Nanocomposites Based on PCL and Halloysite Nanotubes Filled with Lysozyme: Effect of Draw Ratio on the Physical Properties and Release Analysis
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of HNTs-Lysozyme
2.3. Nanocomposites Preparation and Draw Processing
2.4. Methods of Investigation
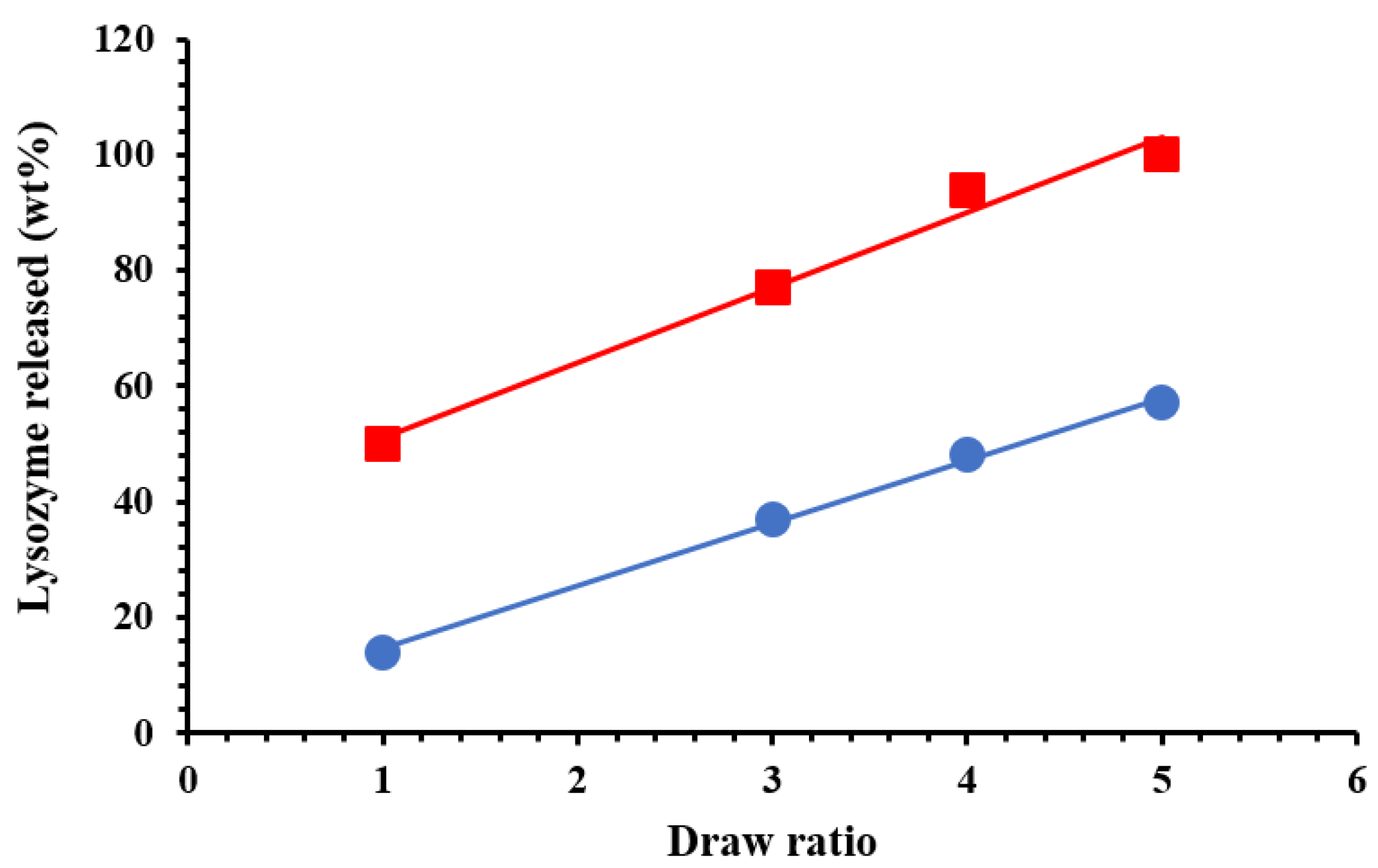
3. Results and Discussion
4. Concluding Remarks
Author Contributions
Conflicts of Interest
References
- Lagaròn, J. Multifunctional and Nanoreinforced Polymers for Food Packaging; Woodhead Publishing: Sawston, Cambridge, UK, 2011. [Google Scholar]
- Benelhadj, S.; Fejji, N.; Degraeve, P.; Attia, H.; Ghorbel, D.; Gharsallaoui, A. Properties of lysozyme/Arthrospira platensis (Spirulina) protein complexes for antimicrobial edible food packaging. Algal Res. 2016, 15, 43–49. [Google Scholar] [CrossRef]
- Xue, M.; Findenegg, G.H. Lysozyme as a pH-Responsive Valve for the Controlled Release of Guest Molecules from Mesoporous Silica. Langmuir 2012, 28, 17578–17584. [Google Scholar] [CrossRef] [PubMed]
- Bonincontro, A.; de Francesco, A.; Onori, G. Influence of pH on Lysozyme Conformation Revealed by Dielectric Spectroscopy. Colloids Surf. B 1998, 12, 1–5. [Google Scholar] [CrossRef]
- Mastromatteo, M.; Conte, A.; Del Nobile, M.A. Advances in controlled release devices for food packaging applications. Trends Food Sci. Technol. 2010, 21, 591–598. [Google Scholar] [CrossRef]
- Bugatti, V.; Acocella, M.; Maggio, M.; Pantani, R.; Gorrasi, G. Release of Lysozyme from cold drawn Poly (ε-caprolactone) at different draw ratios. Marcomol. Mater. Eng. 2017, in press. [Google Scholar]
- Zhang, W.; Ronca, S.; Mele, E. Electrospun Nanofibres Containing Antimicrobial Plant Extracts. Nanomaterials 2017, 7, 42. [Google Scholar] [CrossRef] [PubMed]
- James, S.; McManus, J.J. Thermal and solution stability of lysozyme in the presence of sucrose, glucose, and trehalose. J. Phys. Chem. B 2012, 116, 10182–10188. [Google Scholar] [CrossRef] [PubMed]
- Venkataramani, S.; Truntzer, J.; Coleman, D.R. Thermal stability of high concentration lysozyme across varying pH: A fourier transform infrared study. J. Pharm. Bioallied Sci. 2013, 5, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Al Meslmani, B.M.; Mahmoud, G.F.; Leichtweiß, T.; Strehlow, B.; Sommer, F.O.; Lohoff, M.D.; Bakowsky, U. Covalent immobilization of lysozyme onto woven and knitted crimped polyethylene terephthalate grafts to minimize the adhesion of broad spectrum pathogens. Mater. Sci. Eng. C 2016, 58, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Losso, J.N.; Nakai, S.; Charter, E.A. Lysozyme. In Natural Food Antimicrobial Systems; Naidu, A.S., Ed.; CRC Press LLC: Boca Raton, FL, USA, 2000; pp. 185–210. [Google Scholar]
- Conte, A.; Buonocore, G.G.; Bevilacqua, A.; Sinigaglia, M.; Del Nobile, M.A. Immobilization of lysozyme on polyvinylalcohol films for active packaging applications. J. Food Prot. 2006, 69, 866–870. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.V.; Prevost, N.; Condon, B.; Sethumadhavan, K.; Ullah, J.; Bopp, A. Immobilization of lysozyme on cotton fabrics: Synthesis, characterization, and activity. AATCC Rev. 2011, 11, 73–79. [Google Scholar]
- Muriel-Galet, V.; Talbert, J.N.; Hernandez-Munoz, P.; Gavara, R.; Goddard, J.M. Covalent Immobilization of Lysozyme on Ethylene Vinyl Alcohol Films for Nonmigrating Antimicrobial Packaging Applications. J. Agric. Food Chem. 2013, 61, 6720–6727. [Google Scholar] [CrossRef] [PubMed]
- Farhoodi, M. Nanocomposite Materials for Food Packaging Applications: Characterization and Safety Evaluation. Food Eng. Rev. 2016, 8, 35–51. [Google Scholar] [CrossRef]
- Buonocore, G.G.; Del Nobile, M.A.; Panizza, A.; Corbo, M.R.; Nicolais, L. A general approach to describe the antimicrobial agent release from highly swellable films intended for food packaging applications. J. Control. Release 2003, 90, 97–107. [Google Scholar] [CrossRef]
