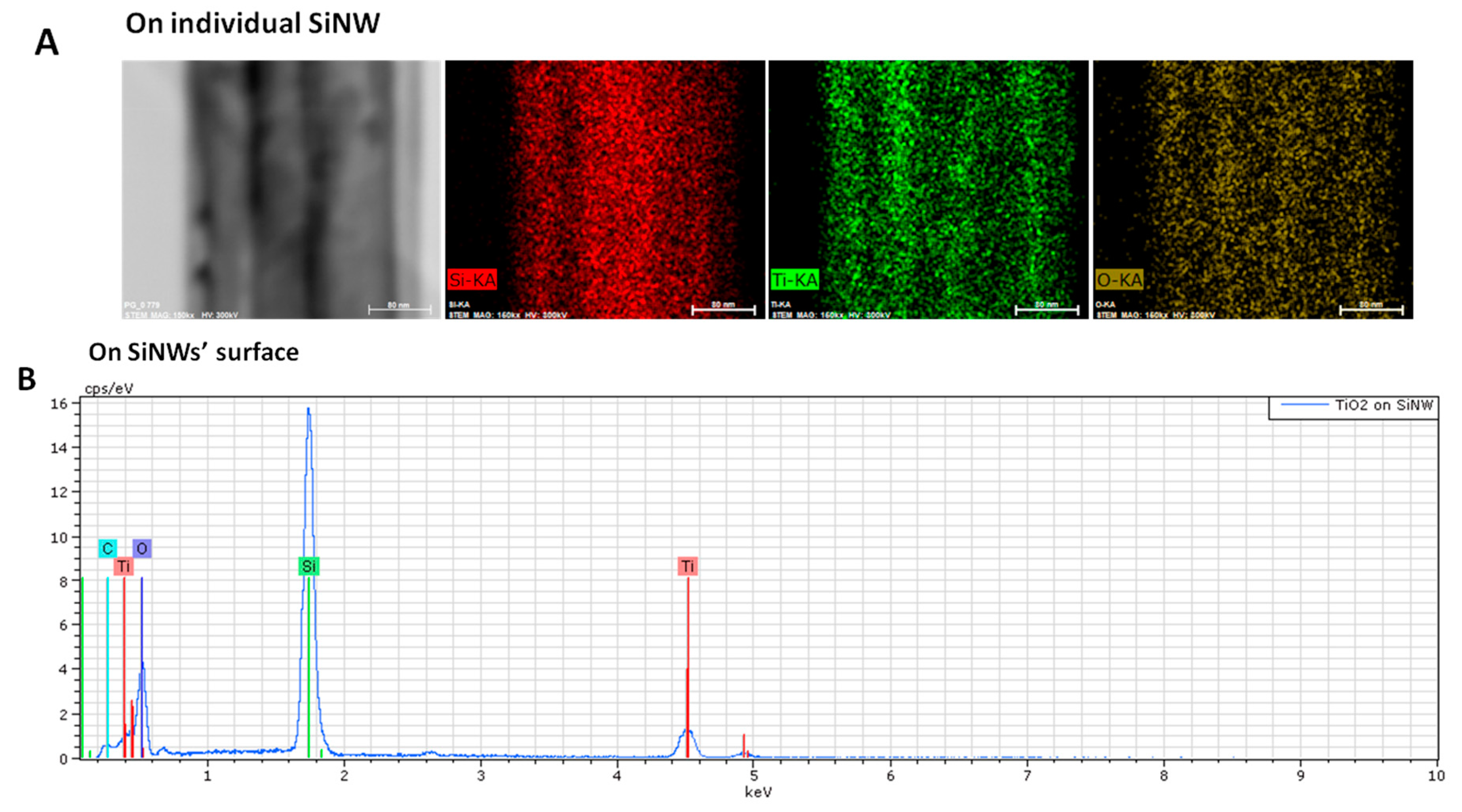
EDX analysis was also performed on TiO
2-SiNWs. On
Figure 4A, we can clearly see that an individual SiNW is uniformly covered by a TiO
2 layer, proving the conformal deposition of TiO
2 on SiNW obtained by ALD. EDX analysis on a larger scale showed the whole coverage of SiNWs by the TiO
2 layer (
Figure 4B). EDX mapping and analysis confirmed the presence of Ti and TiO
2 around the SiNWs.
In addition, and to better assess the chemical state of Ti, X-ray photoelectron spectroscopy (XPS) analyses were also conducted.
