Flexible Carbon Nanotube Modified Separator for High-Performance Lithium-Sulfur Batteries
Abstract
:1. Introduction
2. Results and Discussion
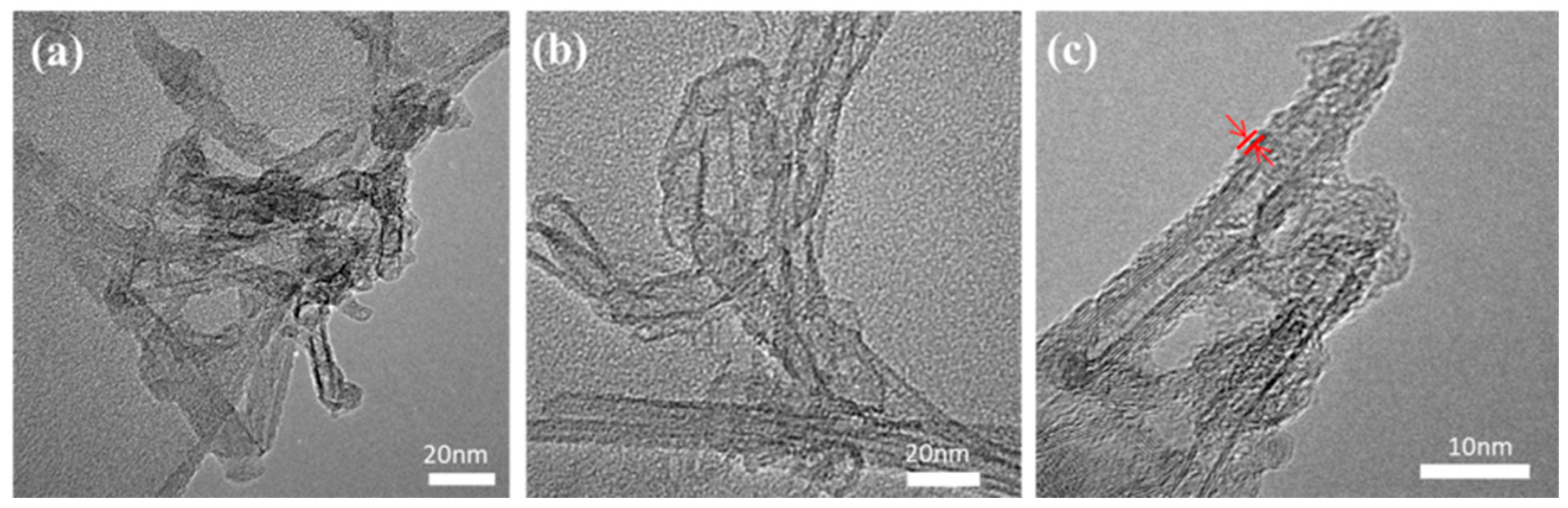
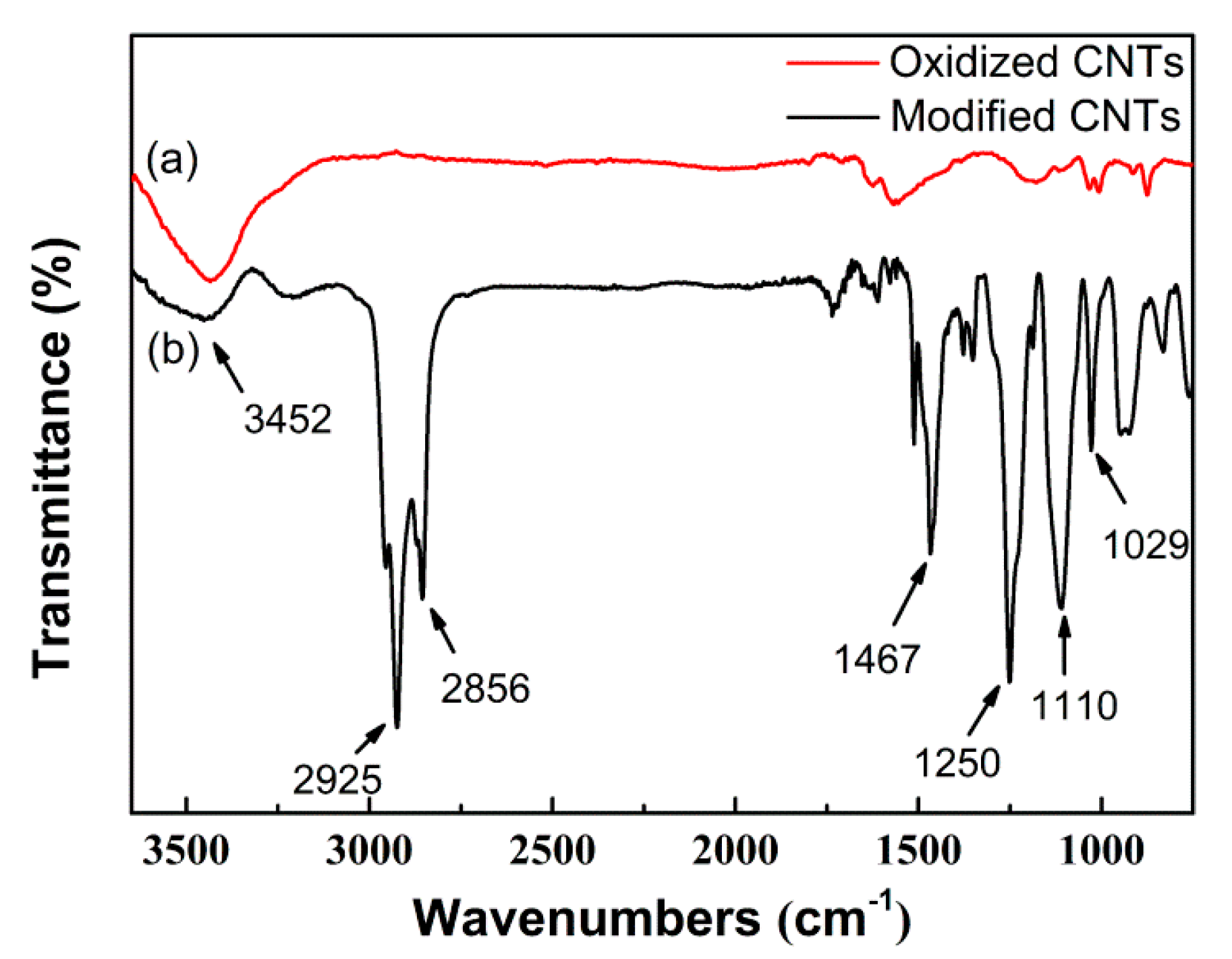
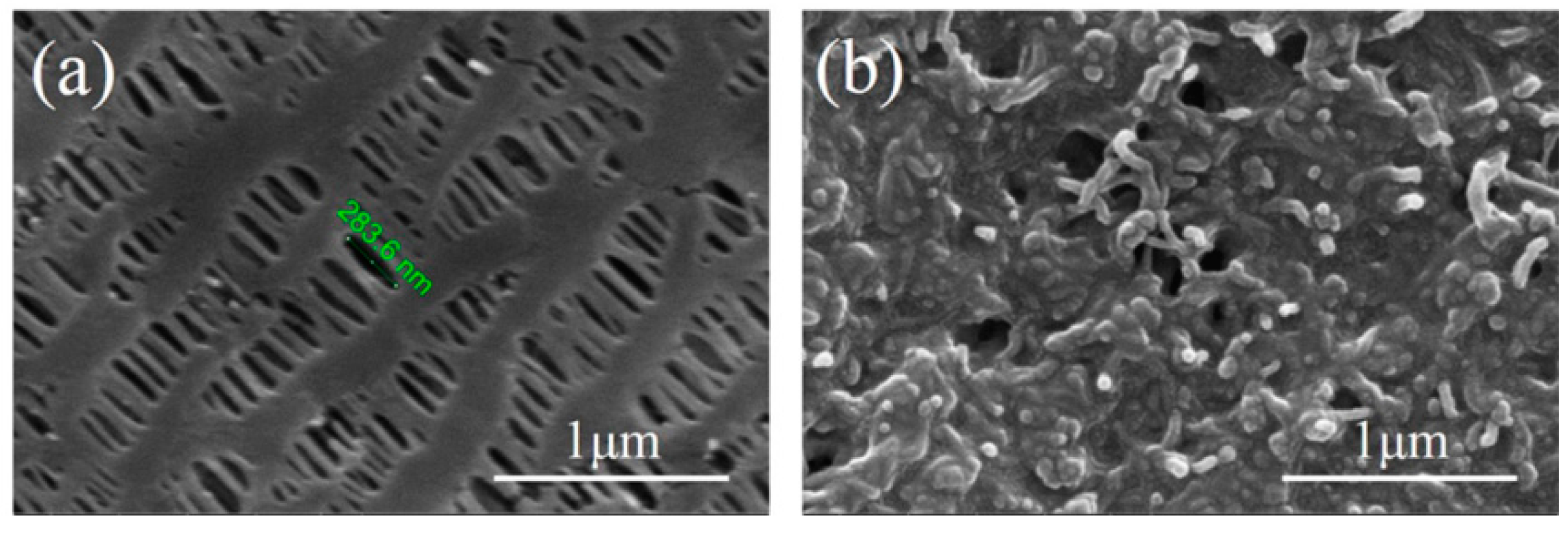
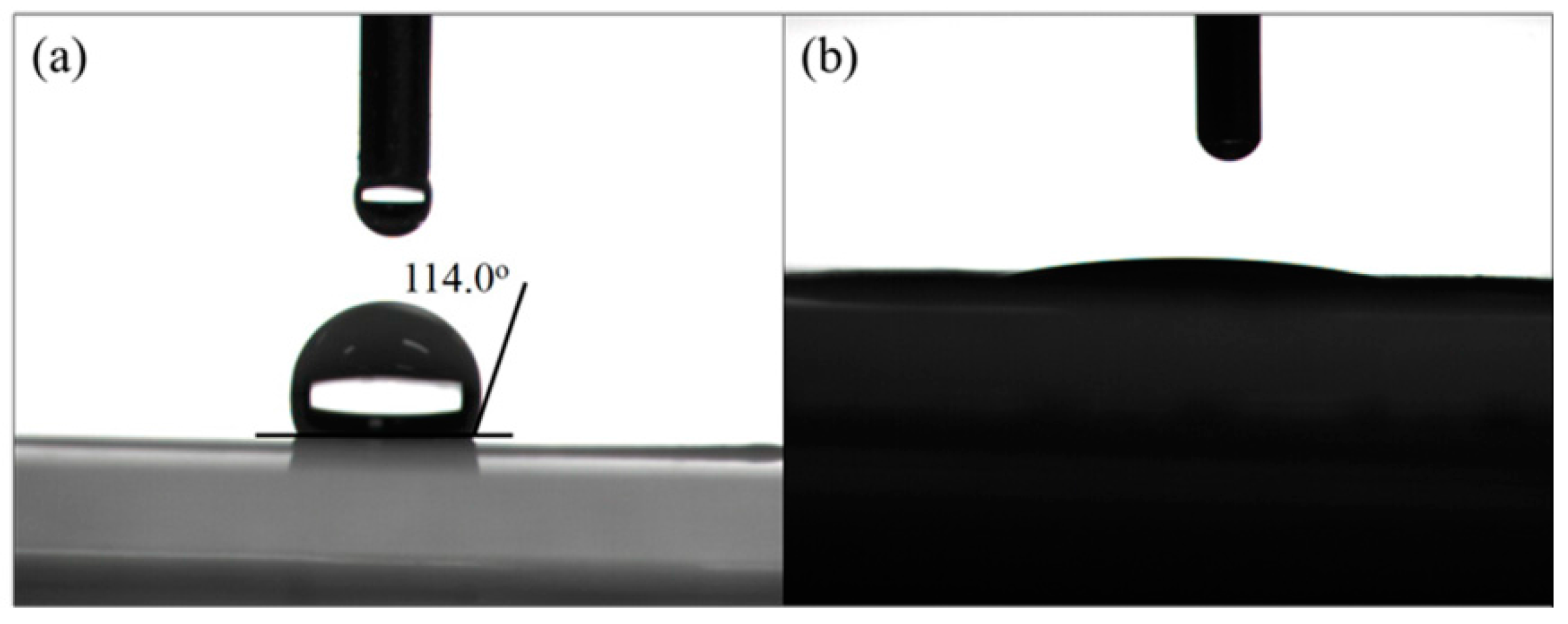
2.1. Morphology and Structural Characterization
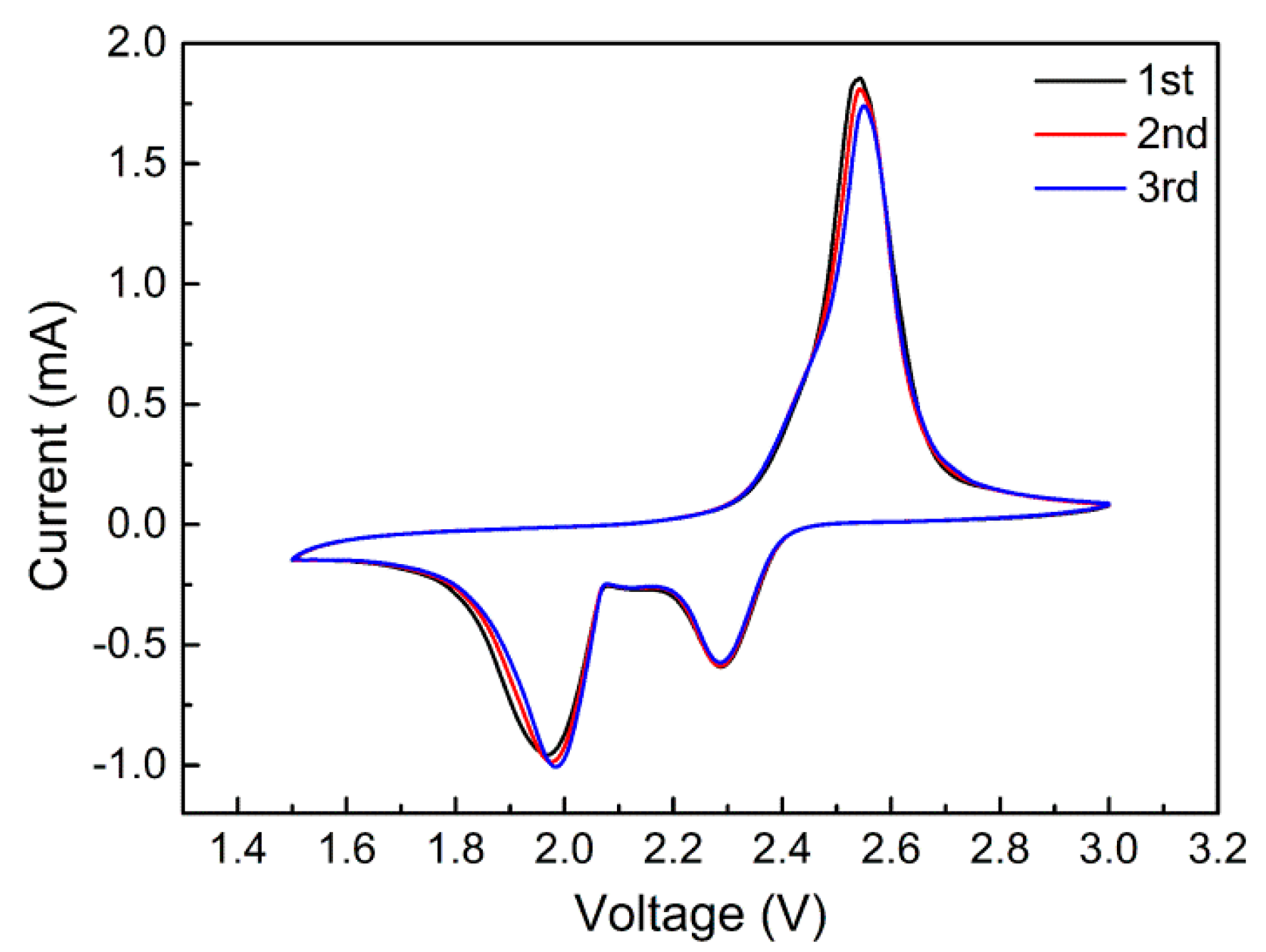
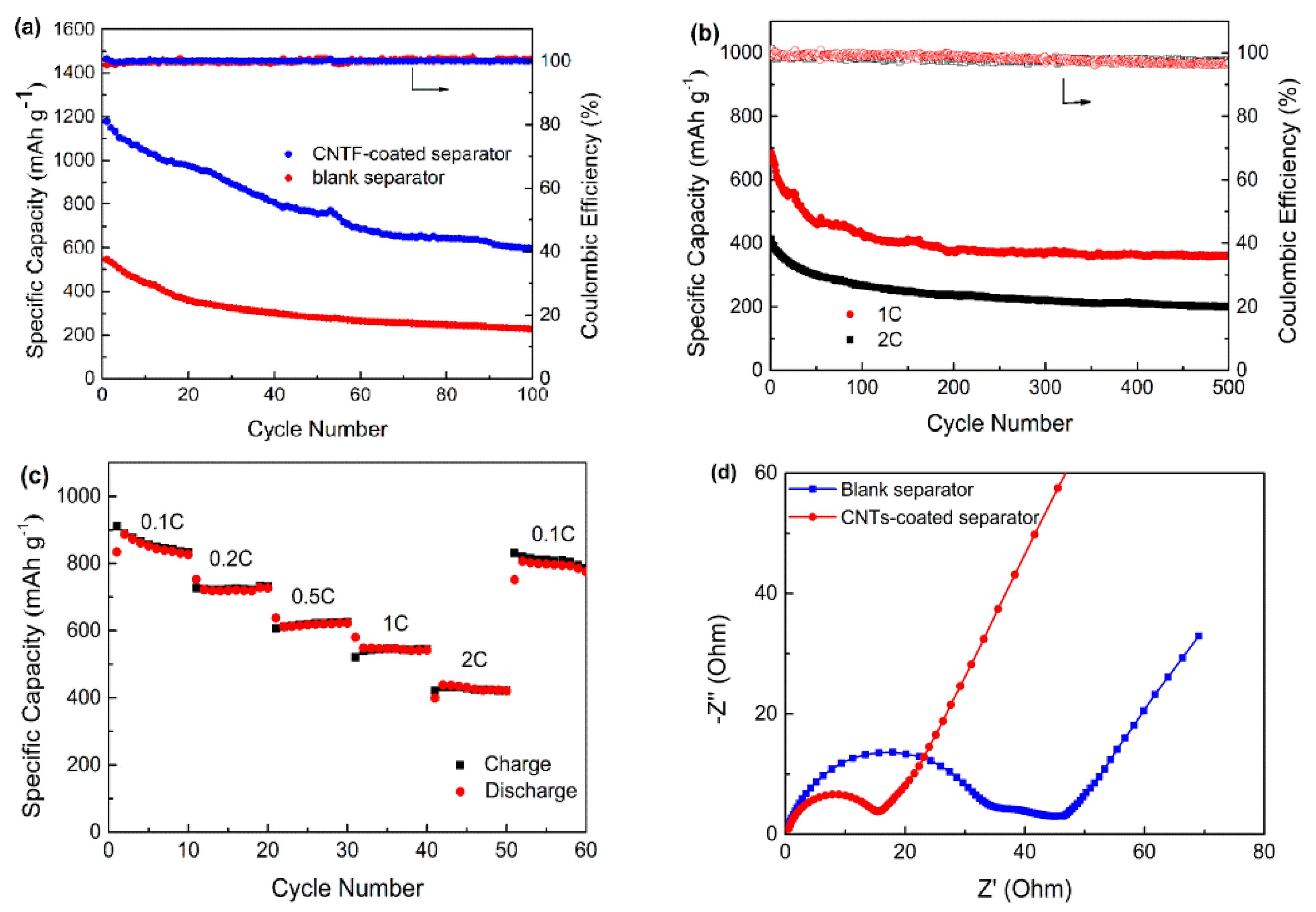
2.2. Electrochemical Performance
3. Materials and Methods
3.1. Materials
3.2. Preparation of CNTs-Coated Separator
3.3. Characterizations
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Adelhelm, P.; Hartmann, P.; Bender, C.L.; Busche, M.; Eufinger, C.; Janek, J. From lithium to sodium: Cell chemistry of room temperature sodium-air and sodium-sulfur batteries. Beilstein J. Nanotechnol. 2015, 6, 1016–1055. [Google Scholar] [CrossRef] [PubMed]
- Whittingham, M.S. Ultimate limits to intercalation reactions for lithium batteries. Chem. Rev. 2014, 114, 11414–11443. [Google Scholar] [CrossRef] [PubMed]