- Buonocore, G.G.; Sinigaglia, M.; Corbo, M.R.; Bevilacqua, A.; La Notte, E.; Del Nobile, M.A. Controlled release of antimicrobial compounds from highly swellable polymers. J. Food Prot. 2004, 67, 1190–1194. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, P.; Balaguer, M.P.; Gomez-Estaca, J.; Gavara, R.; Hernandez-Munoz, P. Chemically modified gliadins as sustained release systems for lysozyme. Food Hydrocoll. 2014, 41, 53–59. [Google Scholar] [CrossRef]
- Min, S.; Rumsey, T.R.; Krochta, J.M. Diffusion of the antimicrobial lysozyme from a whey protein coating on smoked salmon. J. Food Eng. 2008, 84, 39–47. [Google Scholar] [CrossRef]
- Mastromatteo, M.; Lecce, L.; De Vietro, N.; Favia, P.; Del Nobile, M.A. Plasma deposition processes from acrylic/methane on natural fibres to control the kinetic release of lysozyme from PVOH monolayer film. J. Food Eng. 2011, 104, 373–379. [Google Scholar] [CrossRef]
- Conte, A.; Buonocore, G.G.; Nicolais, L.; Del Nobile, M.A. Controlled release of active compounds from antimicrobial films intended for food packaging applications. Ital. J. Food Sci. 2003, 15, 216–218. [Google Scholar]
- Lu, J.R.; Su, T.J.; Howlin, B.J. The Effect of Solution pH on the Structural Conformation of Lysozyme Layers Adsorbed on the Surface of Water. J. Phys. Chem. B 1999, 103, 5903–5909. [Google Scholar] [CrossRef]
- Mendes de Souza, P.; Fernandez, A.; Lopez-Carballo, G.; Gavara, R.; Hernandez-Munoz, P. Modified sodium caseinate films as releasing carriers of lysozyme. Food Hydrocoll. 2010, 24, 300–306. [Google Scholar] [CrossRef]
- Yuan, P.; Tan, D.; Annabi-Bergaya, F. Properties and applications of halloysite nanotubes: Recent research advances and future prospects. Appl. Clay Sci. 2015, 112–113, 75–93. [Google Scholar] [CrossRef]
- Lvov, Y.; Abdullayev, E. Green and functional polymer-clay nanotube composites with sustained release of chemical agents. Prog. Polym. Sci. 2013. [Google Scholar] [CrossRef]
- Lvov, Y.; Shchukin, D.; Möhwald, H.; Price, R. Clay Nanotubes for Controlled Release of Protective Agents—Perspectives. ACS Nano 2008, 2, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Arcudi, F.; Cavallaro, G.; Lazzara, G.; Massaro, M.; Milioto, S.; Noto, R.; Riela, S. Selective Functionalization of Halloysite Cavity by Click Reaction: Structured Filler for Enhancing Mechanical Properties of Bionanocomposite Films. J. Phys. Chem. 2014, 118, 15095–15101. [Google Scholar] [CrossRef] [Green Version]
- Gorrasi, G.; Pantani, R.; Murariu, M.; Dubois, P. PLA/Halloysite Nanocomposite Films: Water Vapor Barrier Properties and Specific Key Characteristics. Macromol. Mater. Eng. 2014, 299, 104–115. [Google Scholar] [CrossRef]
- Liu, M.; Jia, Z.; Jia, D.; Zhou, C. Recent advance in research on halloysite-nanotubes polymer nanocomposites. Prog. Polym. Sci. 2014, 39, 1498–1525. [Google Scholar] [CrossRef]
- Abdullayev, E.; Lvov, Y. Clay Nanotubes for Corrosion Inhibitor Encapsulation: Release Control with End Stoppers. J. Mater. Chem. 2010, 20, 6681–6687. [Google Scholar] [CrossRef]
- Abdullayev, E.; Lvov, Y. Clay Nanotubes for Controlled Release of Protective Agents—A Review. J. Nanosci. Nanotechnol 2011, 11, 10007–10026. [Google Scholar] [CrossRef] [PubMed]
- Abdullayev, E.; Price, R.; Shchukin, D.; Lvov, Y. Halloysite Tubes as Nanocontainers for Anticorrosion Coating with Benzotriazole. Appl. Mater. Int. 2009, 2, 1437–1442. [Google Scholar] [CrossRef] [PubMed]
- Abdullayev, E.; Shchukin, D.; Lvov, Y. Halloysite Clay Nanotubes as a Reservoir for Corrosion Inhibitors and Template for Layer-by-Layer Encapsulation. Mater. Sci. Eng. 2008, 99, 331–332. [Google Scholar]
