Growth Mechanism Studies of ZnO Nanowires: Experimental Observations and Short-Circuit Diffusion Analysis
Abstract
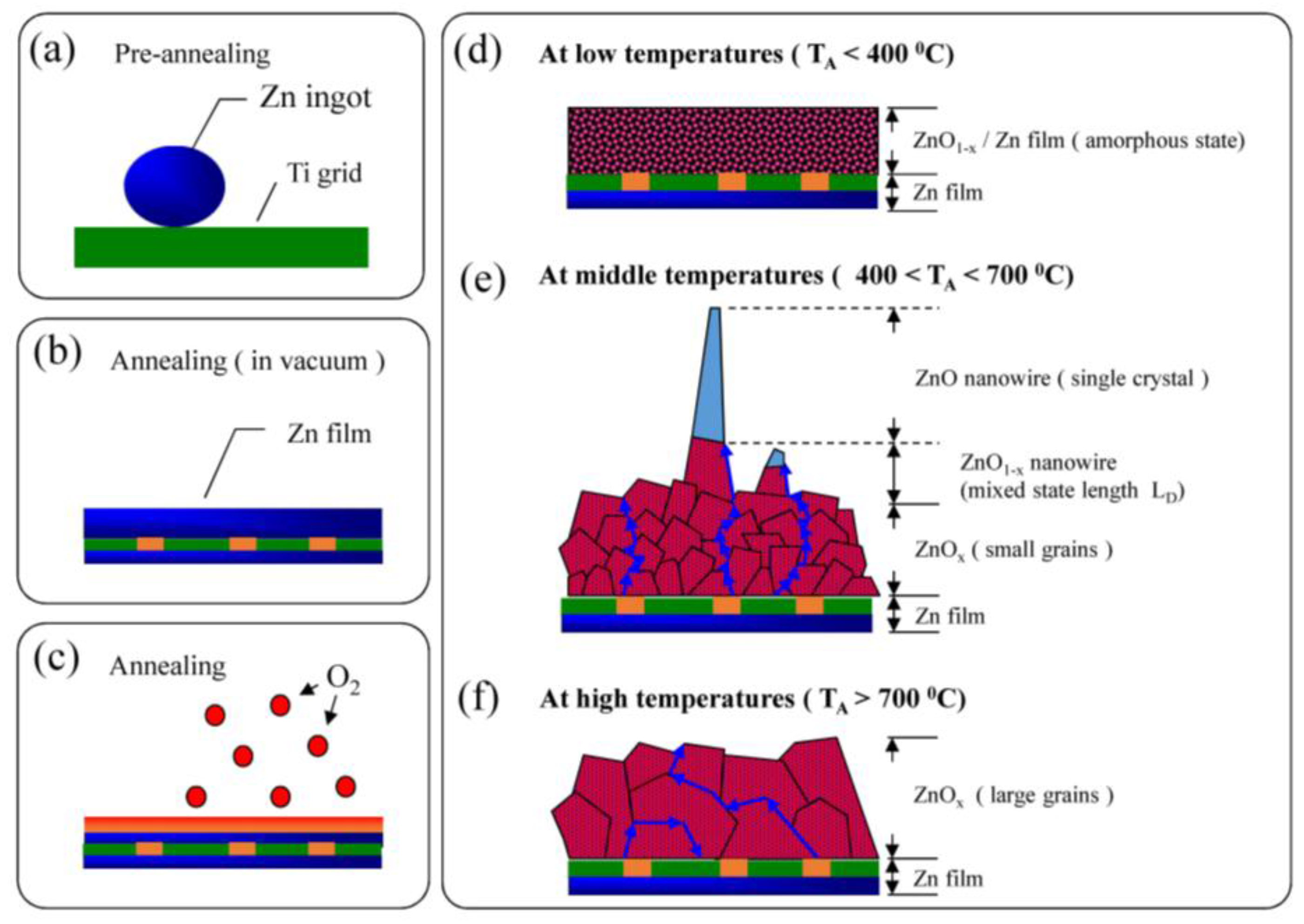
:1. Introduction
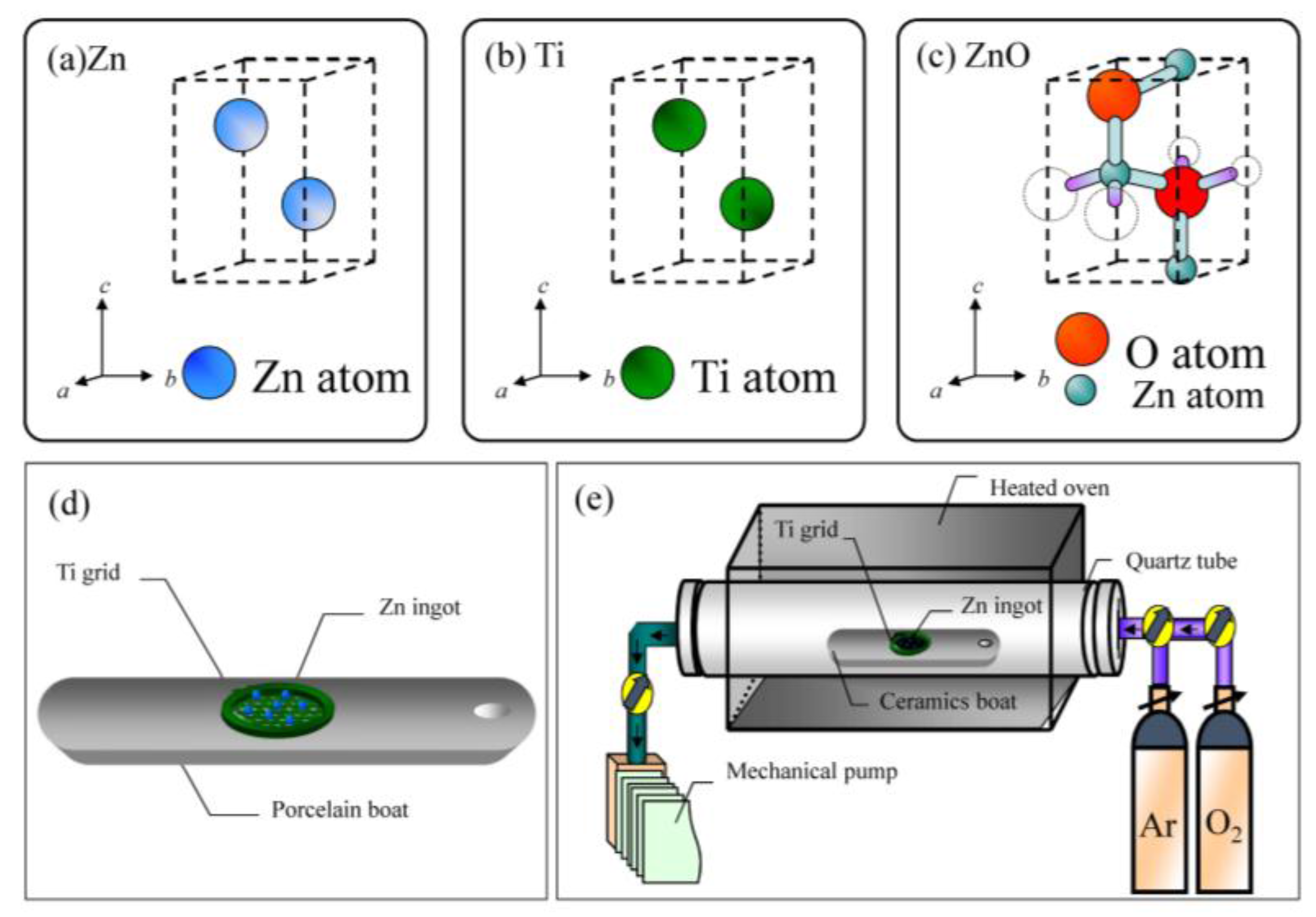
2. Materials and Methods
3. Results and Discussion
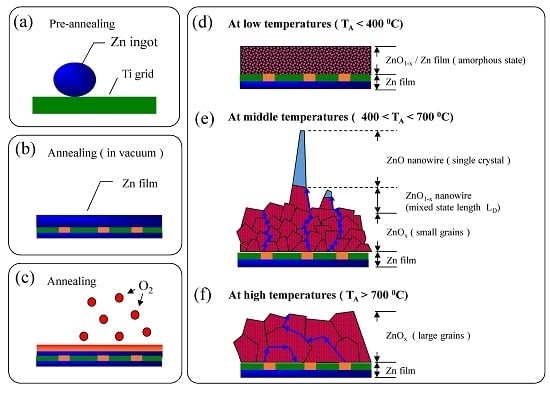
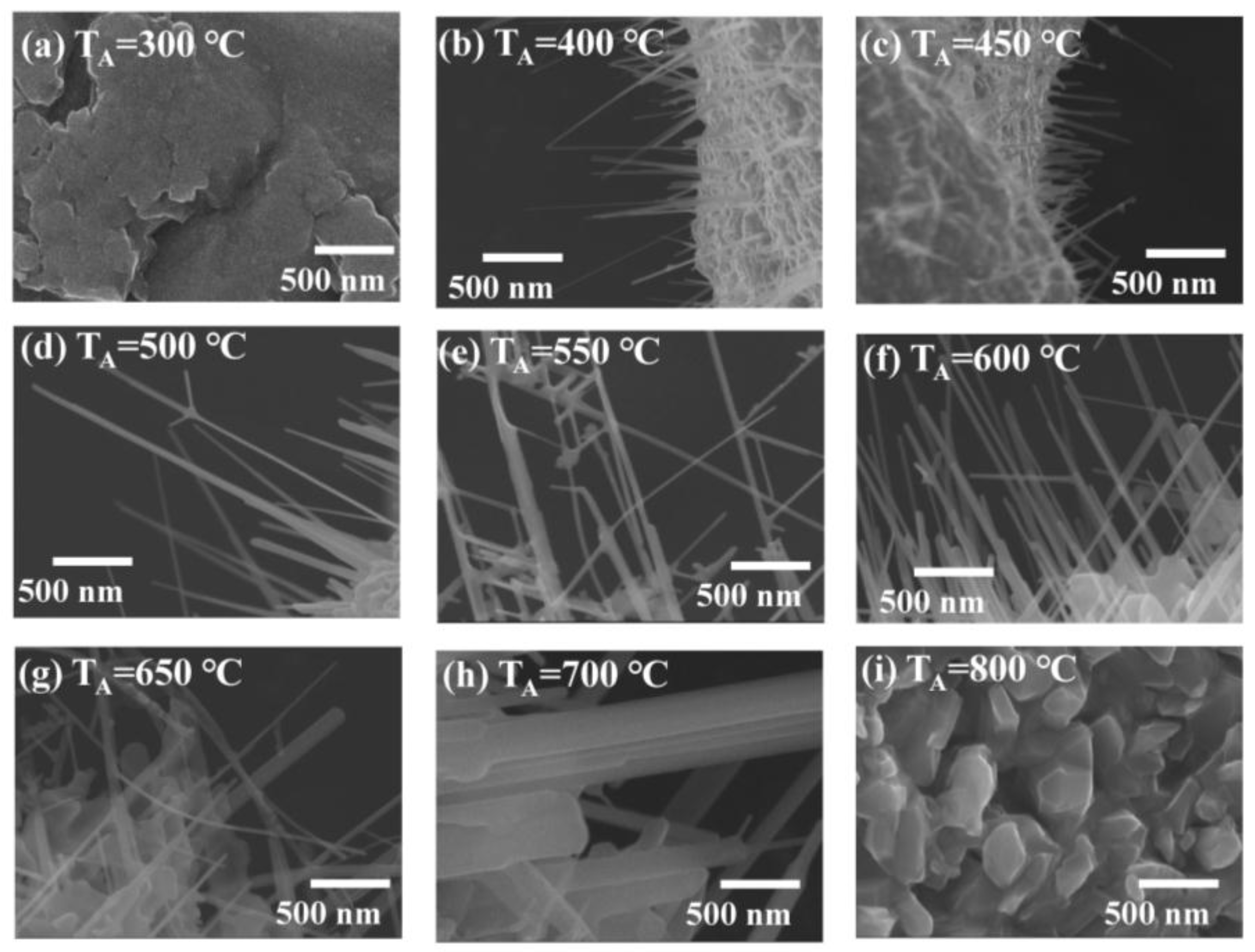
3.1. Morphological Analysis of ZnO Nanowire
3.2. Crystal Structure Analysis of ZnO Nanowires
3.3. Two-Dimensional EDS Mapping
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References and Note
- Janotti, A.; Van de Walle, C.G. Fundamentals of zinc oxide as a semiconductor. Rep. Prog. Phys. 2009, 72, 126501. [Google Scholar] [CrossRef]
- Morkoç, H.; Özgür, Ü. Zinc Oxide: Fundamentals, Materials and Device Technology, 1st ed.; Wiley-VCH: Weinheim, Germany, 2009; p. 477. [Google Scholar]
- Shih, H.Y.; Chen, Y.T.; Huang, N.H.; Wei, C.M.; Chen, Y.F. Size-dependent photoelastic effect in ZnO nanorods. Appl. Phys. Lett. 2009, 94, 021908. [Google Scholar] [CrossRef]
- Huang, M.H.; Mao, S.; Feick, H.; Yan, H.; Wu, Y.; Kind, H.; Weber, E.; Russo, R.; Yang, P. Room-Temperature Ultraviolet Nanowire Nanolasers. Science 2001, 292, 1897–1899. [Google Scholar] [CrossRef] [PubMed]
- Heo, Y.W.; Norton, D.P.; Tien, L.C.; Kwon, Y.; Kang, B.S.; Ren, F.; Pearton, S.J.; LaRoche, J.R. ZnO nanowire growth and devices. Mater. Sci. Eng. R Rep. 2004, 47, 1–47. [Google Scholar] [CrossRef]
- Wang, R.P.; Xu, G.; Jin, P. Size dependence of electron-phonon coupling in ZnO nanowires. Phys. Lett. B 2004, 69, 113303. [Google Scholar] [CrossRef]