- Evers, S.; Nazar, F.L. New Approaches for High Energy Density Lithium-Sulfur Battery Cathodes. Acc. Chem. Res. 2013, 46, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Seh, Z.W.; Sun, Y.; Zhang, Q.; Cui, Y. Designing high-energy lithium-sulfur batteries. Chem. Soc. Rev. 2016, 45, 5605–5634. [Google Scholar] [CrossRef] [PubMed]
- Manthiram, A.; Fu, Y.; Chung, S.H.; Zu, C.; Su, Y.S. Rechargeable lithium-sulfur batteries. Chem. Rev. 2014, 114, 11751–11787. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Shaw, L.L. Recent advances in lithium–sulfur batteries. J. Power Sources 2014, 267, 770–783. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, W.; Jang, J.; Manthiram, A. Sulfur-Embedded Activated Multichannel Carbon Nanofiber Composites for Long-Life, High-Rate Lithium-Sulfur Batteries. Adv. Energy Mater. 2016, 7, 1601943. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.; Chen, L. Reduced graphene oxide film as a shuttle-inhibiting interlayer in a lithium–sulfur battery. J. Power Sources 2013, 242, 65–69. [Google Scholar] [CrossRef]
- Jin, K.; Zhou, X.; Liu, Z. Graphene/Sulfur/Carbon Nanocomposite for High Performance Lithium-Sulfur Batteries. Nanomaterials 2015, 5, 1481–1492. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Jiang, S.; Cai, S.; Wang, X.; Xiang, K.; Ma, Z.; Song, P.; Fisher, A.C. Hierarchical porous carbon modified with ionic surfactants as efficient sulfur hosts for the high-performance lithium-sulfur batteries. Chem. Eng. J. 2017, 313, 404–414. [Google Scholar] [CrossRef]
- Gao, H.; Lu, Q.; Yao, Y.; Wang, X.; Wang, F. Significantly Raising the Cell Performance of Lithium Sulfur Battery via the Multifunctional Polyaniline Binder. Electrochim. Acta 2017, 232, 414–421. [Google Scholar] [CrossRef]
- Yao, H.; Yan, K.; Li, W.; Zheng, G.; Kong, D.; Seh, Z.W.; Narasimhan, V.K.; Liang, Z.; Cui, Y. Improved lithium–sulfur batteries with a conductive coating on the separator to prevent the accumulation of inactive S-related species at the cathode–separator interface. Energy Environ. Sci. 2014, 7, 3381–3390. [Google Scholar] [CrossRef]
- Chung, S.-H.; Manthiram, A. Bifunctional Separator with a Light-Weight Carbon-Coating for Dynamically and Statically Stable Lithium-Sulfur Batteries. Adv. Funct. Mater. 2014, 24, 5299–5306. [Google Scholar] [CrossRef]
