1. Introduction
A linear polymerization reaction between two co-monomers A and B is ideal if its polymer molecules could be strictly expressed as B-[A-B]
n-A while the “
n”, degree of polymerization, approaches infinity. Nevertheless, besides remaining in the reaction solution, the excessive one within the two co-monomers can also bond at both ends of the structure, and thereby serve as an inhibitor to adjust the polymerization degree, or even as a functional group located at both ends. From the perspective of geometry, if a copolymer molecule regularly grows up along one-, two-, or three-dimensional directions, it is possible for the involved excess co-monomer to be bonded at its two ends, outside its planar contour or on its spherical surface. Furthermore, some complicated structures may appear in the copolymer product if it precipitates out of the reaction solution. This type of precipitation is not only pushed forward by its main polymerization reaction, but also by some of the cross-linking or/and crystallization processes, e.g., lamellar crystallization caused by hydrogen bonding among different linear molecules [
1,
2,
3,
4], which are always variable with the reaction time or the molar ratio between its two co-monomers. Therefore, it should be very important to use these copolymerization reaction conditions to modify the polymer precipitates so as to obtain some optimized functional materials.
Melamine-formaldehyde resin (MF) from the polycondensation reaction is well-known due to its wide application in the wood and papermaking industries, as well as dinnerware manufacturing [
5,
6]. Wherein, the MF microspheres can precipitate out of the reaction solution under some special conditions [
7,
8]. MFMSs were introduced into the SERS substrate for the trace analysis of tetramethylthiuram disulfide, uric acid, and other analytes [
9,
10,
11], in which the MFMSs were synthesized by successive catalysis of alkali and acid at a constant formaldehyde/melamine (F:M) molar ratio and the deposition of AgNPs on MF microspheres was carried out by reduction of AgNO
3 using butylamine as the reducing agent. In addition to the complexity of the procedure, what role the F:M molar ratio played in the MFMS synthesis and, furthermore, in the performance of SERS substrates is still unknown.
SERS hot spots between metal nanoparticles can be created by some bottom-up methods [
12,
13], which were shown to be a successful strategy by controlling the distance between the metal nanoparticles using some linkers, e.g., hexamethylenediamine [
14], 1,4-benzenedithiol [
15], and some special copolymers [
16,
17,
18]. This should be further evidence for the SERS hot spots that SERS enhancement on the silver nanoparticles increases with the number of their aggregate-dimensions controlled by some organic molecules increasing from one to two and then to three [
19]. Introducing polymeric carriers into the SERS substrate to enhance the detection sensitivity has become a common practice [
9,
10,
11,
20,
21,
22,
23]. This method depends on the existence of numerous functional groups within the polymer, which are available to the three-dimensional aggregation of the metal nanoparticles. However, it is troublesome that the variable synthetic conditions frequently result in some different characteristics to the polymers, especially to some copolymers [
9,
10,
11,
20,
22,
24], thus, further influencing the properties of the final SERS substrate [
9,
10,
11,
20,
22]. Obviously, how to classify or optimize the polymer involved in the fabrication of SERS substrates is vital.
In our last paper, the types of urea-formaldehyde resin microspheres were investigated where the role of excess monomer in the synthetic process was highlighted [
24]. Indeed, it is a common phenomenon that the copolymer resin will present at least two types of characteristics, which correspond to its modification with the excess one of the two co-monomers. In this paper, MFMSs were synthesized to simply load AgNPs from the colloidal solution for the SERS detection. The excess monomer bonded on the MFMSs was found further playing a crucial role in their SERS substrate, so as to impact on the final SERS detection. The SERS substrate optimized with the excess formaldehyde can be used for the trace analyses of a set of organic pollutant molecules containing mercapto, thiocarbonyl, or quaternary amino groups.