- Dang, H.Y.; Wang, J.; Fan, S.S. The synthesis of metal oxide nanowires by directly heating metal samples in appropriate oxygen atmospheres. Nanotechnology 2003, 14, 738–741. [Google Scholar] [CrossRef]
- Zhang, B.; Zhou, S.M.; Wang, H.W.; Du, Z.L. Raman scattering and photoluminescence of Fe-doped ZnO nanocantilever arrays. Chin. Sci. Bull. 2008, 53, 1639–1643. [Google Scholar]
- Kar, S.; Pal, B.N.; Chaudhuri, S.; Chakravorty, D. One-Dimensional ZnO Nanostructure Arrays: Synthesis and Characterization. J. Phys. Chem. B 2006, 110, 4605–4611. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.X.; Wang, Z.L. Mesoporous Polyhedral Cages and Shells Formed by Textured Self-Assembly of ZnO Nanocrystals. J. Am. Chem. Soc. 2003, 125, 11299–11305. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-L. Nanostructures of zinc oxide. Materialstoday 2004, 7, 26. [Google Scholar] [CrossRef]
- Hu, J.Q.; Li, Q.; Meng, X.M.; Lee, C.S.; Lee, S.T. Thermal reduction route to the fabrication of coaxial Zn/ZnO nanocables and ZnO nanotubes. Chem. Mater. 2003, 15, 305–308. [Google Scholar] [CrossRef]
- Agrawal, R.; Peng, B.; Espinosa, H.D. Elasticity Size Effects in ZnO Nanowires—A Combined Experimental-Computational Approach. Nano Lett. 2009, 8, 3668. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, R.; Peng, B.; Espinosa, H.D. Experimental-Computational Investigation of ZnO nanowires Strength and Fracture. Nano Lett. 2009, 9, 4177. [Google Scholar] [CrossRef] [PubMed]
- Chou, M.H.; Liu, S.B.; Huang, C.Y.; Wu, S.Y.; Cheng, C.L. Confocal Raman spectroscopic mapping studies on a single CuO nanowire. Appl. Surf. Sci. 2008, 254, 7539–7543. [Google Scholar] [CrossRef]