- Bai, S.; Liu, X.; Zhu, K.; Wu, S.; Zhou, H. Metal–organic framework-based separator for lithium–sulfur batteries. Nano Energy 2016, 1, 16094. [Google Scholar] [CrossRef]
- Song, R.; Fang, R.; Wen, L.; Shi, Y.; Wang, S.; Li, F. A trilayer separator with dual function for high performance lithium–sulfur batteries. J. Power Sources 2016, 301, 179–186. [Google Scholar] [CrossRef]
- Guo, J.; Xu, Y.; Wang, C. Sulfur-impregnated disordered carbon nanotubes cathode for lithium-sulfur batteries. Nano Lett. 2011, 11, 4288–4294. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Manthiram, A. Hydroxylated N-doped carbon nanotube-sulfur composites as cathodes for high-performance lithium-sulfur batteries. J. Power Sources 2017, 343, 54–59. [Google Scholar] [CrossRef]
- Chung, S.H.; Manthiram, A. High-Performance Li-S Batteries with an Ultra-lightweight MWCNT-Coated Separator. J. Phys. Chem. Lett. 2014, 5, 1978–1983. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Dong, L.; Fang, J.; Xiong, C. Property-Structure Relationship of Nanoscale Ionic Materials Based on Multiwalled Carbon Nanotubes. ACS Nano 2010, 4, 5797–5806. [Google Scholar] [CrossRef] [PubMed]
- Bourlinos, A.B.; Herrera, R.; Chalkias, N.; Jiang, D.D.; Zhang, Q.; Archer, L.A.; Giannelis, E.P. Surface Functionalized Nanoparticles with Liquid-like Behavior. Adv. Mater. 2005, 17, 234–237. [Google Scholar] [CrossRef]
- Li, J.; Huang, Y.; Zhang, S.; Jiang, W.; Wang, X.; Guo, Y.; Jia, D.; Wang, L. Decoration of Silica Nanoparticles on Polypropylene Separator for Lithium–Sulfur Batteries. ACS Appl. Mater. Interfaces 2017, 9, 7499–7504. [Google Scholar] [CrossRef] [PubMed]
- Zu, C.; Su, Y.S.; Fu, Y.; Manthiram, A. Improved lithium-sulfur cells with a treated carbon paper interlayer. Phys. Chem. Chem. Phys. 2013, 15, 2291–2297. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Chen, J.; Chen, R.; Wu, S.; Li, L.; Chen, S.; Zhao, T. Sulfur/Polythiophene with a Core/Shell Structure: Synthesis and Electrochemical Properties of the Cathode for Rechargeable Lithium Batteries. J. Phys. Chem. C 2011, 115, 6057–6063. [Google Scholar] [CrossRef]