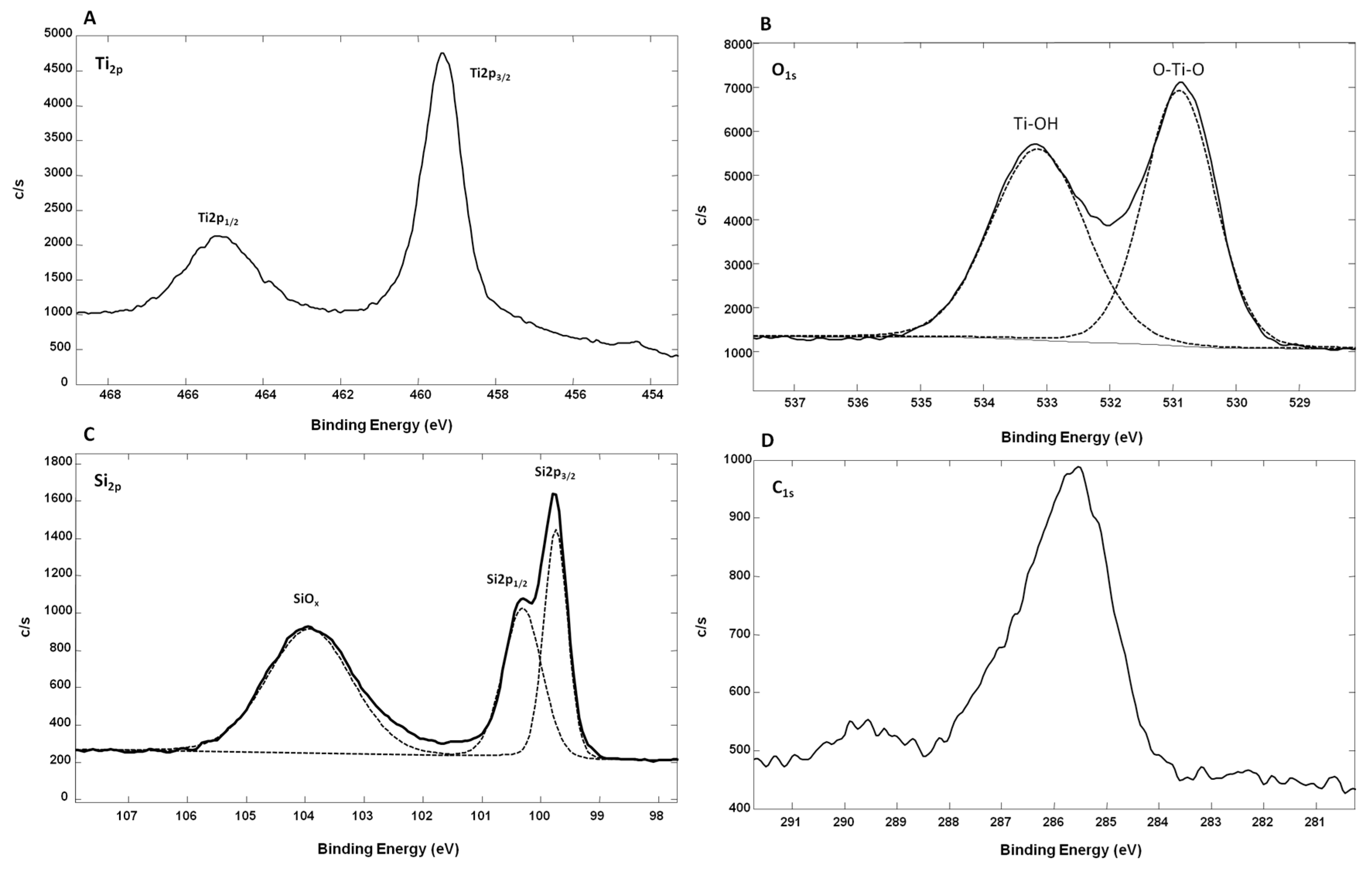
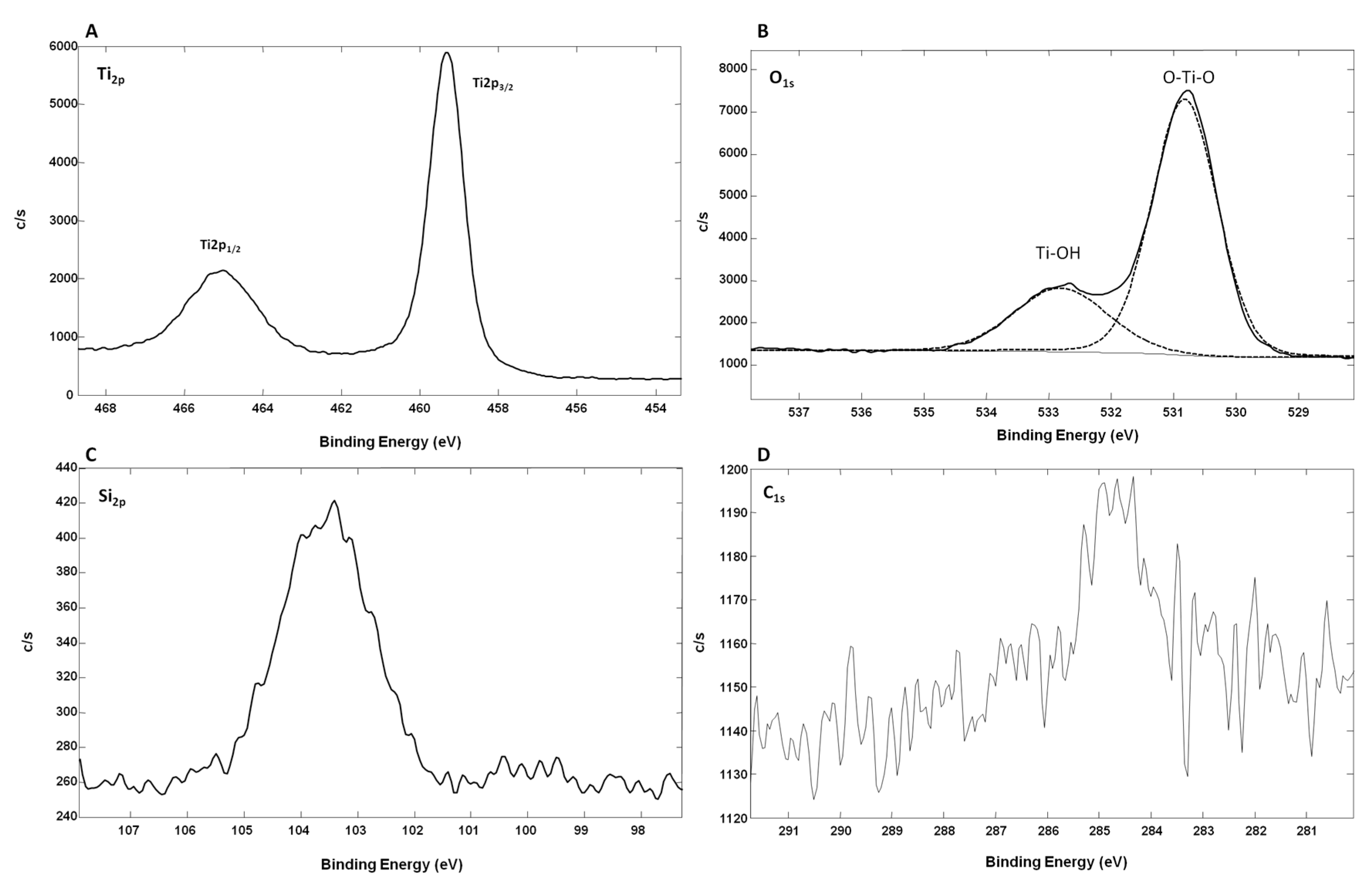
Figure 5 shows the XPS spectra from oxidized Ti-SiNWs. The Ti
2p peak has a significant split spin-orbit component (Δ
metal = 6.1 eV). It is known that the splitting value can vary as a function of the chemical state (e.g., Δ
oxide = 5.7 eV). In the high-resolution XPS spectrum of Ti
2p, we have two main peaks located at 459.34 eV (Ti
2p 3/2) and at 465.14 eV (Ti
2p 1/2) (
Figure 5A). In this case, the split spin-orbit value is 5.8 eV, thus confirming the oxidation of the Ti film. The Ti
2p regions were symmetrical, indicating that Ti
4+ was dominant on the surface [
28]. Also, the Ti to O ratio of oxidized Ti films was 0.22 compared to the standard TiO
2 stoichiometry, which presents a Ti/O ratio value of 0.5. This showed that many oxygen defects are present on the film surface. In addition, as we can see on the Si
2P high-resolution XPS spectrum, oxidized silicon may also contribute to O
1s signal intensity (
Figure 5B). We can also conclude that the observed TiO
2 is coming from partial oxidation of the Ti film deposited on SiNWs. Concerning the high-resolution spectrum of O
1s, this can be fitted with two peaks. The peak at 530.90 eV corresponds to the lattice O (O–Ti–O), while the second peak at 533.14 eV was from the surface hydroxyl groups (Ti–OH) [
29]. The peak corresponding to lattice O showed that Ti
4+ was dominant in oxidized Ti film. In
Figure 5D, we can see the C
1s contribution (8.7 atm. %), probably due to surface contamination by non-oxidized and oxidized carbon species with two peaks at 285.6 eV and 289.73 eV, respectively.
