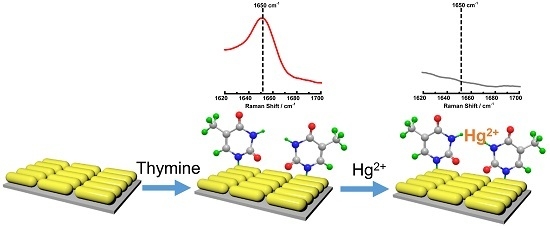
A Simple and Highly Sensitive Thymine Sensor for Mercury Ion Detection Based on Surface Enhanced Raman Spectroscopy and the Mechanism Study
Abstract
:1. Introduction
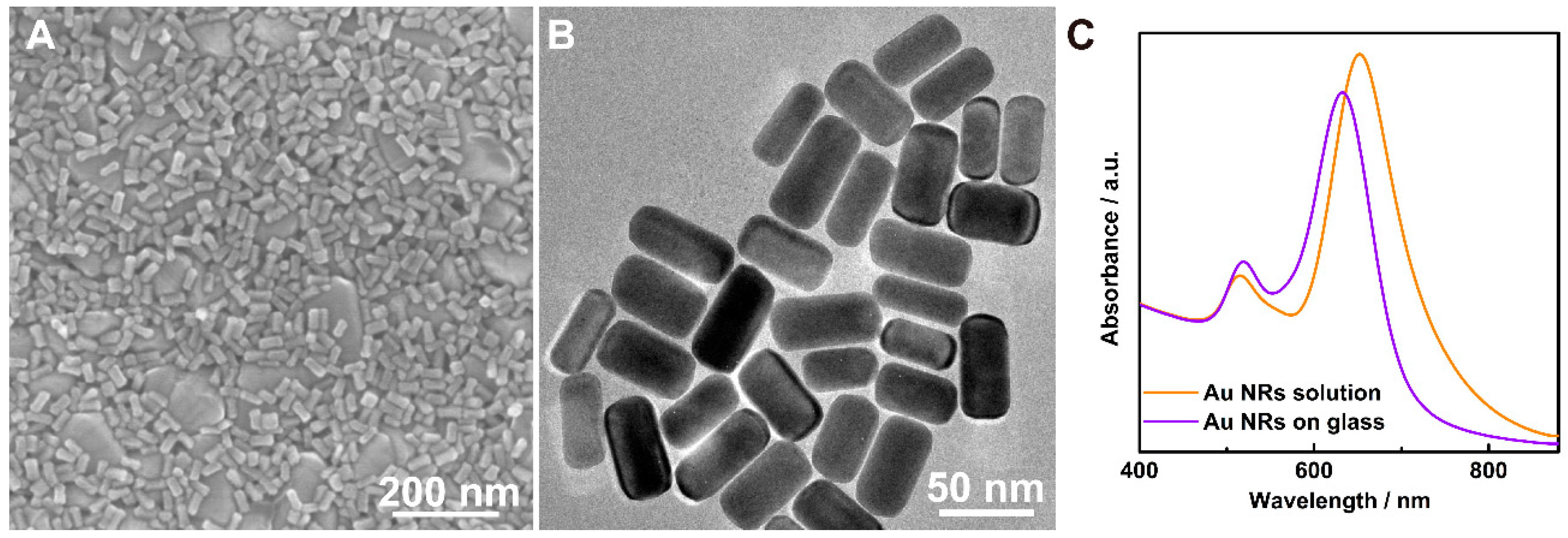
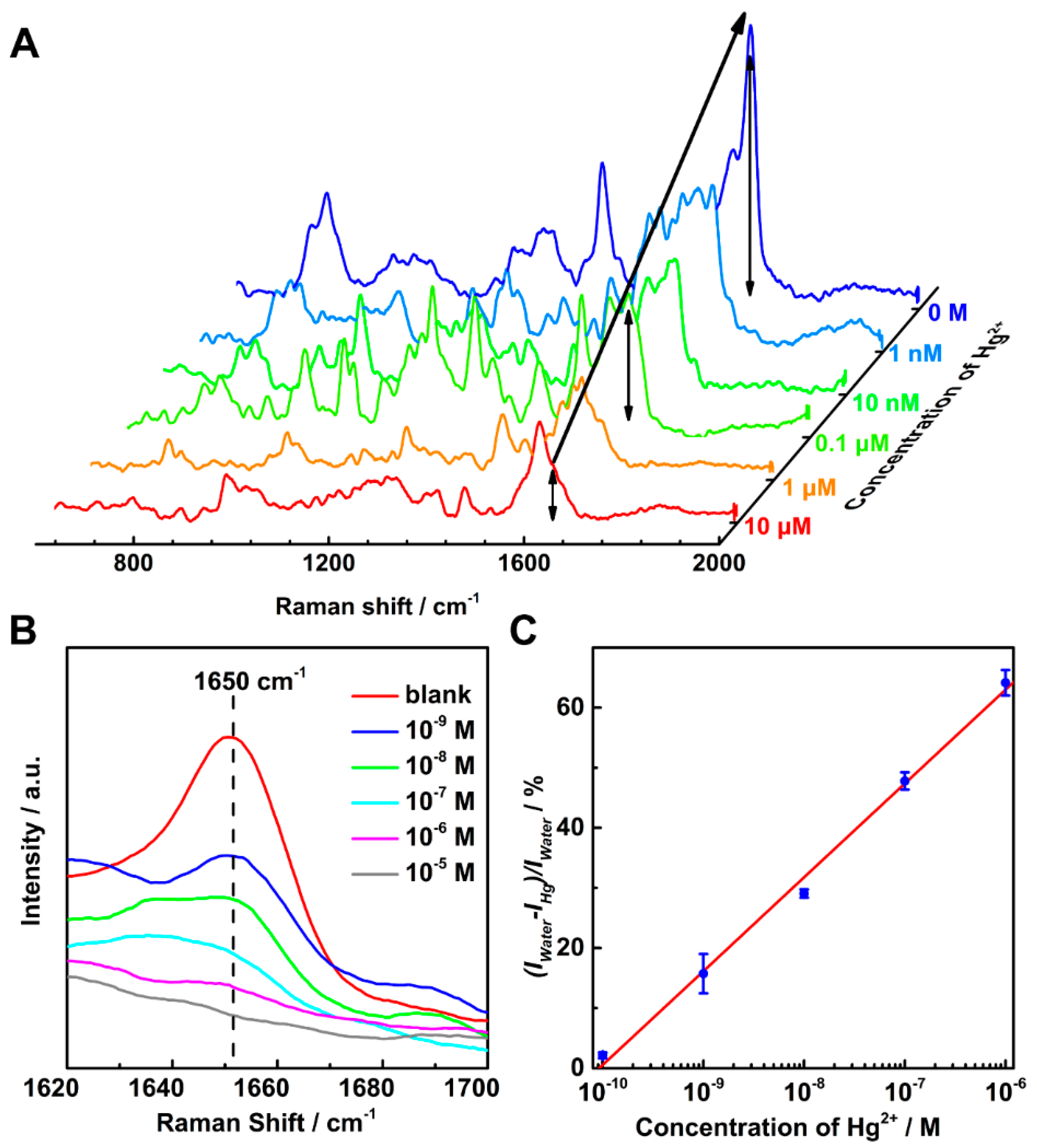
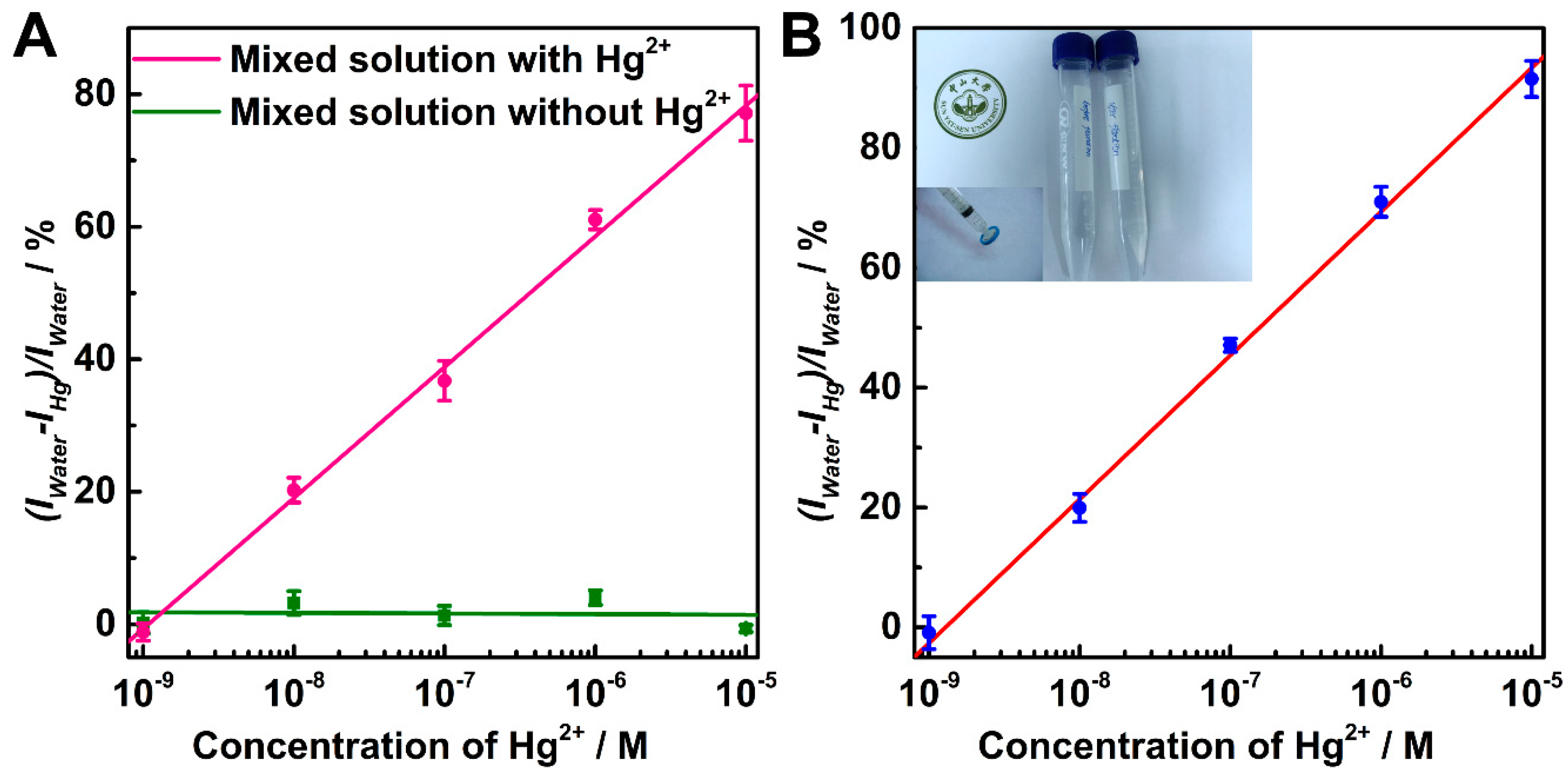
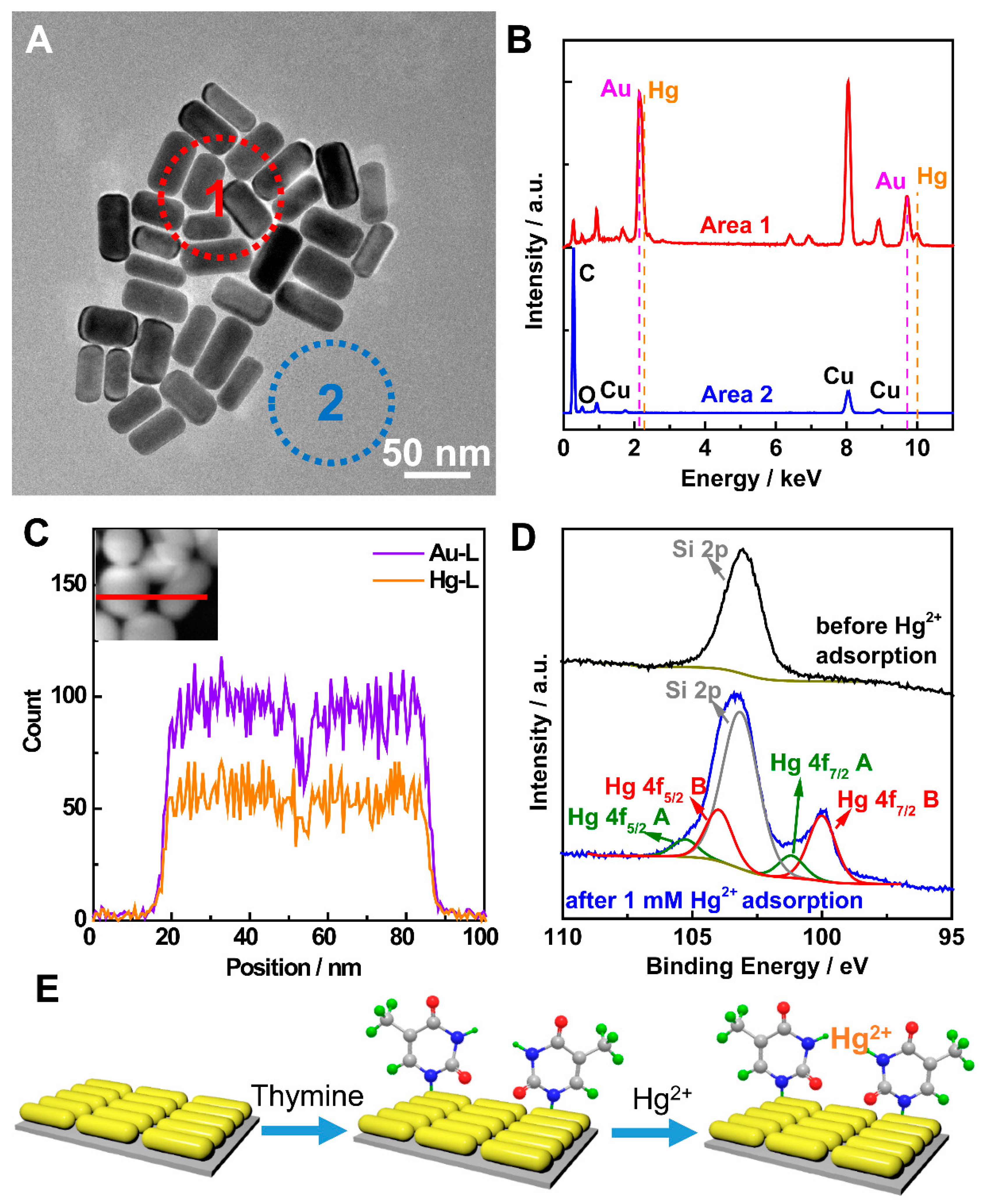
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Preparation of Au NRs
3.3. Fabrication of Thymine Modified Au NRs@T
3.4. Characterization
3.5. Detection of Hg2+ Ion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Forzani, E.S.; Zhang, H.Q.; Chen, W.; Tao, N.J. Detection of heavy metal ions in drinking water using a high-resolution differential surface plasmon resonance sensor. Environ. Sci. Technol. 2005, 39, 1257–1262. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.; Jernelov, A. Biological methylation of mercury in aquatic organisms. Nature 1969, 223, 753–754. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, T.W. The three modern faces of mercury. Environ. Health Perspect. 2002, 110 (Suppl. 1), 11–23. [Google Scholar] [CrossRef] [PubMed]