- Ng, H.T.; Chen, B.; Li, J.; Han, J.; Meyyappan, M. Optical properties of single-crystalline ZnO nanowires on m-sapphire. Appl. Phys. Lett. 2003, 82, 2023. [Google Scholar] [CrossRef]
- Yamg, P.; Yan, H.; Mao, S.; Russo, R.; Johnson, T.; Saykally, R.; Morris, N.; Pham, J.; He, R.; Choi, H.-J. Controlled growth of ZnO nanowires and their optical properties. Adv. Funct. Mater. 2002, 12, 323. [Google Scholar] [CrossRef]
- Wan, Q.; Lin, C.L.; Yu, X.B.; Wang, T.H. Room-temperature hydrogen storage characteristics of ZnO nanowires. Appl. Phys. Lett. 2004, 84, 124. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Zhu, Z. Ammonia sensing characteristics of ZnO nanowires studied by quartz crystal microbalance. Appl. Surf. Sci. 2006, 15, 2404. [Google Scholar] [CrossRef]
- Chang, P.C.; Fan, Z.; Wang, D.; Tseng, W.Y.; Chiou, W.A.; Hong, J.; Lu, J.G. ZnO Nanowires Synthesized by Vapor Trapping CVD Method. Chem. Mater. 2004, 16, 5133. [Google Scholar] [CrossRef]
- Fan, D.H.; Shen, W.Z.; Zheng, M.J.; Zhu, Y.F.; Lu, J.J. Integration of ZnO Nanotubes with Well-Ordered Nanorods through Two-Step Thermal Evaporation Approach. J. Phys. Chem. C 2007, 111, 9116. [Google Scholar] [CrossRef]
- Supplementary materials at https://www.mdpi.com/2079-4991/7/7/188/s1 for the detail of a list of space groups and lattice parameters of Zn, Ti and ZnO (Table S1); Optical images for various annealing temperature of samples (Figure S1); TEM image and corresponding selected-area electron pattern. (Figure S2).