Figure 6 shows the high-resolution XPS spectra of TiO
2-SiNWs. As for oxidized Ti-SiNWs, the high-resolution XPS spectrum of Ti
2p showed two main peaks located at 459.29 eV (Ti
2p 3/2) and at 465.16 eV (Ti
2p 1/2) (
Figure 6A). Both the split spin-orbit value of 5.87 eV and the symmetrical Ti
2p regions showed that Ti
4+ was also dominant on the surface, as already observed for oxidized Ti-SiNWs. However, the Ti to O ratio of TiO
2 film was slightly higher, with a value of 0.31 compared to the standard TiO
2 stoichiometry (Ti/O = 0.5), which correlates well with the deposition of TiO
2 materials. Oxygen defects were still present, but with less influence of Ti–OH groups and SiO
x, as we can see on
Figure 6B,C. Concerning the high-resolution spectrum of O
1s, this can also be fitted with two peaks (
Figure 6B). The peak at 530.82 eV corresponds to the lattice O (O–Ti–O), while the second (less intense than for oxidized Ti-SiNWs) at 532.82 eV was from the surface hydroxyl groups (Ti–OH). The peak corresponding to lattice O showed that Ti
4+ was dominant in oxidized Ti film. On
Figure 6C, the Si
2P high-resolution XPS spectrum shows much lower signal intensity than for that obtained from oxidized Ti-SiNW (2.26 atm. % vs. 19.7 atm. %). This is probably due to the better surface coverage of SiNWs achieved by the ALD method. Finally, in
Figure 6D we can also see the C
1s contribution, probably coming from surface contamination by non-oxidized and oxidized carbon species, with two peaks at 285.48 eV and 289.6 eV, respectively.