- Bisson, T.M.; Xu, Z. Potential hazards of brominated carbon sorbents for mercury emission control. Environ. Sci. Technol. 2015, 49, 2496–2502. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, I.; Sumner, A.; Spitz, L.; Cornblath, D.; Uzzell, B.; Ship, I.; Bloch, P. Neurophysiological and neuropsychological function in mercury-exposed dentists. Lancet 1982, 319, 1147–1150. [Google Scholar] [CrossRef]
- Lee, J.S.; Mirkin, C.A. Chip-based scanometric detection of mercuric ion using DNA-functionalized gold nanoparticles. Anal. Chem. 2008, 80, 6805–6808. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Qu, W.; Chen, W.; Zhang, W.; Wang, Z.; Jiang, X. Highly sensitive, colorimetric detection of mercury (II) in aqueous media by quaternary ammonium group-capped gold nanoparticles at room temperature. Anal. Chem. 2010, 82, 9606–9610. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Hu, L.-L.; Chen, M.-L.; Wang, J.-H. A FRET ratiometric fluorescence sensing system for mercury detection and intracellular colorimetric imaging in live hela cells. Biosens. Bioelectron. 2013, 49, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.C.; Yin, B.C. Highly sensitive detection of mercury (II) ions by fluorescence polarization enhanced by gold nanoparticles. Angew. Chem. Int. Ed. 2008, 47, 8386–8389. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-W.; Jiang, S.-J. Speciation of mercury by reversed-phase liquid chromatography with inductively coupled plasma mass spectrometric detection. J. Anal. Atom. Spectrom. 1993, 8, 681–686. [Google Scholar] [CrossRef]