- Leung, Y.H.; Djurišić, A.B.; Gao, J.; Xie, M.H.; Wei, Z.F.; Xu, S.J.; Chan, W.K. Zinc oxide ribbon and comb structures: Synthesis and optical properties. Chem. Phys. Lett. 2004, 394, 452–457. [Google Scholar] [CrossRef]
- Huang, H.; Yang, S.; Gong, J.; Liu, H.; Duan, J.; Zhao, X.; Zhang, R. Controllable Assembly of Aligned ZnO Nanowires/Belts Arrays. J. Phys. Chem. B 2005, 109, 20746–20750. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Choi, H.J.; Choi, Y.J.; Sohn, S.H.; Park, J.G. Ultrawide ZnO nanosheets. J. Mater. Chem. 2003, 14, 35–36. [Google Scholar] [CrossRef]
- Xu, J.; Pan, Q.; Shun, Y.; Tian, Z. Grain size control and gas sensing properties of ZnO gas sensor. Sens. Actuators 2000, 66, 277–279. [Google Scholar] [CrossRef]
- Giri, P.K.; Bhattacharyya, S.; Singh, D.K.; Kesavamoorthy, R.; Panigrahi, B.K.; Nair, K.G.M. Correlation between microstructure and optical properties of ZnO nanoparticles synthesized by ball milling. J. Appl. Phys. 2007, 102, 093515. [Google Scholar] [CrossRef]
- Zhou, S.M.; Zhang, X.H.; Meng, X.M.; Fan, X.; Wu, S.K.; Lee, S.T. Preparation and photoluminescence of Sc-doped ZnO nanowires. Phys. E Low-Dimens. Syst. Nanostruct. 2005, 25, 587–591. [Google Scholar] [CrossRef]
- Vayssieres, L. Growth of Arrayed Nanorods and Nanowires of ZnO from Aqueous Solutions. Adv. Mater. 2003, 15, 464–466. [Google Scholar] [CrossRef]