2. Results and Discussion
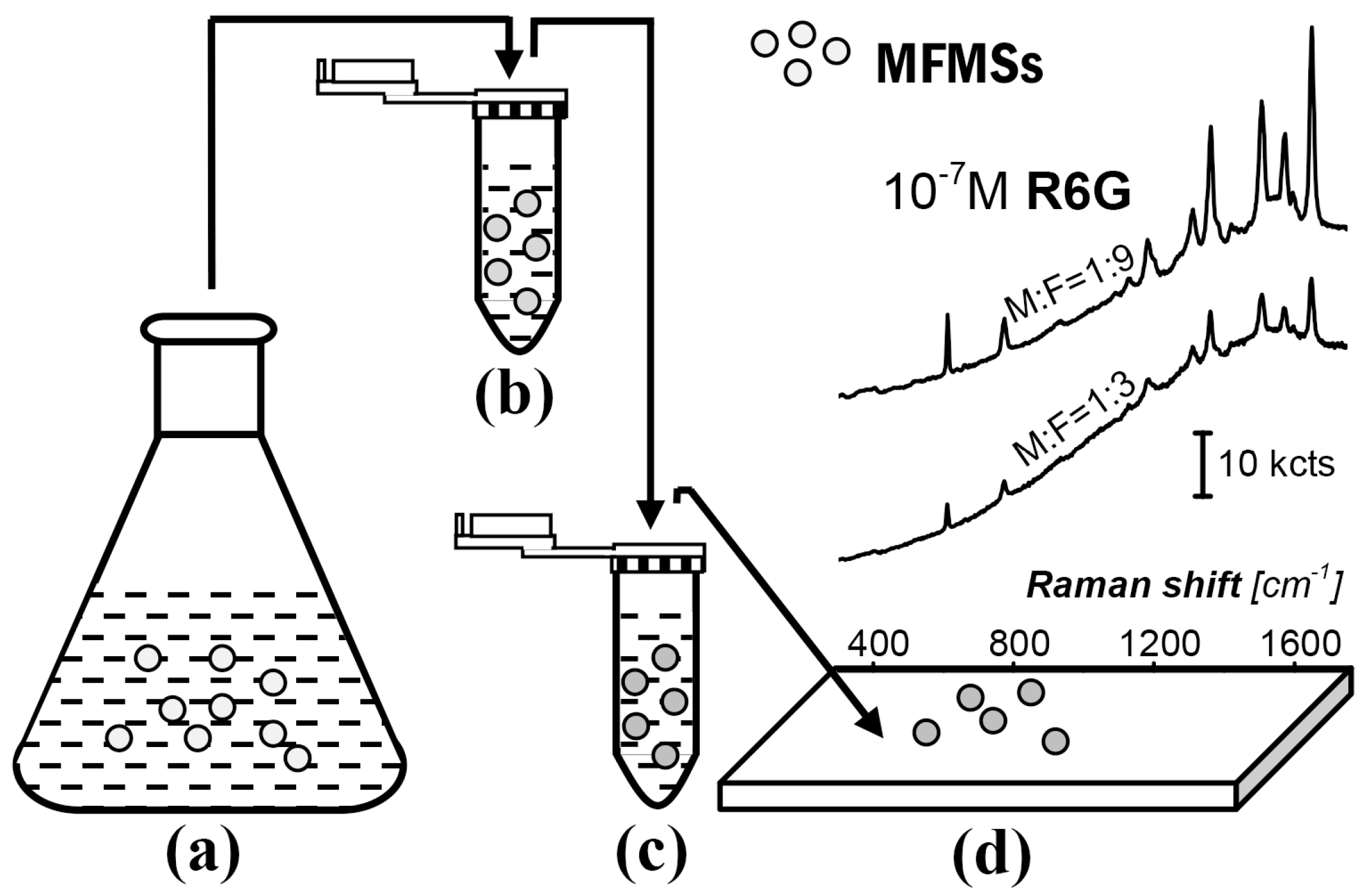
A simple route for the fabrication and use of the SERS substrate, AgNP/MFMSs, is provided in
Figure 1. MFMSs were firstly synthesized with melamine and formaldehyde by catalysis of acetic acid (
Figure 1a). They were transferred into a silver colloidal solution to adsorb AgNPs (
Figure 1b). The AgNP/MFMSs were then transferred into R6G solution to adsorb R6G (
Figure 1c). Finally the incubated AgNP/MFMSs sample was put on a glass slide to interrogate the R6G (
Figure 1d).
AgNPs were loaded on MFMSs within a 2 mL centrifuge tube, followed by an incubation step in a 10
−7 M R6G solution. The colloidal solution was firstly centrifuged at 2000 rpm before use to remove the large AgNPs within itself. The synthesis of the MFMSs was implemented at pH 4.0 and 65 °C with a constant amount of melamine, and either the reaction time or the initial formaldehyde amount was preset as a variable, which further incorporated into the AgNP/MFMSs samples, to investigate their influence on the final SERS performance. Size of the AgNPs was ~120 nm and that of the MFMSs varied from 12.4 ± 2.21 to 5.8 ± 0.47 µm with the F:M molar ratio increasing from 1:1 to 9:1 (see
Table S1, which provided in the
Supplementary Material). The zeta potentials of MF
1 and MF
7 were + 18.7 mV and + 36.6 mV, respectively. After an ultrasonic oscillation step in AgNP colloidal solution for 5 min, the MFMSs were allowed to remain in the solution for 1 h to adsorb the AgNPs, and then collected at a centrifugation rate of 2000 rpm. It was verified that the AgNPs on the MFMSs has an fcc structure, as shown in the x-ray diffraction (XRD) spectra in
Figure S1.