- Bushee, D.S. Speciation of mercury using liquid chromatography with detection by inductively coupled plasma mass spectrometry. Analyst 1988, 113, 1167–1170. [Google Scholar] [CrossRef]
- Jarzynska, G.; Falandysz, J. The determination of mercury in mushrooms by CV-AAS and ICP-AES techniques. J. Environ. Sci. Health A 2011, 46, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wieckowska, A.; Willner, I. Optical analysis of Hg2+ ions by oligonucleotide-gold-nanoparticle hybrids and DNA-based machines. Angew. Chem. Int. Ed. 2008, 47, 3927–3931. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Fu, Y.; Zhang, W.; Ye, S.; Zhang, H.; Xie, F.; Gong, L.; Wei, Z.; Jin, H.; Chen, J. Highly sensitive detection of glucose: A quantitative approach employing nanorods assembled plasmonic substrate. Talanta 2017, 165, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Liu, H.; Yang, L.; Liu, J. Sensitive and selective sers probe for trivalent chromium detection using citrate attached gold nanoparticles. Nanoscale 2012, 4, 6442–6448. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.-J.; Lei, Z.-C.; Li, J.; Zong, C.; Yang, C.J.; Ren, B. Label-free surface-enhanced Raman spectroscopy detection of DNA with single-base sensitivity. J. Am. Chem. Soc. 2015, 137, 5149–5154. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Dai, Z.; Si, S.; Zhang, X.; Wu, W.; Deng, H.; Wang, F.; Xiao, X.; Jiang, C. Ultrasensitive sers substrate integrated with uniform subnanometer scale “hot spots” created by a graphene spacer for the detection of mercury ions. Small 2017, 13. [Google Scholar] [CrossRef]
- Zhang, L.; Chang, H.; Hirata, A.; Wu, H.; Xue, Q.K.; Chen, M. Nanoporous gold based optical sensor for sub-ppt detection of mercury ions. ACS Nano 2013, 7, 4595–4600. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Zhao, M.; Sheng, Y.; Bentolila, L.A.; Tang, Y. Detection of mercury ion by infrared fluorescent protein and its hydrogel-based paper assay. Anal. Chem. 2011, 83, 2324–2329. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, Z.; Qu, W.; Shao, H.; Jiang, X. Colorimetric detection of mercury, lead and copper ions simultaneously using protein-functionalized gold nanoparticles. Biosens. Bioelectron. 2011, 26, 4064–4069. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Tang, Y.; Wang, L.; Zhang, J.; Song, S.; Fan, C.; Wang, S. Optical detection of mercury (II) in aqueous solutions by using conjugated polymers and label-free oligonucleotides. Adv. Mater. 2007, 19, 1471–1474. [Google Scholar] [CrossRef]