- Geng, C.; Jiang, Y.; Yao, Y.; Meng, X.; Zapien, J.A.; Lee, C.S.; Lifshitz, Y.; Lee, S.T. Well-Aligned ZnO Nanowire Arrays Fabricated on Silicon Substrates. Adv. Funct. Mater. 2004, 14, 589–594. [Google Scholar] [CrossRef]
- Zheng, M.J.; Zhang, L.D.; Li, G.H.; Shen, W.Z. Fabrication and optical properties of large-scaleuniform zinc oxide nanowire arrays by one-step electrochemical deposition technique. Chem. Phys. Lett. 2002, 363, 123–128. [Google Scholar] [CrossRef]
- Ren, S.; Bai, Y.F.; Chen, J.; Deng, S.Z.; Xu, N.S.; Wu, Q.B.; Yang, S. Catalyst-free synthesis of ZnO nanowire arrays on zinc substrate by low temperature thermal oxidation. Mater. Lett. 2007, 61, 666–670. [Google Scholar] [CrossRef]
- Rackauskas, S.; Nasibulin, A.G.; Jiang, H.; Tian, Y.; Statkute, G.; Shandakov, S.D.; Lipsanen, H.; Kauppinen, E.I. Mechanistic investigation of ZnO nanowire growth. Appl. Phys. Lett. 2009, 95, 183114. [Google Scholar] [CrossRef]
- Barriga-Castro, E.D.; Mendoza-Resendéz, R.; García, J.; Pridab, V.M.; Luna, C. Pseudo-monocrystalline properties of cylindrical nanowires confinedly grown by electrodeposition in nanoporous alumina templates. RSC Adv. 2017, 7, 13817. [Google Scholar] [CrossRef]
- Shih, P.H.; Hung, H.J.; Ma, Y.R.; Wu, S.Y. Tuning the dimensionality of ZnO nanowires through thermal treatment: An investigation of growth mechanism. Nanoscale Res. Lett. 2012, 7, 354. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.J.; Scholz, R.; Kolb, F.M.; Zacharias, M. Two-dimensional dendritic ZnO nanowires from oxidation of Zn microcrystals. Appl. Phys. Lett. 2004, 85, 4142. [Google Scholar] [CrossRef]