- Gorrasi, G.; Vertuccio, L. Evaluation of zein/halloysite nano-containers as reservoirs of active molecules for packaging applications: Preparation and analysis of physical properties. J. Cereal Sci. 2016, 70, 66–71. [Google Scholar] [CrossRef]
- Veerabadran, N.; Price, R.; Lvov, Y. Clay nanotubes for encapsulation and sustained release of drugs. Nano 2007, 2, 215–222. [Google Scholar] [CrossRef]
- Lvov, Y.; Price, R. Halloysite Nanotubules a Novel Substrate for the Controlled Delivery of Bioactive Molecules. In Bio-Inorganic Hybrid Nanomaterials; Ruiz-Hitzky, E., Ariga, K., Lvov, Y., Eds.; Wiley: London, UK, 2008; pp. 440–478. [Google Scholar]
- Liu, M.; Dai, L.; Shi, H.; Xiong, S.; Zhou, C. In vitro evaluation of alginate/halloysite nanotube composite scaffolds for tissue engineering. Mater. Sci. Eng. C 2015, 49, 700–712. [Google Scholar] [CrossRef] [PubMed]
- Bugatti, V.; Sorrentino, A.; Gorrasi, G. Encapsulation of Lysozyme into halloysite nanotubes and dispersion in PLA: Structural and physical properties and controlled release analysis. Eur. Polym. J. 2017, 93, 495–506. [Google Scholar] [CrossRef]
- Mechanical Properties of Solid Polymers, 3rd ed.; Ward, I.M.; Sweeney, J. (Eds.) Wiley: Hoboken, NJ, USA, 2012. [Google Scholar]
- Koros, W.J.; Burgess, S.K.; Chen, Z. Encyclopedia of Polymer Science and Technology; Wiley: Hoboken, NJ, USA, 2015; pp. 1–96. [Google Scholar] [CrossRef]
- Gorrasi, G. Dispersion of halloysite loaded with natural antimicrobials into pectins: Characterization and controlled release analysis. Carbohydr. Polym. 2015, 127, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Horvath, E.; Frost, R.L.; Mako, E.; Kristof, J.; Cseh, T. Thermal treatment of mechano-chemically activated kaolinite. Thermochim. Acta 2003, 404, 227–235. [Google Scholar] [CrossRef]
- Ciardelli, G.; Chiono, V.; Vozzi, G.; Pracella, M.; Ahluwalia, A.; Barbani, N.; Cristallini, C.; Giusti, P. Blends of Poly(ε-caprolactone) and Polysaccharides in Tissue Engineering Applications. Biomacromolecules 2005, 6, 1961–1976. [Google Scholar] [CrossRef] [PubMed]
- Pantoustier, N.; Alexandre, M.; Degee, P.; Calberg, C.; Jerome, R.; Henrist, C.; Cloots, R.; Rulmont, A.; Dubois, P. Poly(ε-caprolactone)/clay nanocomposites prepared by melt intercalation. Mechanical, thermal and rheological properties. Polymer 2002, 43, 4017–4023. [Google Scholar]
- Du, M.; Guo, B.; Jia, D. Thermal stability and flame retardant effects of halloysite nanotubes on poly(propylene). Eur. Polym. J. 2006, 42, 1362–1369. [Google Scholar] [CrossRef]
- Gorrasi, G.; Senatore, V.; Vigliotta, G.; Belviso, S.; Pucciariello, R. PET-halloysite nanotubes composites for packaging application: Preparation, characterization and analysis of physical properties. Eur. Polym. J. 2014, 61, 145–156. [Google Scholar] [CrossRef]
- Bugatti, V.; Costantino, U.; Gorrasi, G.; Nocchetti, M.; Tammaro, L.; Vittoria, V. Nano-hybrids incorporation into poly(ε-caprolactone) for multifunctional applications: Mechanical and barrier properties. Eur. Polym. J. 2010, 46, 418–427. [Google Scholar] [CrossRef]
- Koo, J.H. Mechanical Properties of Polymer Nanocomposites. In Fundamentals, Properties, and Applications of Polymer Nanocomposites; Cambridge University Press: Cambridge, UK, 2016; Chapter 7; pp. 273–331. [Google Scholar]
- Quintavalla, S.; Vicini, L. Antimicrobial food packaging in meat industry. Meat Sci. 2002, 62, 373–380. [Google Scholar] [CrossRef]
- Bugatti, V. Dispersion of Inorganic Fillers in Polymeric Matrices for Food Packaging Applications. Ph.D. Thesis, University of Salerno, Italy, 2012. Available online: http://hdl.handle.net/10556/274 (accessed on 18 May 2012).
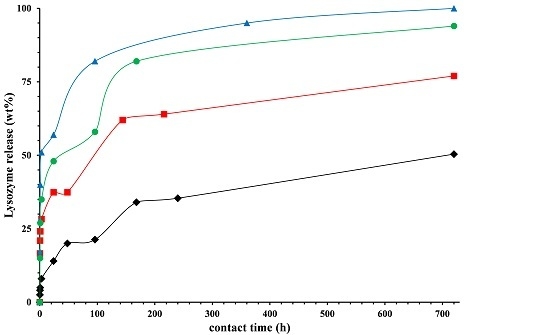
 ), Lysozyme (
), Lysozyme (  ), nano-hybrid HNTs/Lysozyme (
), nano-hybrid HNTs/Lysozyme (  ).