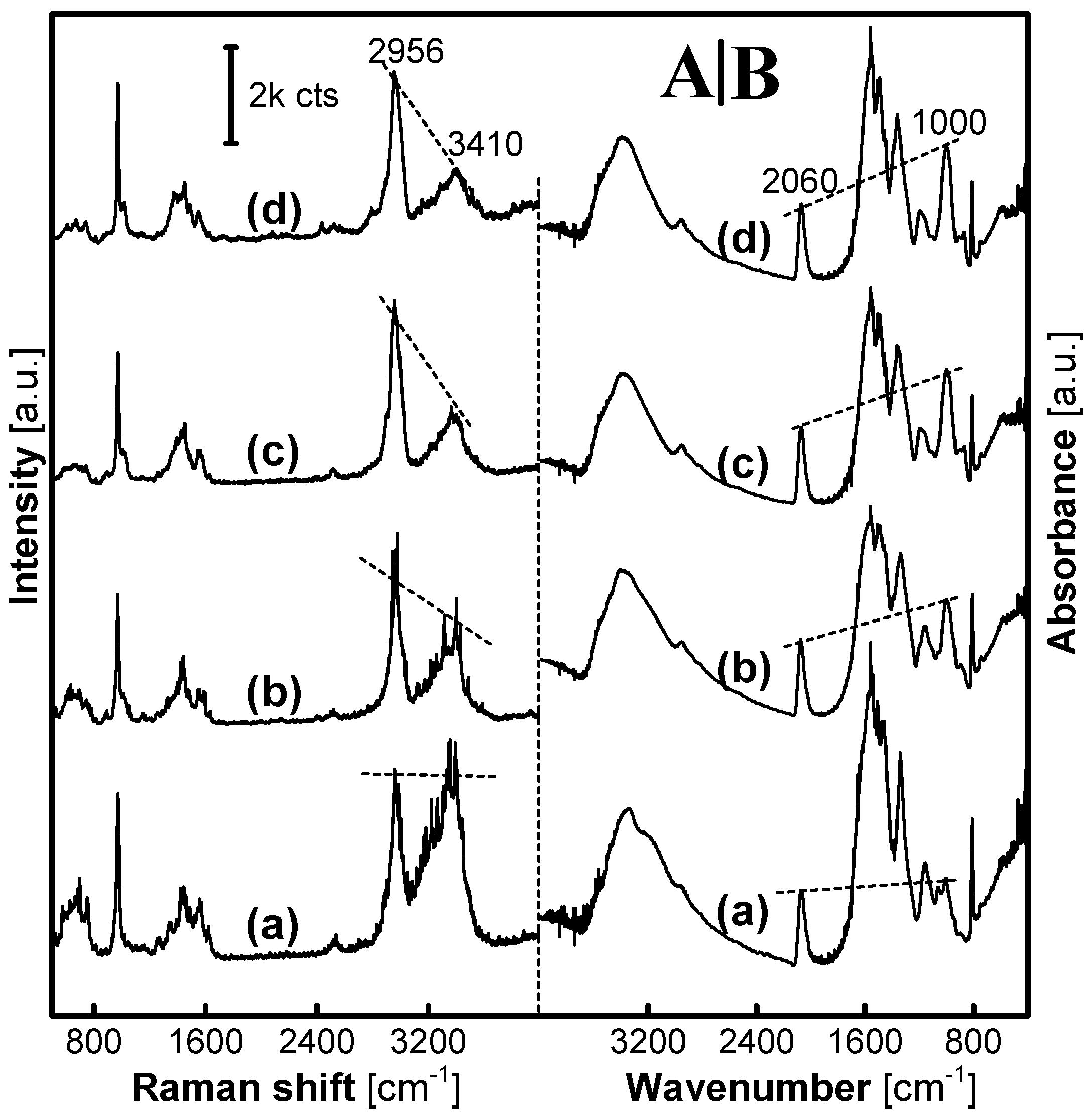
Figure 2 gives Raman and Infrared (IR) spectra of a set of MFMS samples. With the F:M molar ratio increased from 1:1 to 9:1, a Raman scattering peak at 2900 cm
−1 that could be assigned to the stretching vibration of C–H in the groups –CH
2– or/and –CH
2OH was enhanced along with a gradual decline of another one around 3400 cm
−1, which was attributed to the vibration of N–H and/or O–H in the –NH–, –NH
2 and/or –CH
2OH, as shown in
Figure 2A. IR spectra, as shown in
Figure 2B, used Pb(SCN)
2 (50 wt%) as an internal standard with its characteristic peak at 2060 cm
−1 to evaluate the change of the peak intensity at 1000 cm
−1. The adsorption peak at 1000 cm
−1 was assigned to the C–O vibration that was associated with the existence of –NHCH
2OH groups in the MFMSs. Herein, with the F:M molar ratio increasing from 1:1 to 9:1, the intensity of the peak at 1000 cm
−1 increased. Therefore, it is an important conclusion from the results of Raman and IR analyses that the number of the –NHCH
2OH groups in the MFMSs increases with the F:M molar ratio increasing from 1:1 to 9:1 in the synthesis of MFMSs. Herein, it is also possible for X-ray diffraction and particle size, as well as the yield of resin materials to change gradually, e.g., as reported in the synthesis of urea-formaldehyde resin microspheres [
24,
25].