- Gandhi, A.C.; Hung, H.J.; Shih, P.H.; Cheng, C.L.; Ma, Y.R.; Wu, S.Y. In Situ Confocal Raman Mapping Study of a Single Ti-Assisted ZnO Nanowire. Nanoscale Res. Lett. 2010, 5, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.L.; Ma, Y.R.; Chou, M.H.; Huang, C.Y.; Yeh, V.; Wu, S.Y. Direct observation of short-circuit diffusion during the formation of a single cupric oxide nanowire. Nanotechnology 2007, 18, 245604. [Google Scholar] [CrossRef]
- Herchl, R.; Khoi, N.N.; Homma, T.; Smeltzer, W.W. Short-circuit diffusion in the growth of nickel oxide scales on nickel crystal faces. Oxid. Met. 1972, 4, 35–49. [Google Scholar] [CrossRef]
- Li, S.B.; Bei, G.P.; Zhai, H.X.; Zhang, Z.L.; Zhou, Y.; Li, C.W. The origin of driving force for the formation of Sn whiskers at room temperature. J. Mater. Res. 2007, 22, 3226. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Y.; Cai, R.; Jiang, Q.; Wang, J.; Li, B.; Sharma, A.; Zhou, G. The origin of hematite nanowire growth during the thermal oxidation of iron. Mater. Sci. Eng. B 2012, 177, 327. [Google Scholar]
- Xu, C.H.; Woo, C.H.; Shi, S.Q. Formation of CuO nanowires on Cu foil. Chem. Phys. Lett. 2004, 399, 62. [Google Scholar] [CrossRef]
- Lu, Y.; Zhou, G. Enhanced CuO Nanowire Formation by Thermal Oxidation of Roughened Copper. J. Electrochem. Soc. 2012, 159, C205. [Google Scholar]