From EDX and XPS analyses, we have demonstrated that both deposition methods led to the coating of SiNWs by TiO
2 layers. In the case of the TiO
2-SiNW surface, we have demonstrated that ALD provides a high quality of conformal film deposition with lower amounts of hydroxyl groups present on the surface. The anatase phase of TiO
2, which has been already characterized by XRD analysis in one of our recent studies should be noted [
30]. Concerning the thermal evaporation of metallic titanium film, its exposure to ambient air at room temperature led to the spontaneous formation of a passive oxide film. It is known that this film is amorphous and composed of three layers (TiO, Ti
2O
3 and TiO
2) with an overall thickness ranging from 5 nm to 10 nm. Ti
2O
3 is the intermediate layer, whereas the layer in contact with the environment is the anatase TiO
2 layer [
31].
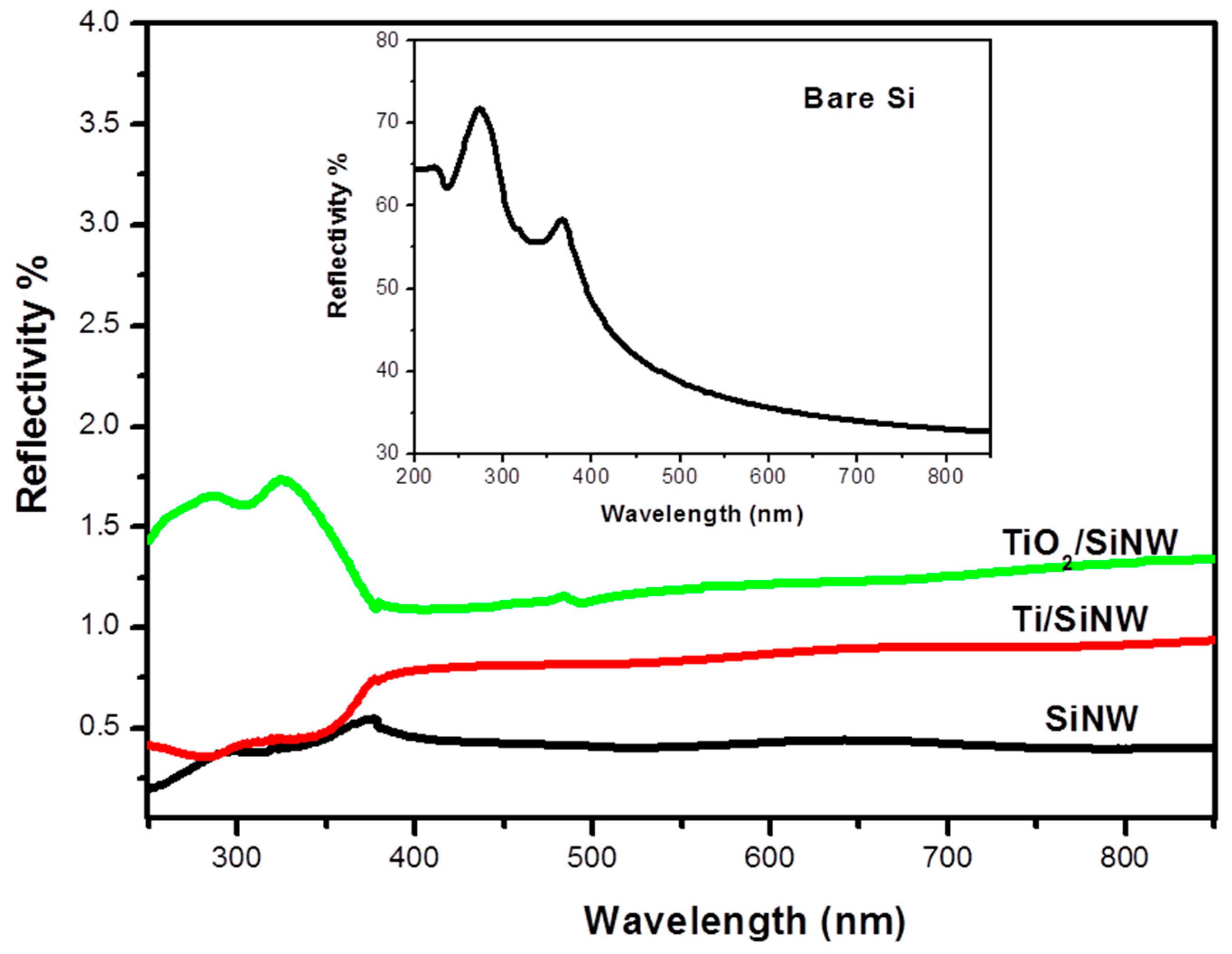
The optical properties of our interfaces were also assessed. To do so, we performed reflectivity measurements of oxidized Ti-SiNWs, TiO
2-SiNWs and SiNWs surfaces. All surfaces exhibited anti-reflective properties at λ = 355 nm (mass spectrometer UV laser wavelength) compared to bulk silicon (
Figure 7 and insert).