- Sun, J.; Huang, Y.; Wang, W.; Yu, Z.; Wang, A.; Yuan, K. Application of gelatin as a binder for the sulfur cathode in lithium–sulfur batteries. Electrochim. Acta 2008, 53, 7084–7088. [Google Scholar] [CrossRef]
- Choi, J.-W.; Cheruvally, G.; Kim, D.-S.; Ahn, J.-H.; Kim, K.-W.; Ahn, H.-J. Rechargeable lithium/sulfur battery with liquid electrolytes containing toluene as additive. J. Power Sources 2008, 183, 441–445. [Google Scholar] [CrossRef]
- Fu, Y.; Manthiram, A. Orthorhombic Bipyramidal Sulfur Coated with Polypyrrole Nanolayers as a Cathode Material for Lithium–Sulfur Batteries. J. Phys. Chem. C 2012, 116, 8910–8915. [Google Scholar] [CrossRef]
- Zhou, G.; Pei, S.; Li, L.; Wang, D.W.; Wang, S.; Huang, K.; Yin, L.C.; Li, F.; Cheng, H.M. A graphene-pure-sulfur sandwich structure for ultrafast, long-life lithium-sulfur batteries. Adv. Mater. 2014, 26, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Ma, J.; Li, B.; Zuo, Y.; Xia, D. Enhanced cycle performance of lithium-sulfur batteries using a separator modified with a PVDF-C layer. ACS Appl. Mater. Interfaces 2014, 6, 20276–20281. [Google Scholar] [CrossRef] [PubMed]