- Ono, A.; Togashi, H. Highly selective oligonucleotide-based sensor for mercury (II) in aqueous solutions. Angew. Chem. Int. Ed. 2004, 43, 4300–4302. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, Y.X.; Jin, J.Y.; Yang, R.H. Gold nanoparticle-based colorimetric and “turn-on” fluorescent probe for mercury (II) ions in aqueous solution. Anal. Chem. 2008, 80, 9021–9028. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.N.; Ren, W.X.; Kim, J.S.; Yoon, J. Fluorescent and colorimetric sensors for detection of lead, cadmium, and mercury ions. Chem. Soc. Rev. 2012, 41, 3210–3244. [Google Scholar] [CrossRef] [PubMed]
- Mardis, E.R. A decade’s perspective on DNA sequencing technology. Nature 2011, 470, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Z.; Han, J.; Zhang, J.; Zhao, H.; Jiang, L. Method for detection of Hg2+ based on the specific thymine-Hg2+-Thymine interaction in the DNA hybridization on the surface of quartz crystal microbalance. Colloid Surf. B 2011, 87, 289–292. [Google Scholar] [CrossRef] [PubMed]
- EPA, U.S. Mercury Update: Impact on Fish Advisories; United States Environmental Protection Agency, Office of Water: Washington, DC, USA, 2001; EPA-823-F-01-011.