However, the SiNWs sample exhibited the lowest reflectance in the whole range (250–850 nm), with a value attaining 0.5% of reflectivity, as already shown in our previous work [
22]. After Ti thermal evaporation and air oxidation, reflectivity was below 0.5% until 380 nm, and then increased slightly to 0.75% from 380 nm to 850 nm. By contrast, with TiO
2 deposition by ALD (10 nm), a slight increase of the reflectivity was observed, with 1.5% of reflectivity in the spectral range 250–400 nm and 1.2% of reflectivity from 400 nm to 800 nm. Such anti-reflective surfaces will provide higher photon absorption during the LDI process.
MOAC is one of the most widely used techniques for phosphopeptide enrichment while limiting non-specific interactions. Titanium oxide (TiO
2) has amphoteric properties, which means that it can behave as either a Lewis acid or a Lewis base as a function of the pH of its environment. Under acidic condition, TiO
2 acts as a Lewis acid with positive charges that can display an ion-exchange feature [
32]. The mechanism of interaction is believed to be based on both ion exchange and Lewis acid/base interactions, leading to high specificity [
33]. However, as for the IMAC method, it is true that acidic residues can also bind TiO
2 with high affinity, thereby capturing acidic peptides and phosphopeptides as well. Nevertheless, the addition of strong organic acids (like trifluoroacetic acid (TFA)) to the interaction buffer, minimizes the binding of carboxyl groups (from Aspartic acid or Glutamic acid for instance) to TiO
2 and thus can prevent non-specific interactions resulting in greater specificity for phosphopeptides [
17,
34]. Indeed, at pH 2, acidic amino acid residues such as Aspartic acid (D) or Glutamic acid (E) will be protonated (E pKa ≈ 4.3 and D pKa ≈ 3.7) and no interactions could happen. By contrast, phosphate groups have two ionization steps. The first ionization is around pH ≈ 1.5 and the second around pH ≈ 6.3, meaning that at pH ≈ 2 phosphate groups have negative charge and can interact with the positively charged TiO
2 surface. The ability of our interfaces to selectively capture phosphopeptides was then investigated. To do so, a phosphopeptide mixture composed of both non-phosphorylated and phophosrylated peptides was used in acetonitrile/water/TFA solution (50/49.9/0.1
v/
v/
v). Trifluoroacetic acid (TFA, 0.1%
v/
v, pH ≈ 2) was supplemented in the loading buffer to reduce non-specific interactions [
17]. This mixture contained: Angiotensin II (1046.54
m/
z, DRVYIHPF); Angiotensin I (1296.68
m/
z, DRVYIHPFHL); Myelin basic protein, fragment 104–118 (1578.85
m/
z, GKGRGLSLSRFSWGA); pTpY peptide (MAP kinase fragment 177–189) (1669.67
m/
z, DHTGFL
pTE
pYVATR); pY peptide (insulin receptor, fragment 1142–1153) (1702.75
m/
z, TRDI
pYETDYYRK); pT peptide (1720.89
m/
z, VPIPGRFDRRV
pTVE); pS peptide (PKA RII peptide, fragment 81–99) (2192.08
m/
z, DLDVPIPGRFDRRV
pSVAAE) (see
Table 1).