These trends in the structure of the MFMSs are further transferred into a gradually-increasing SERS performance from R6G loaded on the AgNPs. As presented from
Figure 3a–d, the intensity of SERS signals from R6G on the loaded AgNPs were strikingly enhanced with the F:M molar ratio increasing from 1:1 to 9:1, while the Raman signals of the MFMSs themselves were faint, and almost only an intense fluorescence background was observed on the AgNP/MF
1MSs (see
Figure 3a). Only one percent of the 20 mW laser power was adopted in the detection of SERS spectra, reflecting the superiority of SERS analysis on the AgNP/MFMSs (herein “AgNP” and “MF” in the expression AgNP/MF
aMSs denote AgNPs and MFMSs, respectively, and the subscript “a” is the molar ratio between formaldehyde and melamine preset in the MF polycondensation reaction).
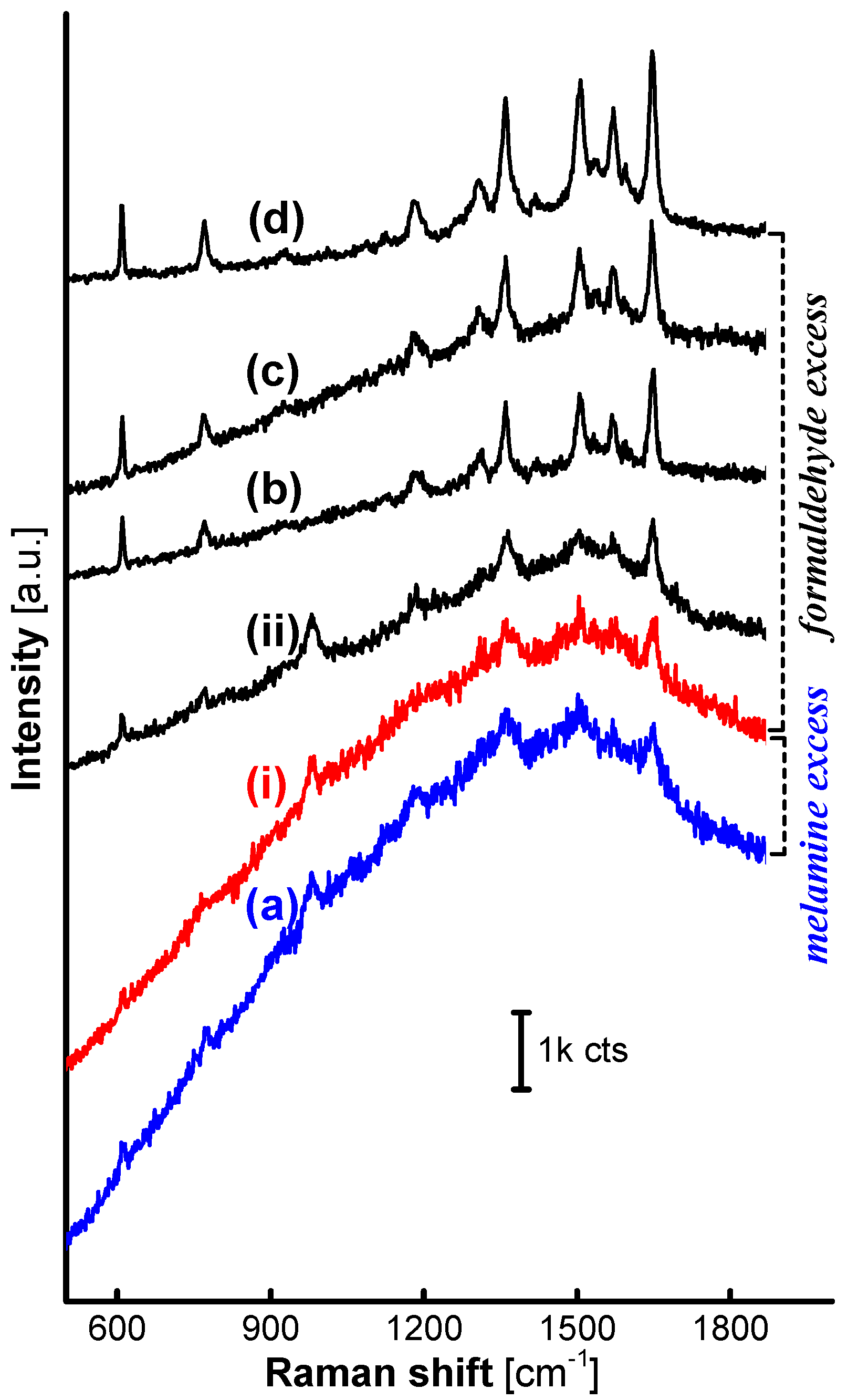
Obviously, there must be a specific value of the F:M molar ratio between 1:1 and 3:1 that can demarcate the characteristics of the MFMS surfaces and thereby divide the loaded AgNPs into active and inactive ones based on the SERS performance. Two MFMS samples were further synthesized at the F:M molar ratio of 1.5:1 (MF
1.5) and 2:1 (MF
2), respectively, to evaluate their SERS performance. SERS signals originating from R6G on the AgNP/MF
2MSs (ii) emerged clearly, though there existed an intense fluorescence background in each one of the spectra collected on AgNP/MF
2MSs (ii), AgNP/MF
1.5MSs (i) and AgNP/MF
1MSs (a) as shown in
Figure 3. Although the exact structure of the MF
1.5 is still waiting for verification, it is evident from the SERS effectiveness that the initial F:M molar ratio of 1.5:1 in the MFMS synthesis reaction is a valuable reference, on which it is reasonable for the surface characteristics of the MFMSs to be demarcated by the excess formaldehyde [
24] or melamine.