- Wen, J.; Zhang, H.; Chen, H.; Zhang, W.; Chen, J. Stretchable plasmonic substrate with tunable resonances for surface-enhanced Raman spectroscopy. J. Opt. 2015, 17, 114015. [Google Scholar] [CrossRef]
- Madzharova, F.; Heiner, Z.; Guhlke, M.; Kneipp, J. Surface-enhanced hyper-Raman spectra of adenine, guanine, cytosine, thymine, and uracil. J. Phys. Chem. C 2016, 120, 15415–15423. [Google Scholar] [CrossRef] [PubMed]
- Etchegoin, P.G.; Meyer, M.; Blackie, E.; Le Ru, E.C. Statistics of single-molecule surface enhanced Raman scattering signals: Fluctuation analysis with multiple analyte techniques. Anal. Chem. 2007, 79, 8411–8415. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Seong, N.H.; Dlott, D.D. Measurement of the distribution of site enhancements in surface-enhanced Raman scattering. Science 2008, 321, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Palla, M.; Bosco, F.G.; Rindzevicius, T.; Alstrom, T.S.; Schmidt, M.S.; Boisen, A.; Ju, J.; Lin, Q. Surface-enhanced Raman spectroscopy based quantitative bioassay on aptamer-functionalized nanopillars using large-area Raman mapping. ACS Nano 2013, 7, 5350–5359. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Hu, Z.; Chang, X.; He, Q.; Tu, Z.; Zhang, L.; Gao, R. Chemically modified activated carbon with s-benzyldithiocarbazate as a new solid-phase extractant for preconcentration of mercury prior to ICP-AES determination. Int. J. Environ. Anal. Chem. 2011, 91, 1024–1034. [Google Scholar] [CrossRef]
- Kang, T.; Yoo, S.M.; Kang, M.; Lee, H.; Kim, H.; Lee, S.Y.; Kim, B. Single-step multiplex detection of toxic metal ions by au nanowires-on-chip sensor using reporter elimination. Lab Chip 2012, 12, 3077–3081. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.D.; Li, Y.F.; Ling, J.; Huang, C.Z. A localized surface plasmon resonance light-scattering assay of mercury (II) on the basis of Hg2+-DNA complex induced aggregation of gold nanoparticles. Environ. Sci. Technol. 2009, 43, 5022–5027. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ni, Y.; Kokot, S. The use of DNA self-assembled gold nano-rods for novel analysis of lead and/or mercury in drinking water. Anal. Met. 2015, 7, 4514–4520. [Google Scholar] [CrossRef]
- Han, D.; Lim, S.Y.; Kim, B.J.; Piao, L.; Chung, T.D. Mercury (II) detection by sers based on a single gold microshell. Chem. Commun. 2010, 46, 5587. [Google Scholar] [CrossRef] [PubMed]
- Behra, P.; Bonnissel-Gissinger, P.; Alnot, M.; Revel, R.; Ehrhardt, J.J. XPS and XAS study of the sorption of Hg (II) onto pyrite. Langmuir 2001, 17, 3970–3979. [Google Scholar] [CrossRef]
| Raman Shift (cm−1) | Assignment |
|---|---|
| 1650 | C=O stretching vibration |
| 1435 | N–H deformation |
| 1369 | N–H and C–H in-plane bending |
| 1230 | Ring stretch |
| 1013 | Ring stretch |
| 984 | N–H wagging |
| 804 | Ring deformation bend |
| 738 | Ring breathing |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Ye, S.-B.; Fu, Y.; Zhang, W.; Xie, F.; Gong, L.; Fang, P.-P.; Chen, J.; Tong, Y. A Simple and Highly Sensitive Thymine Sensor for Mercury Ion Detection Based on Surface Enhanced Raman Spectroscopy and the Mechanism Study. Nanomaterials 2017, 7, 192. https://doi.org/10.3390/nano7070192
Yang H, Ye S-B, Fu Y, Zhang W, Xie F, Gong L, Fang P-P, Chen J, Tong Y. A Simple and Highly Sensitive Thymine Sensor for Mercury Ion Detection Based on Surface Enhanced Raman Spectroscopy and the Mechanism Study. Nanomaterials. 2017; 7(7):192. https://doi.org/10.3390/nano7070192
Chicago/Turabian StyleYang, Hao, Sui-Bo Ye, Yu Fu, Weihong Zhang, Fangyan Xie, Li Gong, Ping-Ping Fang, Jian Chen, and Yexiang Tong. 2017. "A Simple and Highly Sensitive Thymine Sensor for Mercury Ion Detection Based on Surface Enhanced Raman Spectroscopy and the Mechanism Study" Nanomaterials 7, no. 7: 192. https://doi.org/10.3390/nano7070192