- Metselaar, R. Diffusion in solids. Part I: Introduction to the theory of diffusion. J. Mater. Educ. 1984, 6, 229. [Google Scholar]
- Gao, F.; Chino, N.; Naik, S.P.; Sasaki, Y.; Okubo, T. Photoelectric properties of nano-ZnO fabricated in mesoporous silica film. Mater. Lett. 2007, 61, 3179–3184. [Google Scholar] [CrossRef]
- Tomlins, G.W.; Routbort, J.L.; Mason, T.O. Zinc self-diffusion, electrical properties, and defect structure of undoped, single crystal zinc oxide. J. Appl. Phys. 2000, 87, 117. [Google Scholar] [CrossRef]
- Kofstad, P. Nonstoichiometry, Diffusion, and Electrical Conductivity in Binary Metal Oxides; Wiley: New York, NY, USA, 1972. [Google Scholar]
- Brass, A.M.; Chanfreau, A. Accelerated diffusion of hydrogen along grain boundaries in nickel. Acta Mater. 1996, 44, 3823–3831. [Google Scholar] [CrossRef]
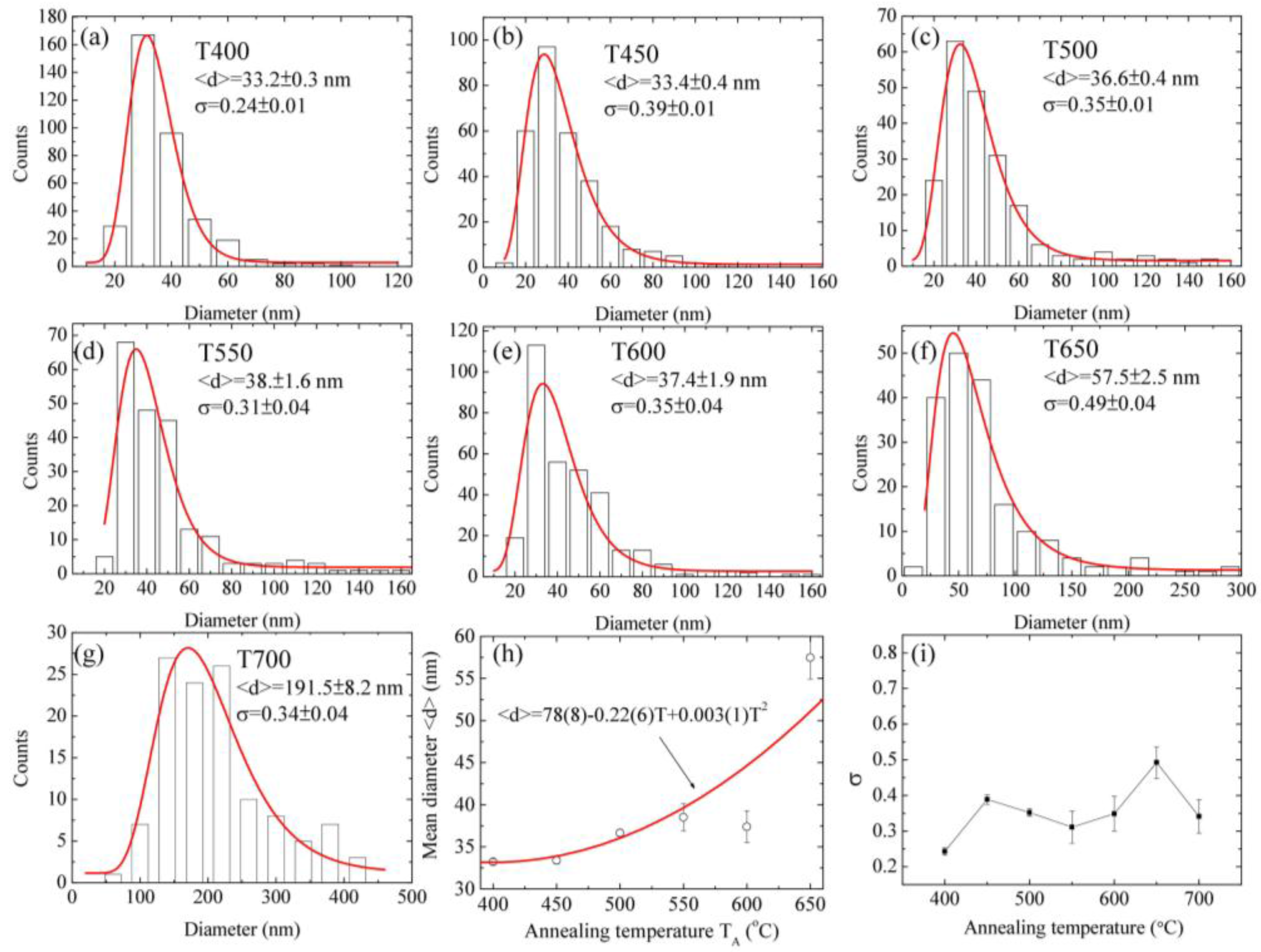
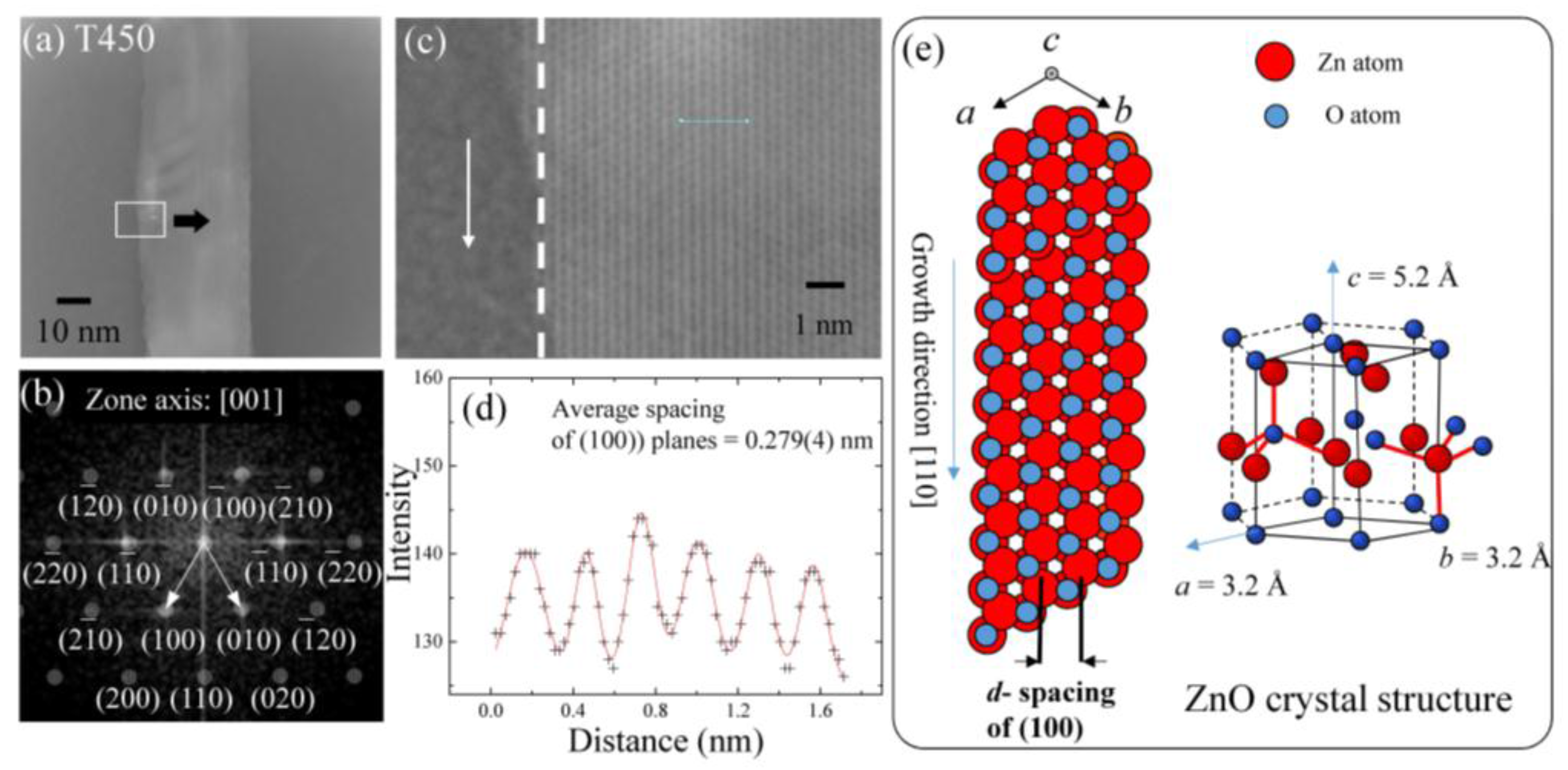
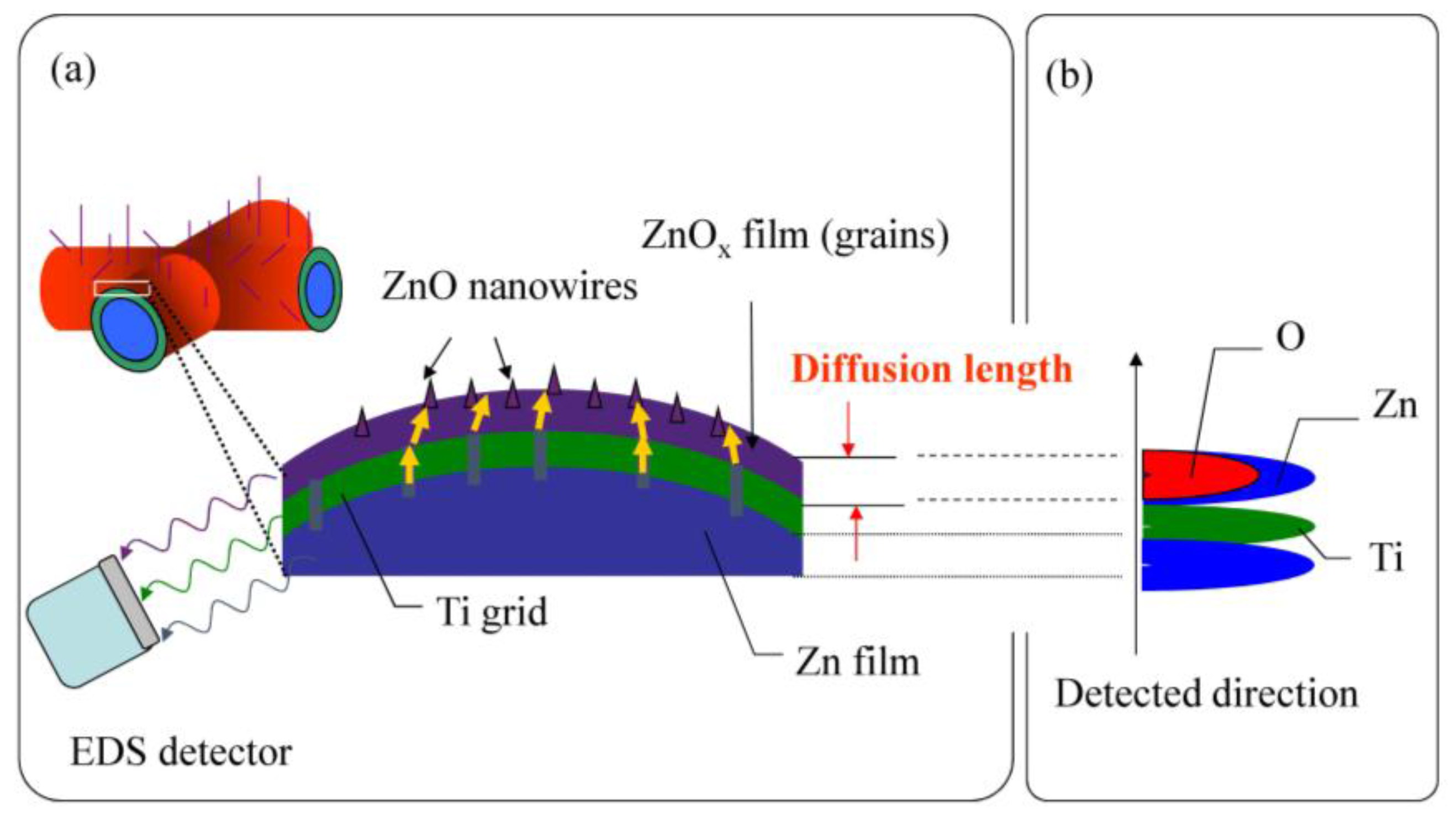
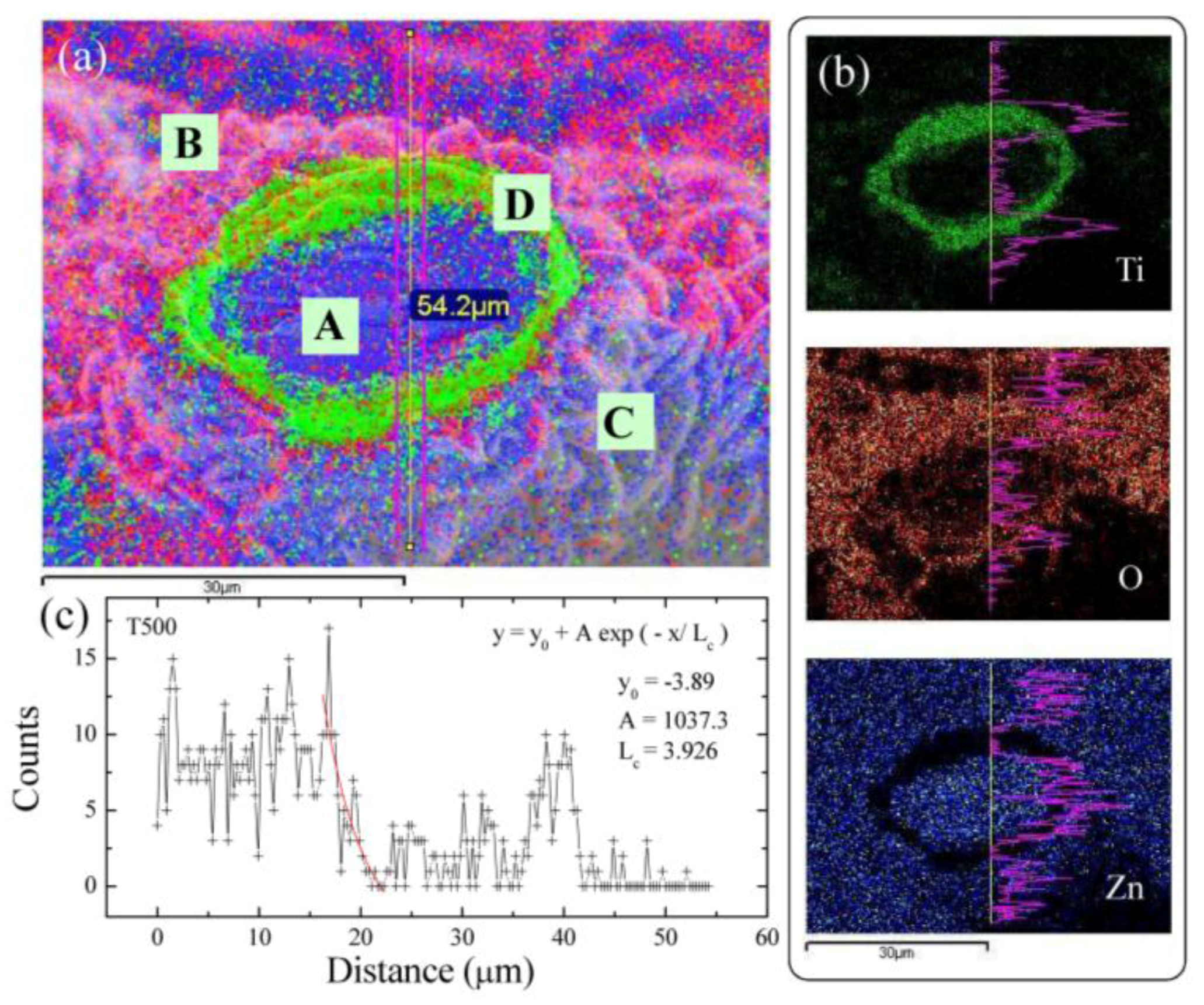

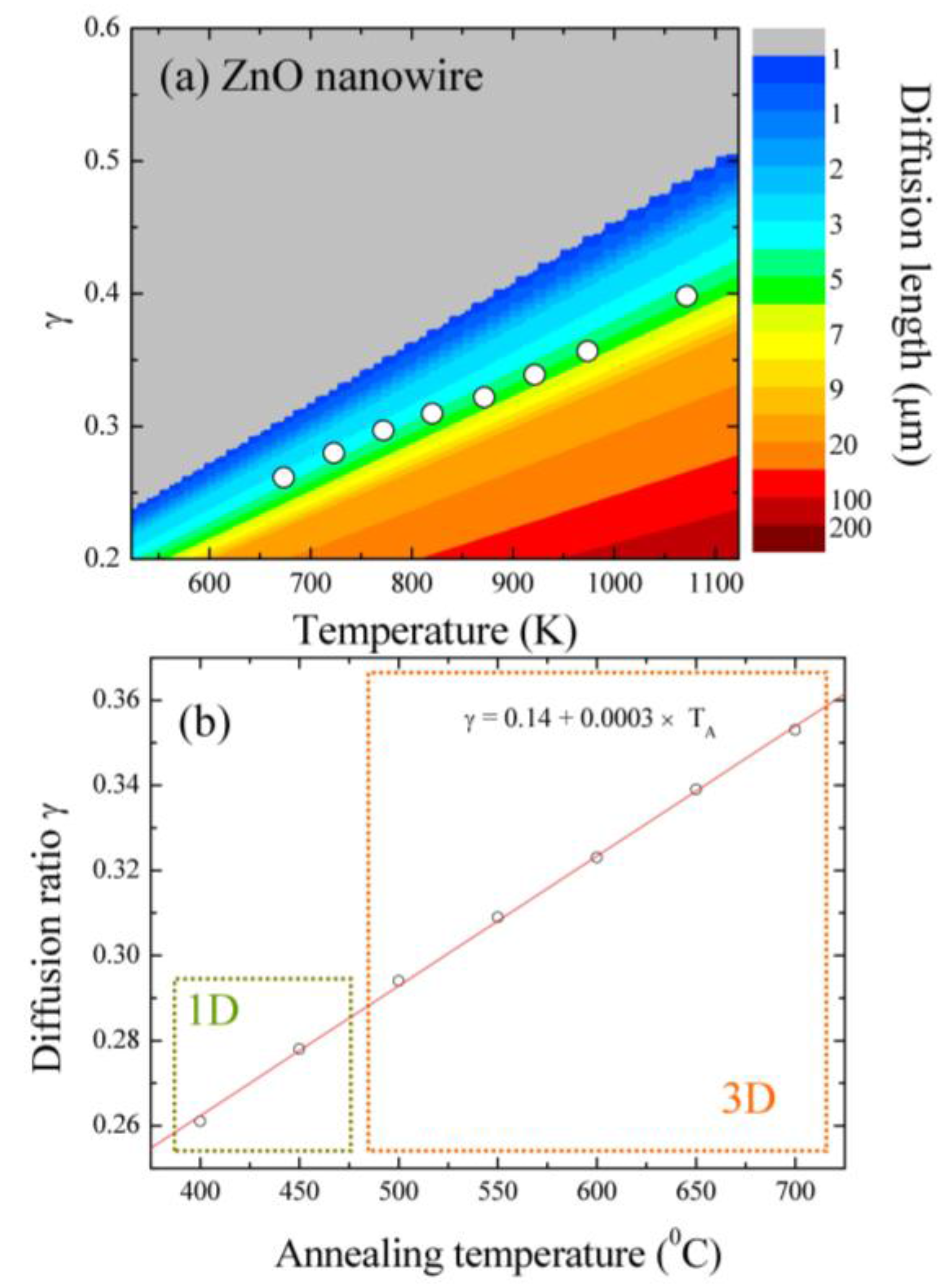
| TA (°C) | Sample | <d> (nm) | δ (nm) | Area | R2 |
|---|---|---|---|---|---|
| 400 | T400 | 33.2 ± 0.3 | 0.24 ± 0.01 | 3219 ± 113 | 0.99 |
| 450 | T450 | 33.4 ± 0.4 | 0.39 ± 0.01 | 2789 ± 78 | 0.99 |
| 500 | T500 | 36.6 ± 0.4 | 0.35 ± 0.01 | 1834 ± 204 | 0.99 |
| 550 | T550 | 38.5 ± 1.6 | 0.31 ± 0.04 | 1834 ± 204 | 0.92 |
| 600 | T600 | 37.4 ± 1.9 | 0.35 ± 0.04 | 2817 ± 331 | 0.88 |
| 650 | T650 | 57.5 ± 2.5 | 0.49 ± 0.04 | 3348 ± 441 | 0.97 |