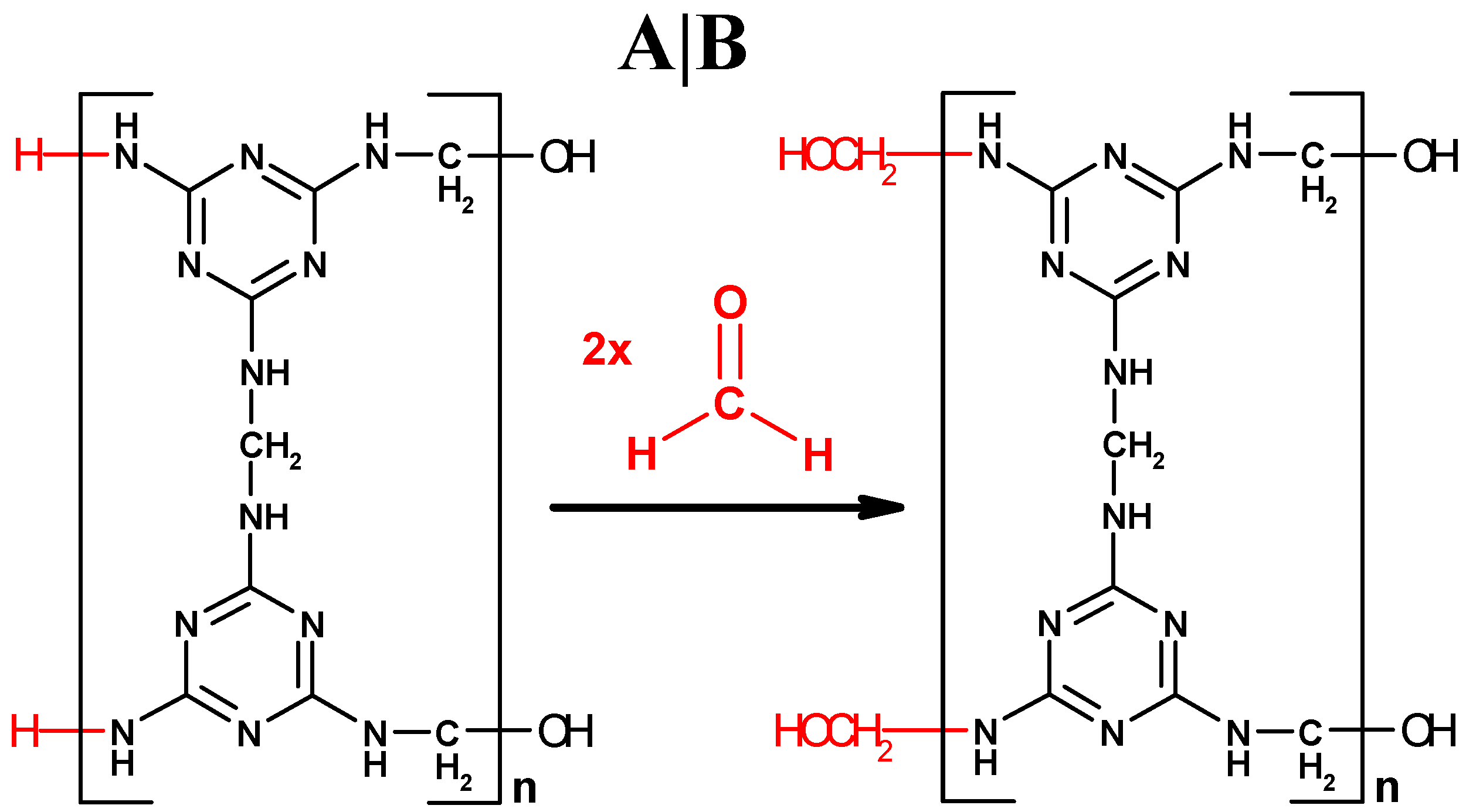
Assuming that the MF polycondensation reaction strictly complies with the F:M molar ratio of 1.5:1, an ideal structure in linear molecular form is argued as
Figure 4A, based on its ideal polymerized unit C
9N
12H
12 within
Figure 4A. In the structure
Figure 4A, all the –NH
2 groups from the reactant melamine are changed into –NHCH
2– after the polycondensation reaction, except the two remaining at the left end of the resin molecule.