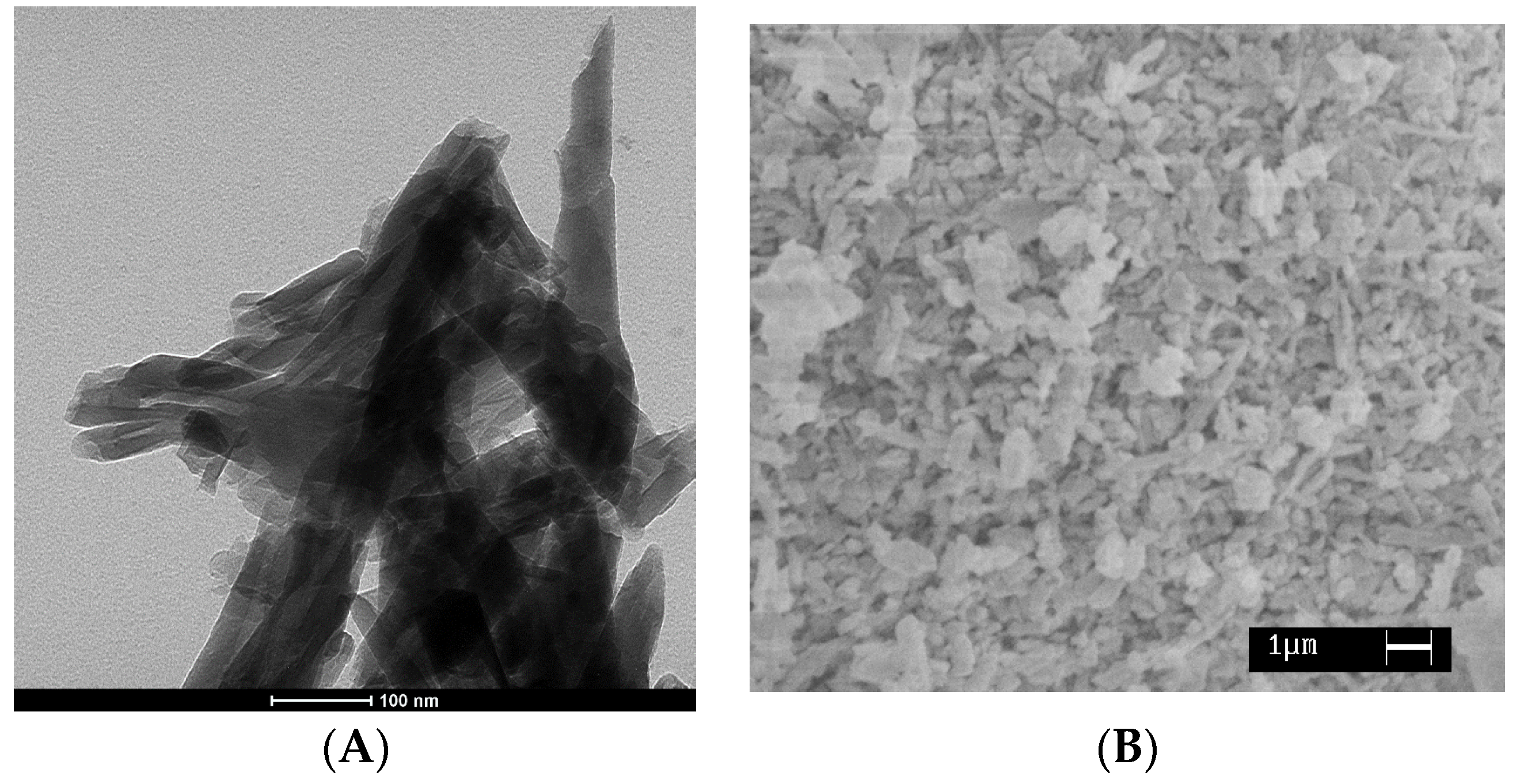
).
 ), drawn at λ = 3 (
), drawn at λ = 3 (  ), drawn at λ = 4 (
), drawn at λ = 4 (  ), and drawn at λ = 5 (
), and drawn at λ = 5 (  ); and (B) the ratio between absorbance bands of symmetrical (2850 cm−1) and asymmetrical (2930 cm−1) C–H stretching for drawn samples divided by the absorbance of undrawn samples.
); and (B) the ratio between absorbance bands of symmetrical (2850 cm−1) and asymmetrical (2930 cm−1) C–H stretching for drawn samples divided by the absorbance of undrawn samples.
 ), drawn at λ = 3 (
), drawn at λ = 3 (  ), drawn at λ = 4 (
), drawn at λ = 4 (  ), and drawn at λ = 5 (
), and drawn at λ = 5 (  ); and (B) the ratio between absorbance bands of symmetrical (2850 cm−1) and asymmetrical (2930 cm−1) C–H stretching for drawn samples divided by the absorbance of undrawn samples.