The concentration varied from 400 fmol/µL to 4 fmol/µL. Then, different SiNWs with TiO
2 or oxidized Ti films of different thicknesses were assessed. Thicknesses of 2 nm, 5 nm and 10 nm and 5 nm and 10 nm for TiO
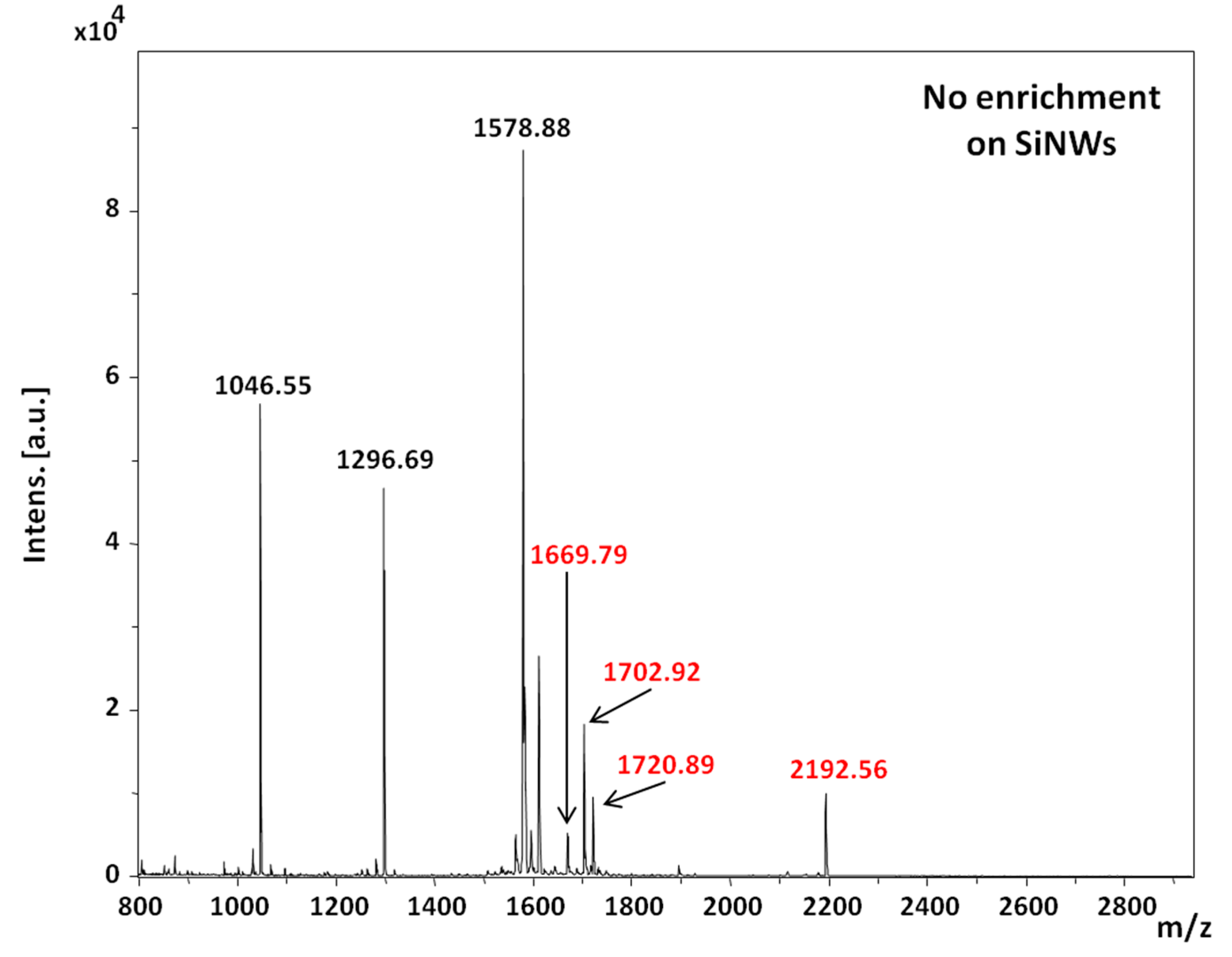
2 films deposited by ALD and oxidized Ti deposited by thermal evaporation followed by air oxidation, respectively, were used. When 1 µL was allowed to dry on all surfaces, no enrichment was obviously observed, as can be seen in
Figure 8, where all the seven peptides were detected on the SiNWs surface.
Table 2 summarizes the enrichment results obtained from the phosphopeptide mixture (400 fmol/µL) on different surfaces. As can be seen, the best enrichment was obtained for the oxidized Ti-SiNWs’ surface with 10 nm of Ti, whereas the 10 nm TiO
2-SiNWs’ surface was only able to detect three phosphopeptides. We can attempt to explain this by the fact that it is well known that the properties of TiO
2 films (optical, thermal, electrical etc.) strongly depend on the conditions of the mode of film deposition, which leads to different surface behavior and performance [
35,
36]. In our case, the air oxidation of the metallic Ti layer induced an increased amount of hydroxyl groups compared to TiO
2 deposited by ALD (
Figure 5B and
Figure 6B). However, at this stage, no assumption concerning the influence of hydroxyl groups on the phosphopeptide enrichment can be drawn. Other systematic studies (especially on the material itself) are needed to confirm this hypothesis.
From
Table 2, we can also notice that the silicon nanowires covered by 2 nm of TiO
2 (ALD) were not able to detect any phosphorylated peptide, whereas 5 nm of TiO
2 allowed the detection of two phosphopeptides. This difference could be due to incomplete coverage of the SiNWs by TiO
2. Indeed, even if the ALD deposition method allows a conformal coverage on well-defined structures such as on a periodic network of nanowires through lithography and dry etching, this is not the case in our study. In fact, since our surface morphology resembles strongly packed nanowires, we can suppose that, for low thicknesses, the overall nanowires are not totally covered by TiO
2 film, thus explaining the lower performances. The same conclusion can be drawn when the enrichment performances for 5 nm and 10 nm of oxidized Ti films are compared.