We would like to emphasize the “overreaction” between the –NH
2 groups in the MF resin molecule and the excess formaldehyde remaining in the reaction solution. If the MF polycondensation reaction occurs in the solution with more amount of formaldehyde than it needs, the formaldehyde will excessively bond at the ends of MF resin molecules after the melamine in the reaction solution is exhausted. The two –NH
2 groups remained at the left end of the linear molecular structure will be further consumed by their overreaction with the excess formaldehyde, as shown from (A) to (B) in
Figure 4. This process is similar if a resin molecular structure grows in two- or three-dimensions, where some of the excess monomer in the reaction solution will be bonded at more or all terminal sites as the polycondensation reaction is over. The terminal overreaction between the –NH
2 groups in a two-dimensional MF resin molecule and the excess formaldehyde remaining in the reaction solution is further illustrated in
Figure S2.
Although a MFMS is not a single resin macromolecule grown in three-dimensions, the “terminal overreaction” could obviously be used to understand its growth process. The MFMS will have to be exposed in the polycondensation mother liquor and it is thereby possible for the excessive reactant in the solution to be bonded enough on the surface of it. When the F:M molar ratio is more than its ideal F:M molar ratio of 1.5:1, the surface of the MFMSs with bonded –NH2 groups will finally be transferred into the one that was enriched with –NHCH2OH, because there is excessive formaldehyde in the reaction solution throughout the synthesis reaction.
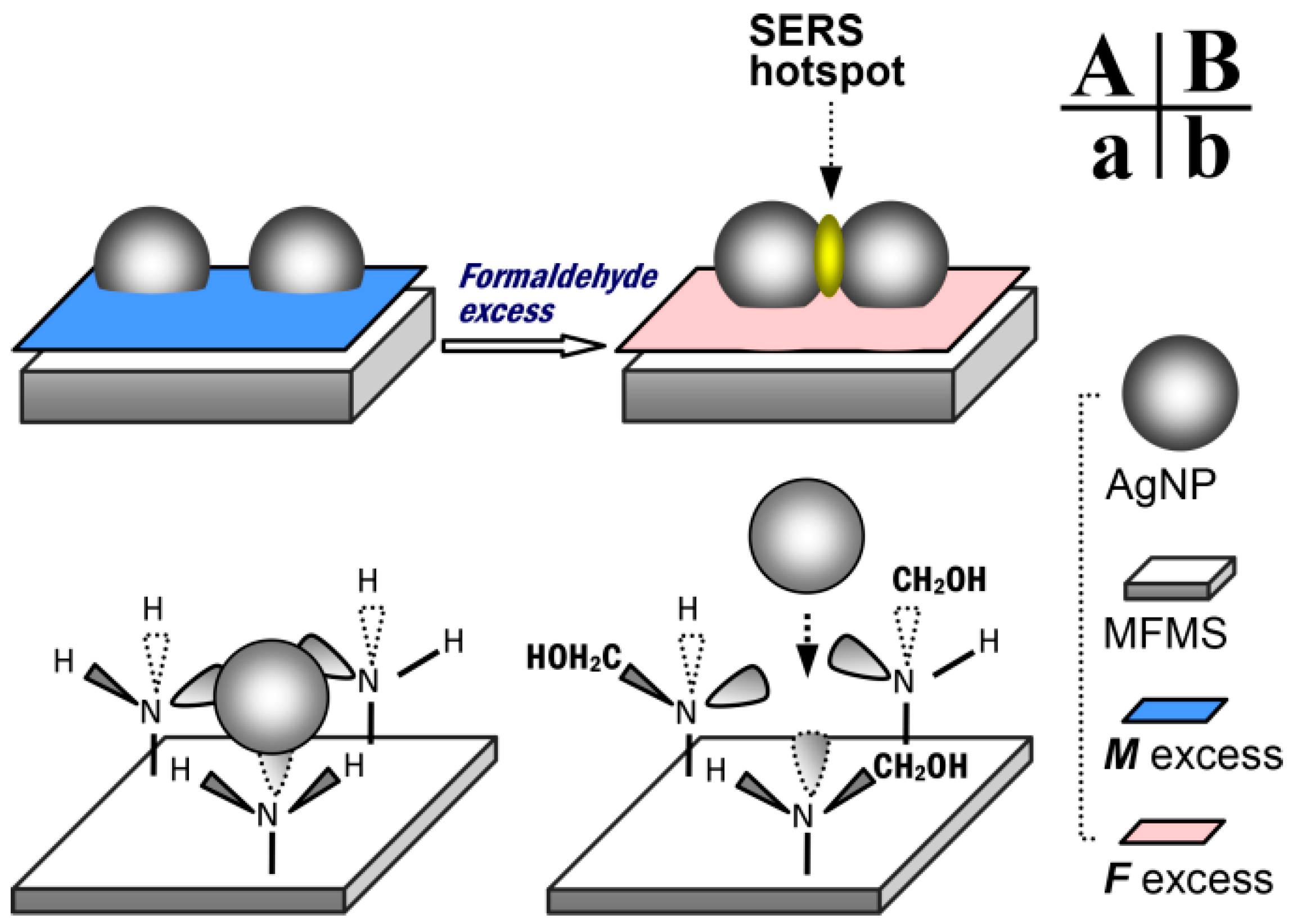
Figure 5 gives a possible explanation for the formation of SERS hot spots on the AgNP/MFMSs. On the surface of MFMS with formaldehyde excess, the –NH– in the –NHCH
2OH group has a weak capability to complex with AgNP, thereby allowing two AgNPs to interact with each other sufficiently to form an effective SERS hot spot, as shown in
Figure 5B,b.