); and (B) the ratio between absorbance bands of symmetrical (2850 cm−1) and asymmetrical (2930 cm−1) C–H stretching for drawn samples divided by the absorbance of undrawn samples.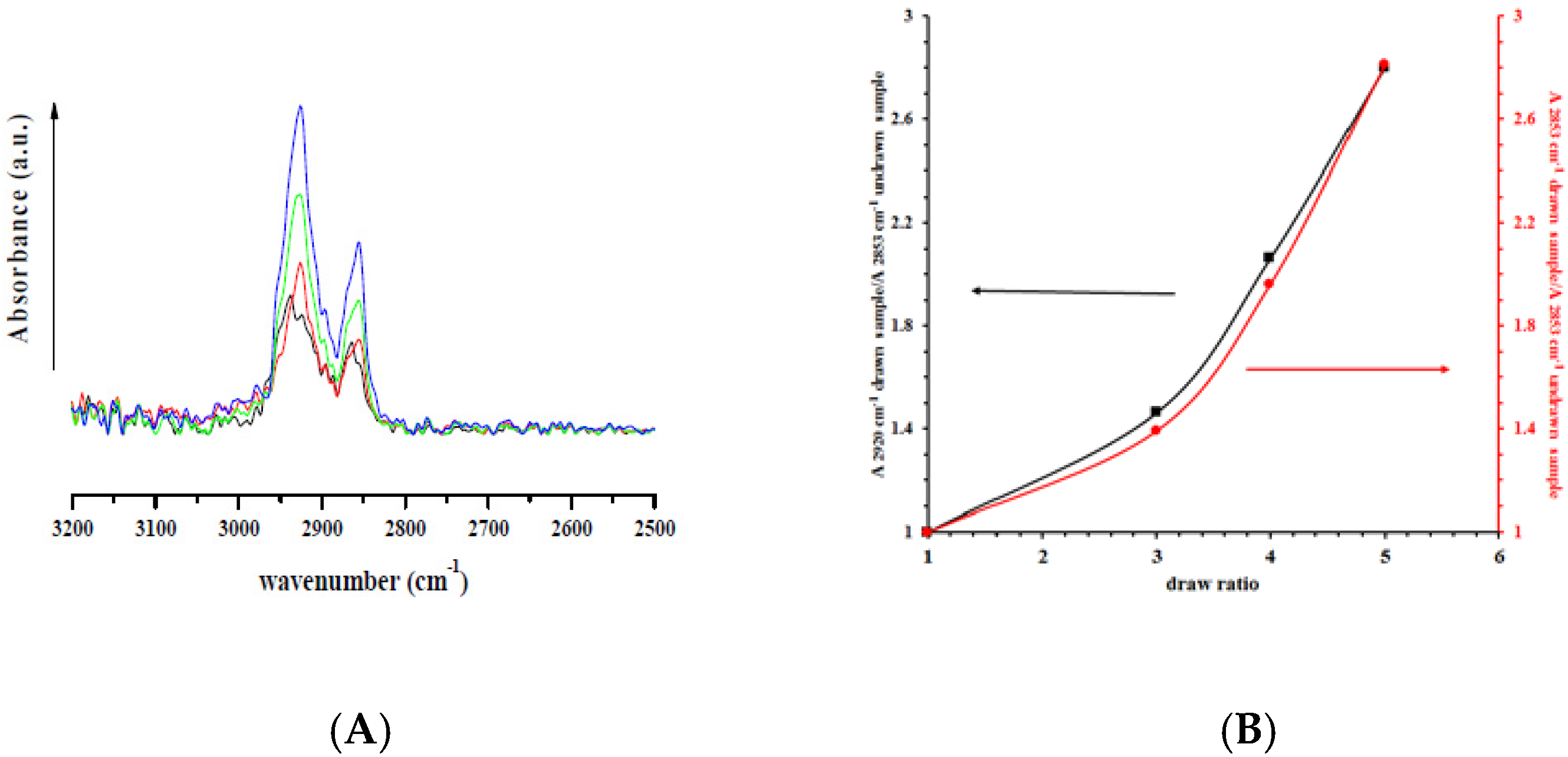
 ), undeformed nanocomposite (
), undeformed nanocomposite (  ), drawn at λ = 3 (
), drawn at λ = 3 (  ), drawn at λ = 4 (
), drawn at λ = 4 (  ), and drawn at λ = 5 (
), and drawn at λ = 5 (  ).
).
 ), undeformed nanocomposite (
), undeformed nanocomposite (  ), drawn at λ = 3 (
), drawn at λ = 3 (  ), drawn at λ = 4 (
), drawn at λ = 4 (  ), and drawn at λ = 5 (
), and drawn at λ = 5 (  ).
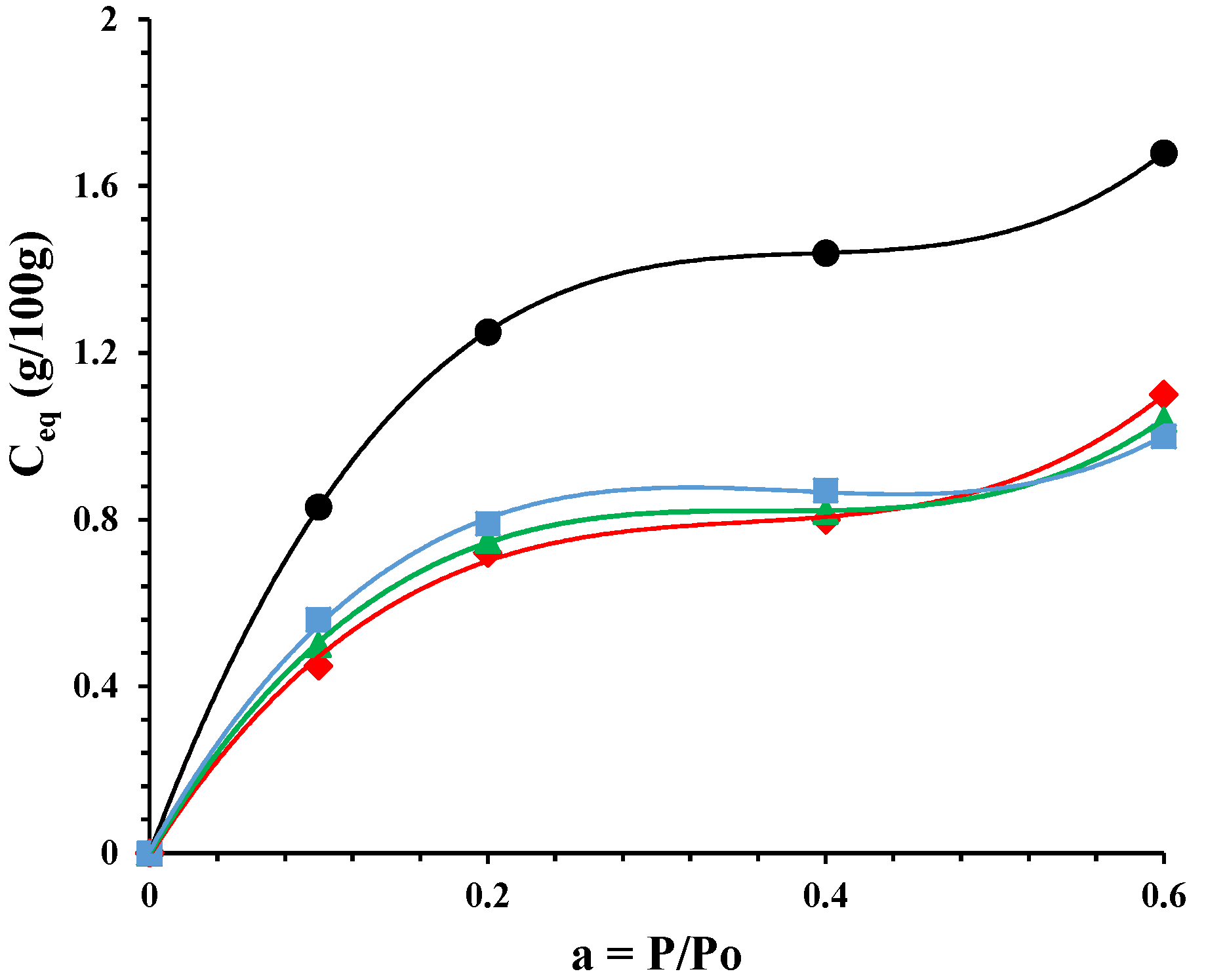
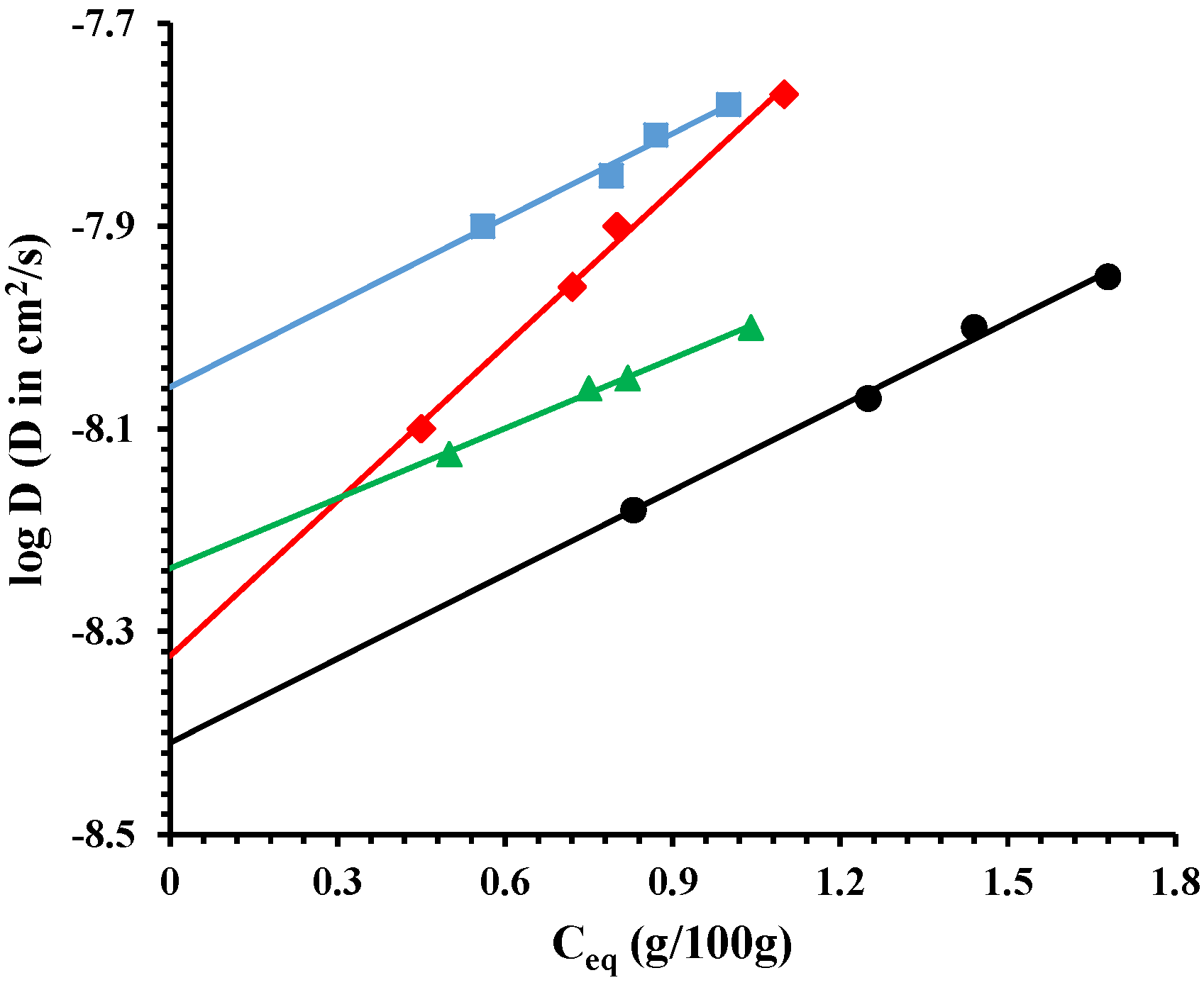