| Separator | Shrinkage of Separator (%) | ||||
|---|---|---|---|---|---|
| 120 °C | 130 °C | 140 °C | 150 °C | 155 °C | |
| Blank | 6.7 | 10.3 | 21.7 | 38.3 | 43.0 |
| CNTs-coated | 5.0 | 9.3 | 16.0 | 25.0 | 35.0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Wu, X.; Wang, S.; Tang, Z.; Yang, Q.; Hu, G.-H.; Xiong, C. Flexible Carbon Nanotube Modified Separator for High-Performance Lithium-Sulfur Batteries. Nanomaterials 2017, 7, 196. https://doi.org/10.3390/nano7080196
Liu B, Wu X, Wang S, Tang Z, Yang Q, Hu G-H, Xiong C. Flexible Carbon Nanotube Modified Separator for High-Performance Lithium-Sulfur Batteries. Nanomaterials. 2017; 7(8):196. https://doi.org/10.3390/nano7080196
Chicago/Turabian StyleLiu, Bin, Xiaomeng Wu, Shan Wang, Zhen Tang, Quanling Yang, Guo-Hua Hu, and Chuanxi Xiong. 2017. "Flexible Carbon Nanotube Modified Separator for High-Performance Lithium-Sulfur Batteries" Nanomaterials 7, no. 8: 196. https://doi.org/10.3390/nano7080196




