Non-Noble Metal Oxide Catalysts for Methane Catalytic Combustion: Sonochemical Synthesis and Characterisation
Abstract
:1. Introduction
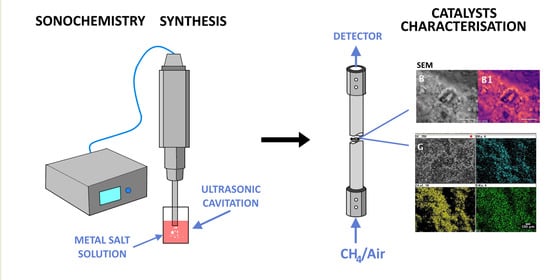
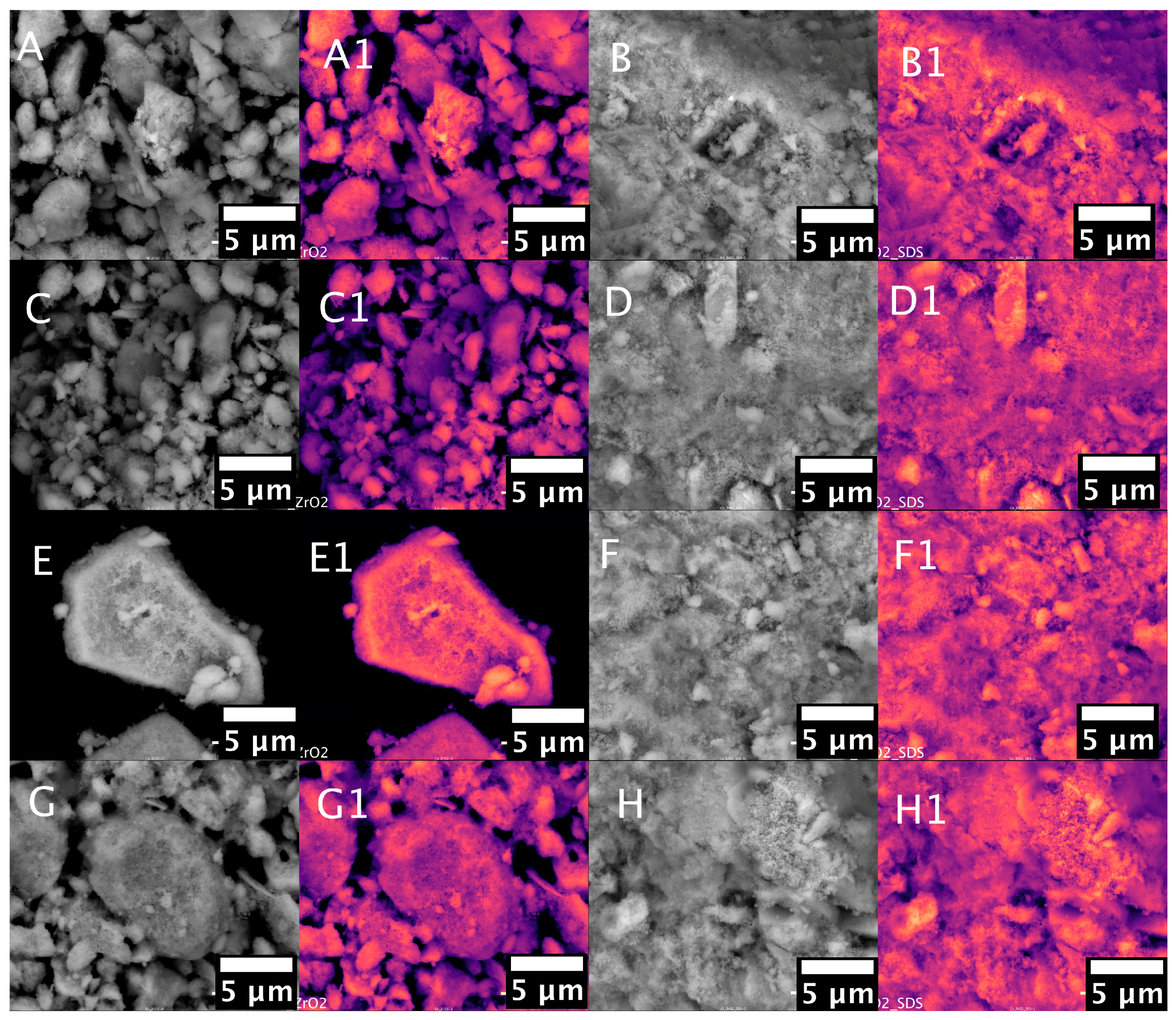
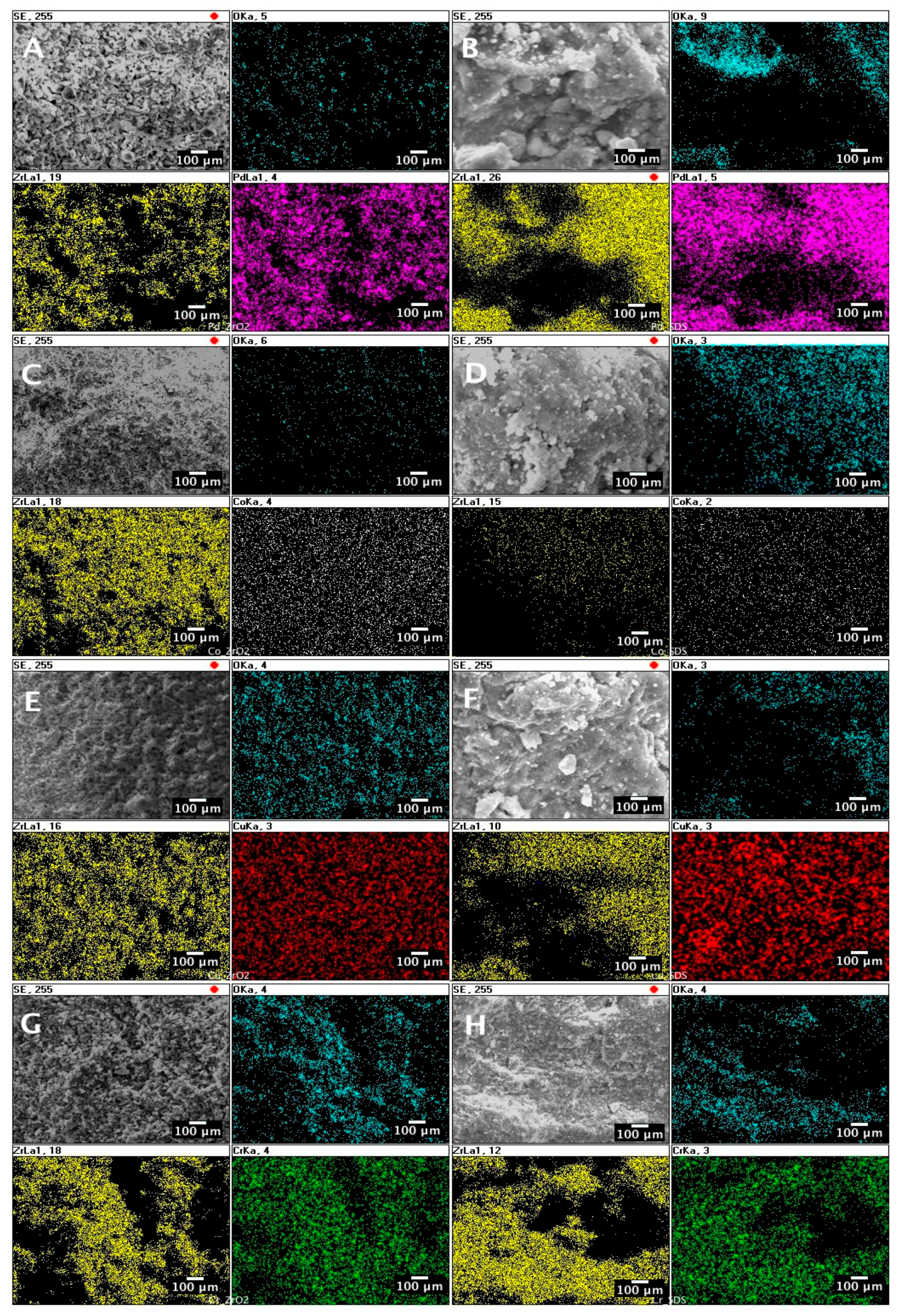
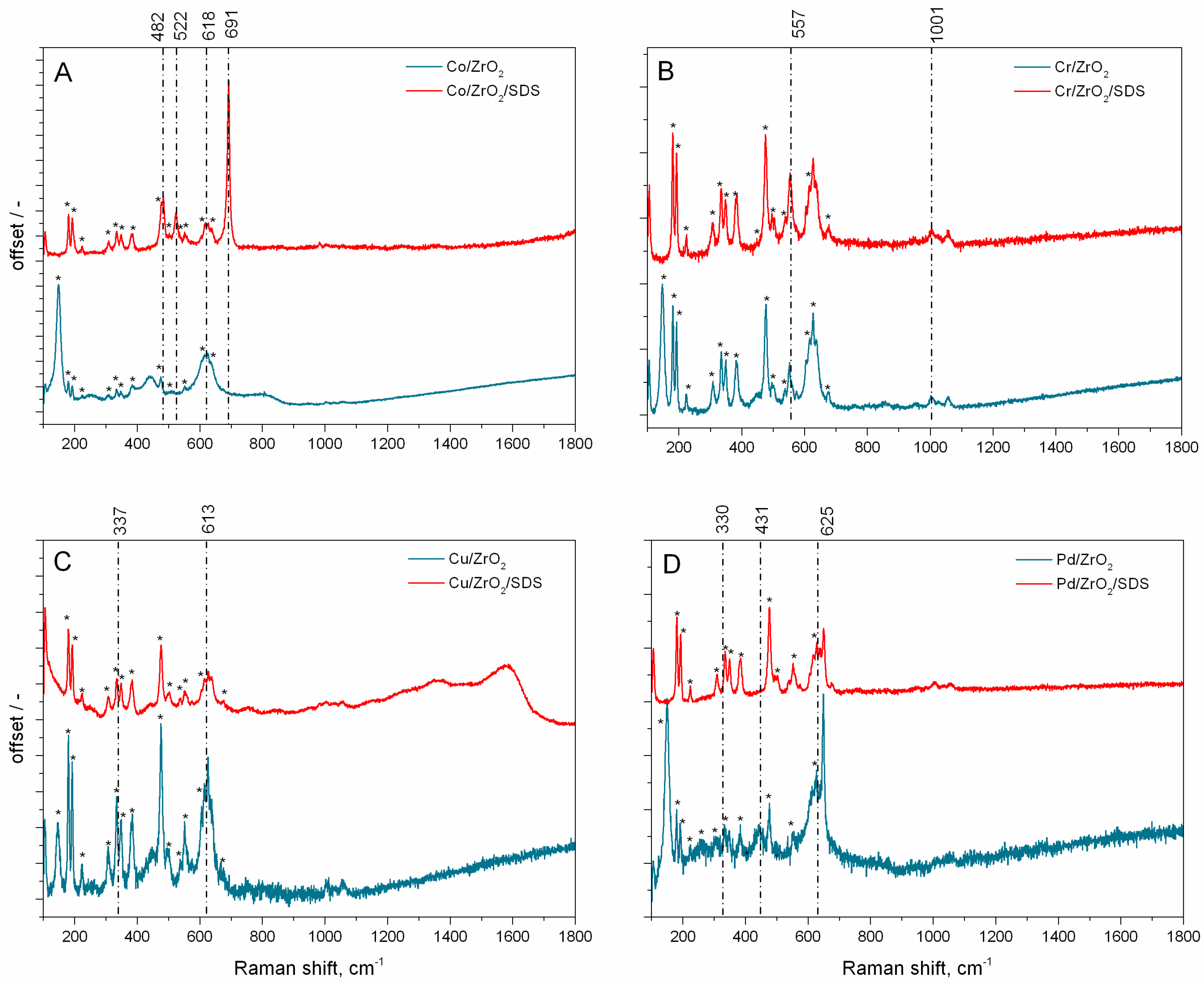
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation
3.3. Catalyst Characterisation
3.4. Catalytic Activity Tests
4. Conclusions
- (1)
- There is no correlation between the addition of stabiliser and the metal loading in any of the prepared catalyst samples.