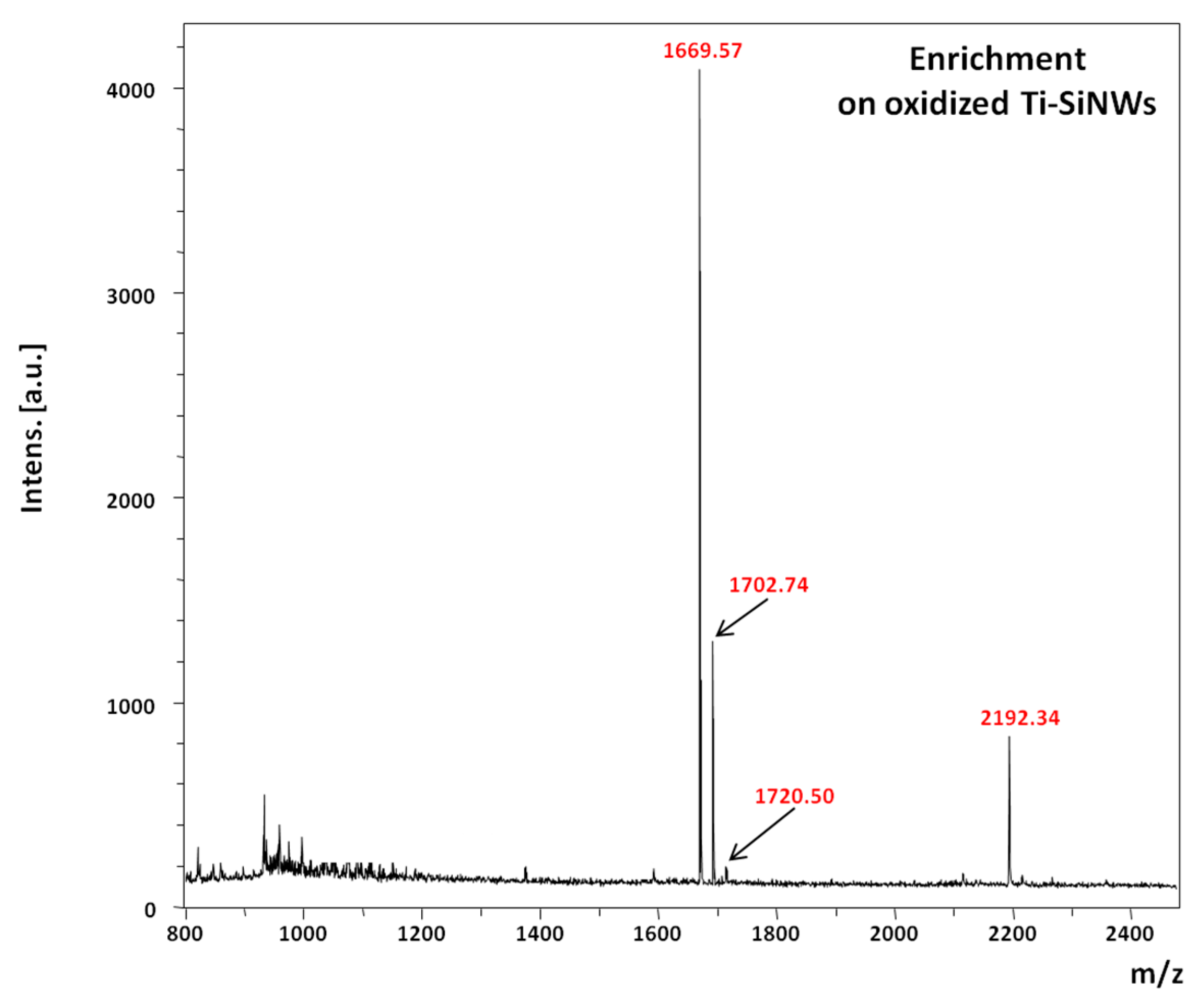
Figure 9 shows the MS spectrum of phosphopeptide enrichment performed on oxidized Ti-SiNWs from 400 fmol/μL solution and after 10 min of incubation, rinsing and drying prior to LDI-MS. It should be noted that increasing the incubation time had no effect on the improvement of the detection sensitivity.
Despite the fact that the reproducibility and repeatability of SiNWs (without a Ti coating) was already proven, the same experiments have been performed on oxidized Ti-SiNWs as well [
22,
23]. All experiments were repeated at least three times to ensure satisfactory spectral reproducibilities (inter-spot, intra-spot and surface-to-surface variations). Good reproducibilities with a relative standard deviation (RSD) below 15% were obtained, except for the pT peptide (1720.86
m/
z) for which RSD was much higher (RSD ≈ 40%). This is probably due to weaker interactions of the pT peptide with the interface because of its basic behavior (isolectric point ≈ 10.56). To assess the sensitivity of detection, a dilution of peptide mixture was performed to obtain a concentration of 40 and 4 fmol/µL. In both cases, oxidized Ti-SiNWs (10 nm) gave the best enrichment properties, i.e., the four phosphorylated peptides were detected. Nevertheless, some attempts were made at concentrations below 4 fmol/mL with modest success and poor reliability, meaning that below this concentration no satisfactory results can be reached.