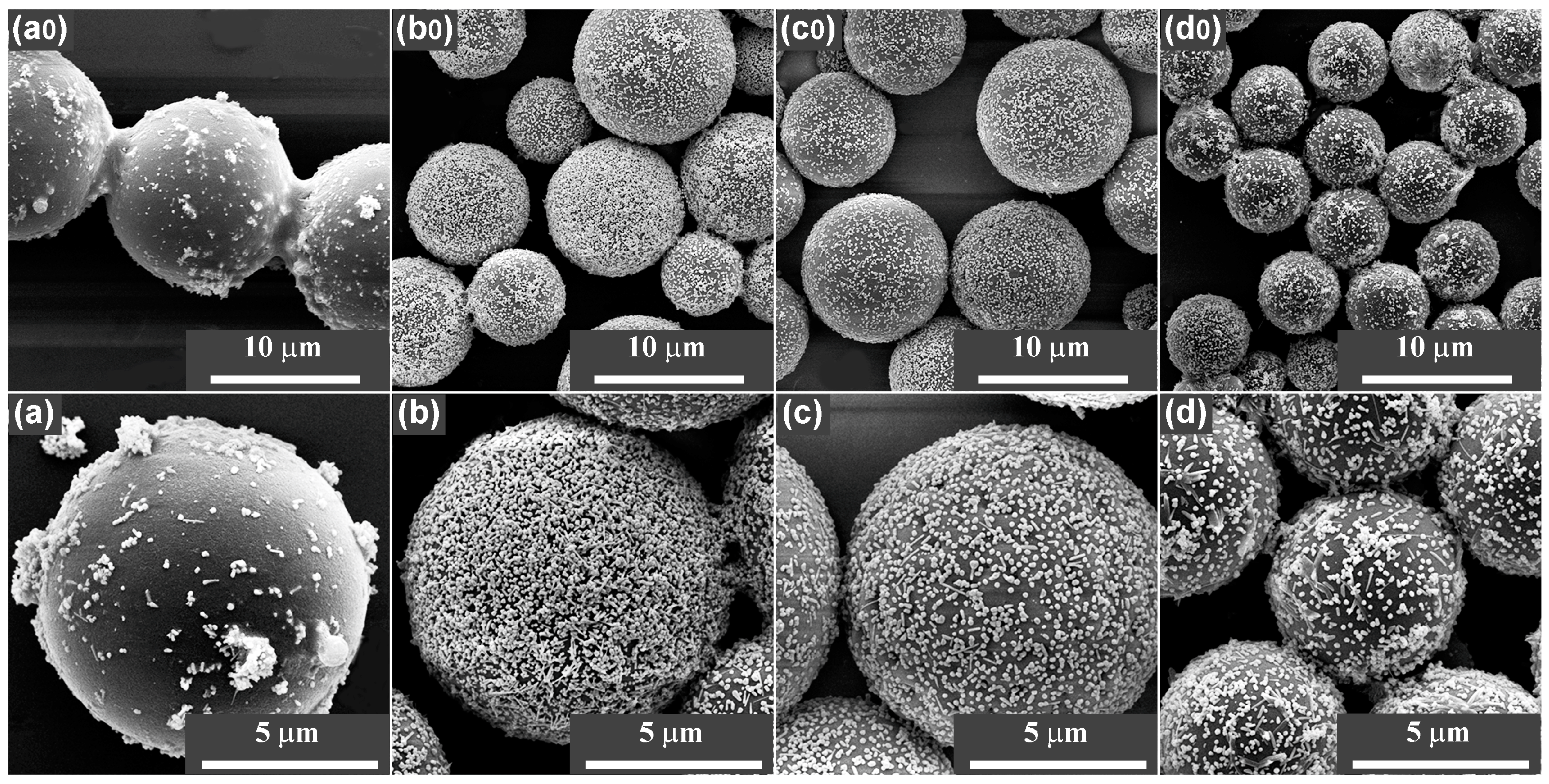
Dispersion characteristics of the AgNPs on the MFMSs are shown in their scanning electron microscope (SEM) images (
Figure 6). The AgNPs had been perfectly dispersed on the samples of AgNP/MF
3MSs (
Figure 6(b,b0)), AgNP/MF
7MSs (
Figure 6(c,c0)), and AgNP/MF
9MSs (
Figure 6(d,d0)), while they were severely aggregated on AgNP/MF
1MSs (also see
Figure S3). The density of the AgNPs on the surface AgNP/MF
3MSs seem to be the highest, indeed the contents of AgNPs in all the AgNP/MFMSs samples were roughly consistent (5.02–6.42%, as shown in
Table S2). These results demonstrated that the SERS performance of the substrate is independent of the silver loading efficiency, but strongly related to the assembly of AgNPs on the surface of MFMSs. The surface with excess formaldehyde favors the assembly of AgNPs and, thus, the formation of SERS hot spots.