| 700 | T700 | 191.5 ± 8.2 | 0.34 ± 0.04 | 4177 ± 442 | 0.93 |
| Sample | Trunk | Branch | ||
|---|---|---|---|---|
| Diameter (nm) | Lattice Constant a (nm) | Diameter (nm) | Lattice Constant a (nm) | |
| T400 | 13.0 | 0.32 (2) | ||
| T450 | 32.1 | 0.32 (3) | ||
| T500 | 54.1 | 0.33 (1) | 19.1 | 0.33 (3) |
| T550 | 37.1 | 0.32 (3) | 25.8 | 0.32 (3) |
| T600 | 65.2 | 0.32 (2) | 56.4 | 0.32 (6) |
| T650 | 83.3 | 0.32 (3) | 33.3 | 0.32 (3) |
| Dimensionality | Sample | Diffusion Length (μm) | Diffusion Ratio γ |
|---|---|---|---|
| 1-D | T400 | 3.404 | 0.261 |
| 1-D | T450 | 3.674 | 0.278 |
| 3-D | T500 | 3.927 | 0.294 |
| 3-D | T550 | 4.310 | 0.309 |
| 3-D | T600 | 4.861 | 0.323 |
| 3-D | T650 | 5.080 | 0.339 |
| 3-D | T700 | 5.542 | 0.353 |
| Large grains | T800 | 4.462 | 0.402 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shih, P.-H.; Wu, S.Y. Growth Mechanism Studies of ZnO Nanowires: Experimental Observations and Short-Circuit Diffusion Analysis. Nanomaterials 2017, 7, 188. https://doi.org/10.3390/nano7070188
Shih P-H, Wu SY. Growth Mechanism Studies of ZnO Nanowires: Experimental Observations and Short-Circuit Diffusion Analysis. Nanomaterials. 2017; 7(7):188. https://doi.org/10.3390/nano7070188
Chicago/Turabian StyleShih, Po-Hsun, and Sheng Yun Wu. 2017. "Growth Mechanism Studies of ZnO Nanowires: Experimental Observations and Short-Circuit Diffusion Analysis" Nanomaterials 7, no. 7: 188. https://doi.org/10.3390/nano7070188