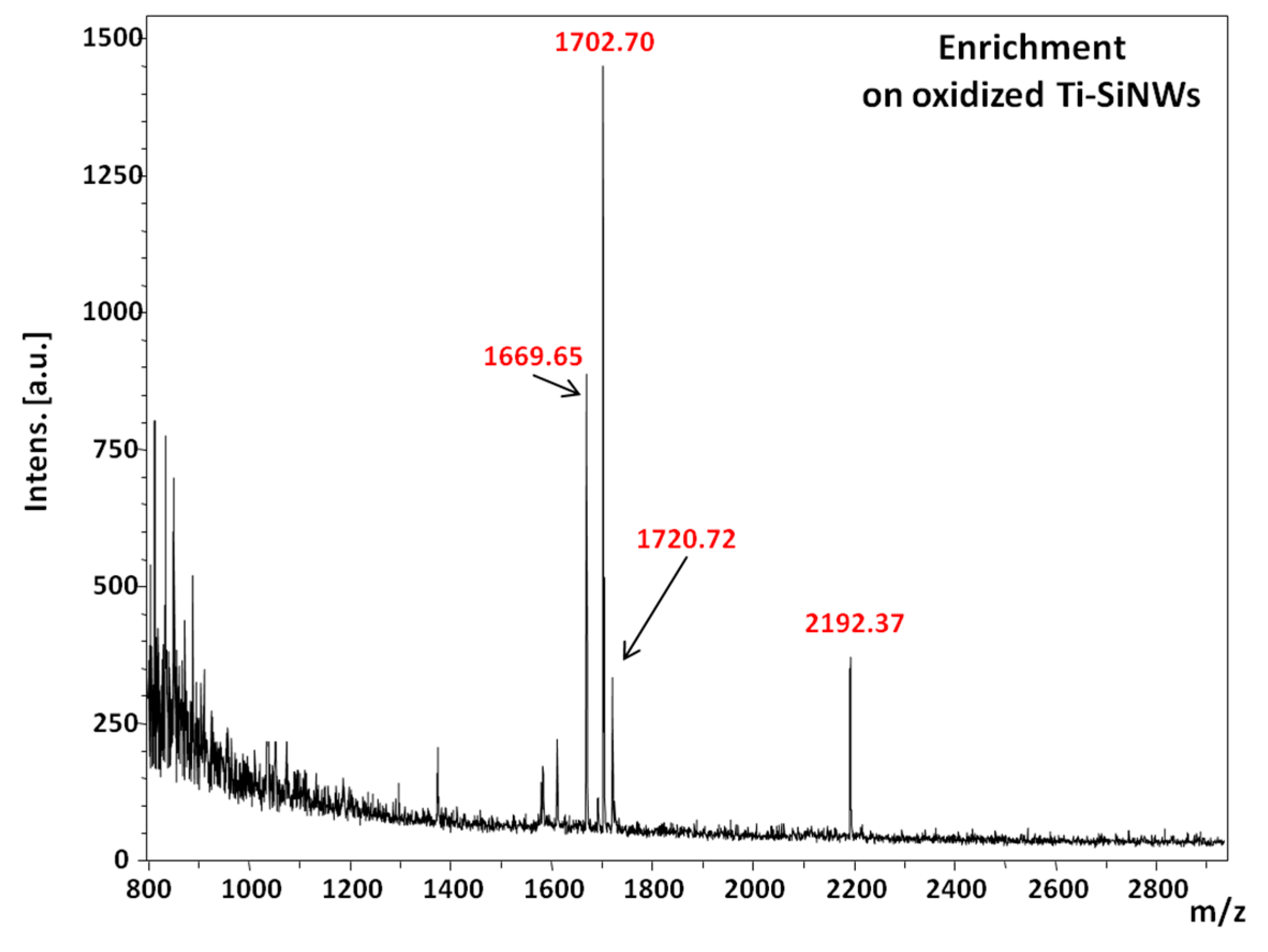
Finally, the performance of the oxidized Ti-SiNWs (10 nm) interface was examined in a more complex biological sample. To do so, we used a solution of serum composed of 50% fetal bovine serum (FBS) in phosphate buffer saline (PBS) (
v/
v). Then, a spike of phosphopeptide mixture (to reach 400 fmol/µL), without any further treatment, was made. 3 µL of the resulting mixture was incubated on the oxidized Ti-SiNWs’ surface for 10 min, followed by appropriate rinsing and drying as already described above.
Figure 10 shows the MS spectrum of the phosphopeptide mixture spiked in FBS solution. As we can see, only peaks related to [M + H]
+ ions of phosphorylated peptides were detected at 1669.65
m/
z, 1702.70
m/
z, 1720.72
m/
z and 2192.37
m/
z.