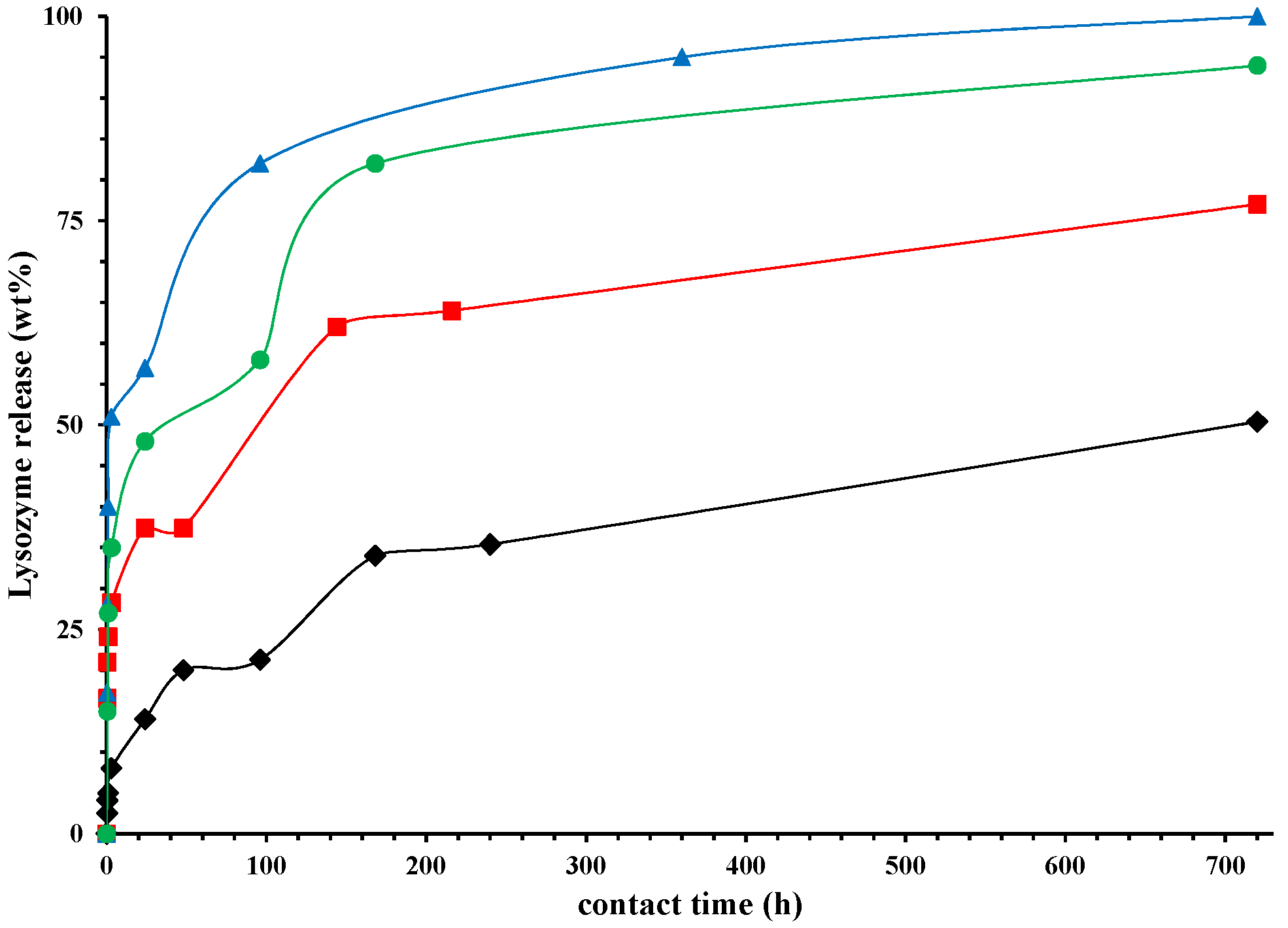
).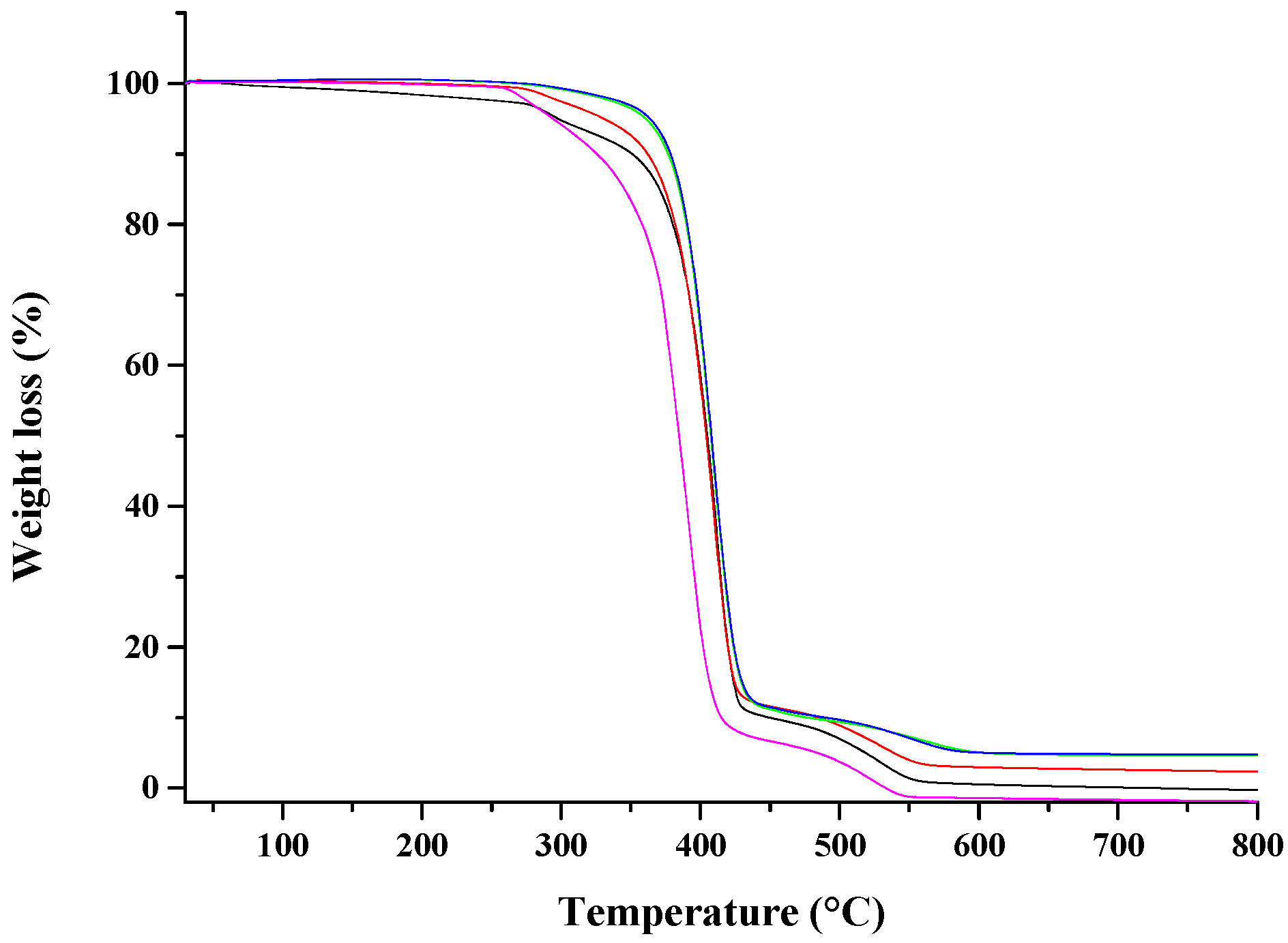
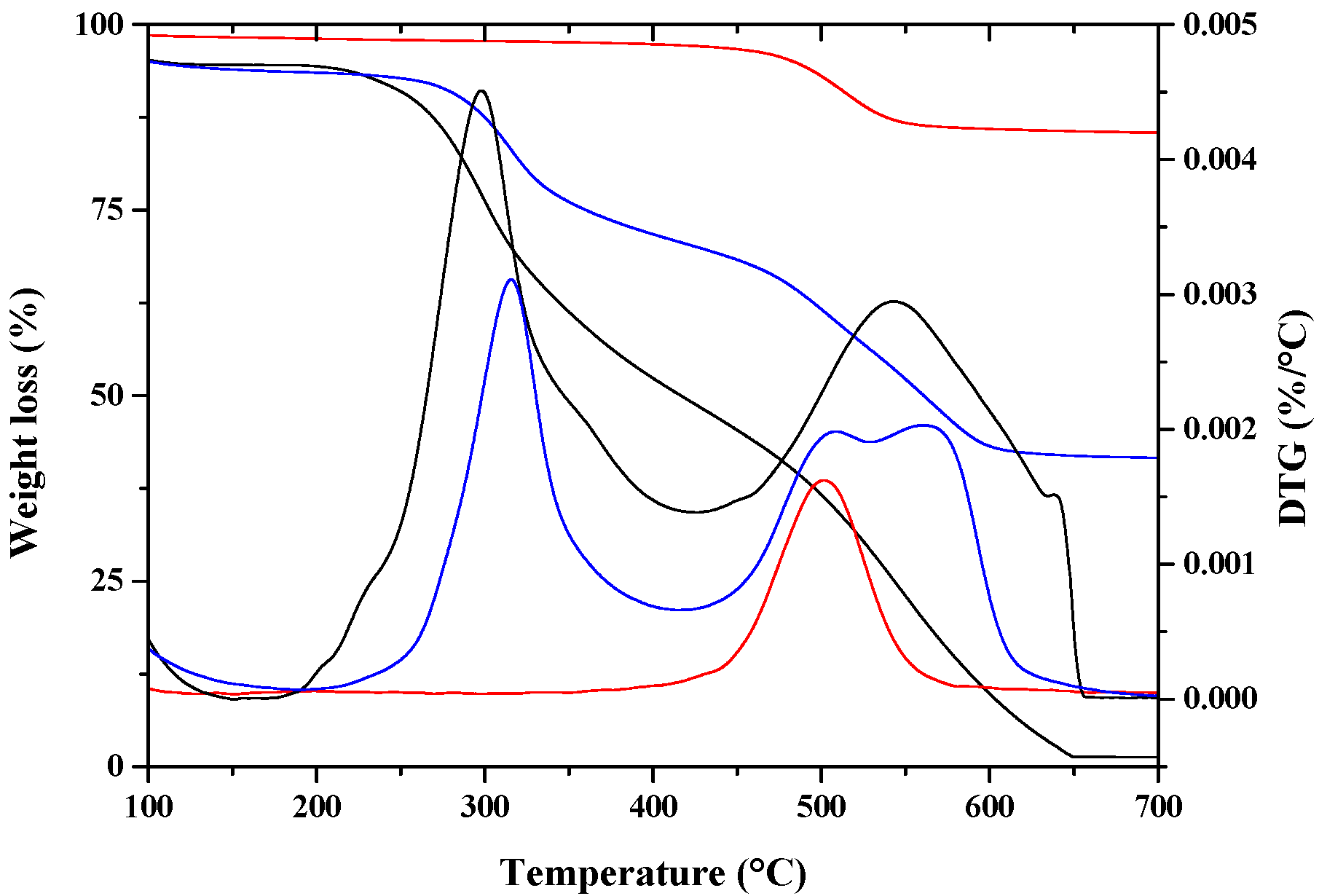
| Sample | Td (10% Weight Loss) | Td (50% Weight Loss) | Td (95% Weight Loss) |
|---|---|---|---|
| PCL | 320 °C | 385 °C | 480 °C |
| PCL drawn at λ = 5 | 325 °C | 390 °C | 505 °C |
| PCL/10%HNTs-Lysozyme | 350 °C | 403 °C | 518 °C |
| Nano-composite drawn at λ = 3 | 360 °C | 405 °C | 537 °C |
| Nano-composite drawn at λ = 4 | 373 °C | 407 °C | 573 °C |
| Nano-composite drawn at λ = 5 | 377 °C | 409 °C | 583 °C |
| Sample | E (MPa) | σy (MPa) | εy (%) | σb (MPa) | εb (%) |
|---|---|---|---|---|---|
| PCL # | 185 ± 24 | 9.95 ± 0.23 | 11.50 ± 3.1 | 15.88 ± 0.23 | 616 ± 14.22 |