The reaction time in the MFMS synthesis is another important variable. The excess co-monomer will remain in the solution following the exhaustion of the other inadequate one and be bonded on the surface of MFMSs to the maximum extent at the end of the synthesis reaction. With the reaction time increased from 20 min to 8 h, the fluorescence background in the SERS spectra on AgNP/MF
3MSs declined, but the SERS signals gained intensity gradually, as shown in
Figure 7. Herein, the amount of formaldehyde bonded on the MFMS surface was speculated to increase with the exhaustion of the melamine in the reaction solution, and the AgNPs that were thereby activated on the surface modified gradually until completion of the reaction.
The specific demarcation F:M molar ratio, 1.5:1, was again confirmed by the SERS spectra on AgNPs loaded on the MFMSs that were synthesized in 8 h, as shown in
Figure 8. Herein, the SERS signals all gained striking intensity if the incorporated MFMSs synthesized at the F:M molar ratio more than 1.5:1, except those faint on MF
1.5 (i) and no SERS signals on MF
1 (a) that were obviously inactivated by the excess melamine bonded on the surface.
Oligomers incorporated in MFMSs were suspected to be involved in the aggregation of the AgNPs, to thereby influence the SERS enhancement. If they did, they should be dissolvable to a certain extent in water because of the existence of hydrophilic melamine and/or formaldehyde bonded to them, and might work as linkers involved in the construction of SERS hot spots between the AgNPs [
14,
15,
16,
17,
18]. To evaluate the influence of MF oilgomers on the SERS enhancement, MFMSs were dispersed into water under ultrasonic oscillation, impregnated for 12 h, and then removed via centrifugation. Herein, the AgNPs, separated via centrifugation between 2000 and 6000 rpm, were treated by the impregnating solution and then used directly for the SERS detection following its R6G incubation.
With the F:M molar ratio increased, the AgNPs gave SERS signals following a trend of the intensity just similar to that observed on the AgNP/MFMSs samples (compare
Figure 9 with
Figure 3), but the intensity of the SERS signals was only about one-twentieth the value observed on the AgNP/MFMSs. Obviously, the oligomers impregnated out at ambient temperature are effective in the SERS detection, but only play a minor role in the presence of MFMSs. These results, again, verified the importance of excess formaldehyde based on the small molecular level, while providing additional evidence for the complexing rationale from the –NH– groups to AgNPs, as shown in
Figure 5.
SERS spectra measured on AgNP/MFMSs incubated with a set of R6G concentrations are shown in
Figure S4. With the concentration decreased from 10
−7 to 10
−13 M, the SERS signal from R6G gradually declined to a R6G detection limit of 10
−13 M. The SERS enhancement factor (EF) is estimated to be about 1.24 × 10
8 (
Figure S5) [
26,
27]. The relative standard deviation (RSD) of the SERS peak intensity in detection of 10
−7 M R6G was found to be less than 15%, as shown in
Figure S6 and
Table S3. Therefore, AgNP/MFMSs can be used as a simple and effective SERS substrate.
AgNP/MFMSs as a SERS substrate can be used to detect organic molecules with mercapto or thiocarbonyl groups that can covalently bond with Ag atoms, such as
p-hydroxythiophenol and tetramethylthiuram disulfide in ethanol solution, as shown in
Figure 10a,b. It is competent for analyzing organic amine salts in aqueous solution, e.g., trace amounts of Rhodamine 6G, malachite green, methylene blue, basic violet 14, and crystal violet, as shown in
Figure 10c–f, which is significant in the detection of environmental and food contaminants. We would like to explain this feature by the similar compatibility between the organic amino molecules and the MFMSs, and it should be helpful for the organic amino molecules to be collected and enriched by the MFMSs in the incubation step.