| PCL drawn at λ = 5 | 530 ± 22 | 36.45 ± 0.67 | 13.57 ± 3.4 | 32.52 ± 0.34 | 320 ± 15.34 |
| PCL/10%HNTs-Lysozyme | 320 ± 16 | 10.24 ± 0.34 | 8.79 ± 3.7 | 18.14 ± 0.65 | 570 ± 12.31 |
| Nano-composite drawn at λ = 3 | 335 ± 15 | 16.72 ± 0.46 | 9.31 ± 2.4 | 28.98 ± 0.47 | 157 ± 16.26 |
| Nano-composite drawn at λ = 4 | 347 ± 23 | 23.60 ± 0.42 | 14.08 ± 3.6 | 36.10 ± 0.36 | 162 ± 8.420 |
| Nano-composite drawn at λ = 5 | 447 ± 20 | 39.40 ± 0.57 | 14.06 ± 4.8 | 54.61 ± 0.74 | 102 ± 13.27 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bugatti, V.; Viscusi, G.; Naddeo, C.; Gorrasi, G. Nanocomposites Based on PCL and Halloysite Nanotubes Filled with Lysozyme: Effect of Draw Ratio on the Physical Properties and Release Analysis. Nanomaterials 2017, 7, 213. https://doi.org/10.3390/nano7080213
Bugatti V, Viscusi G, Naddeo C, Gorrasi G. Nanocomposites Based on PCL and Halloysite Nanotubes Filled with Lysozyme: Effect of Draw Ratio on the Physical Properties and Release Analysis. Nanomaterials. 2017; 7(8):213. https://doi.org/10.3390/nano7080213
Chicago/Turabian StyleBugatti, Valeria, Gianluca Viscusi, Carlo Naddeo, and Giuliana Gorrasi. 2017. "Nanocomposites Based on PCL and Halloysite Nanotubes Filled with Lysozyme: Effect of Draw Ratio on the Physical Properties and Release Analysis" Nanomaterials 7, no. 8: 213. https://doi.org/10.3390/nano7080213