- (2)
- The TEM analyses confirmed the decrease of particle sizes for the catalysts prepared via the sonochemical method with the addition of SDS.
- (3)
- XRD analysis partly confirmed the presence of oxidised metal nanoparticles. The determination of the catalysts’ structure was performed by µRaman analysis. The nanoparticles are in the form of PdO, Co3O4, CuO, and Cr2O3.
- (4)
- An SEM analysis in backscattered electron mode confirmed the presence of metal nanoparticles at the catalysts’ surfaces.
- (5)
- An SEM/EDS mapping revealed that the active metals (Pd, Co, Cr, and Cu) are uniformly dispersed over the catalyst.
- (6)
- The activity of the catalysts prepared via the sonochemical method is higher than the activity of their counterparts prepared by the incipient wetness method. SDS addition affects only the Pd-based catalyst, but this (about 50% of total activity) is eliminated using ultrasound irradiation.
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zheng, N.; Stucky, G.D. A general synthetic strategy for oxide-supported metal nanoparticle catalysts. J. Am. Chem. Soc. 2006, 128, 14278–14280. [Google Scholar] [CrossRef] [PubMed]
- Gawande, M.B.; Goswami, A.; Felpin, F.X.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and Cu-Based Nanoparticles: Synthesis and Applications in Catalysis. Chem. Rev. 2016, 116, 3722–3811. [Google Scholar] [CrossRef] [PubMed]
- Prieto, G.; Tüysüz, H.; Duyckaerts, N.; Knossalla, J.; Wang, G.-H.; Schüth, F. Hollow Nano- and Microstructures as Catalysts. Chem. Rev. 2016, 116, 14056–14119. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Liu, B.; Zhang, Q.; Deng, W.; Wang, Y.; Yang, Y. Recent advances in heterogeneous selective oxidation catalysis for sustainable chemistry. Chem. Soc. Rev. 2014, 43, 3480–3524. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Feng, J.; Yan, S.; Zou, Z. Solar fuel production: Strategies and new opportunities with nanostructures. Nano Today 2015, 10, 468–486. [Google Scholar] [CrossRef]
- Wang, H.; Guo, Z.; Wang, S.; Liu, W. One-dimensional titania nanostructures: Synthesis and applications in dye-sensitized solar cells. Thin Solid Films 2014, 558, 1–19. [Google Scholar] [CrossRef]
- Terranova, M.L.; Orlanducci, S.; Tamburri, E.; Guglielmotti, V.; Rossi, M. Si/C hybrid nanostructures for Li-ion anodes: An overview. J. Power Sources 2014, 246, 167–177. [Google Scholar] [CrossRef]
- Zhang, D.; Pan, C.; Shi, L.; Huang, L.; Fang, J.; Fu, H. A highly reactive catalyst for CO oxidation: CeO2 nanotubes synthesized using carbon nanotubes as removable templates. Microporous Mesoporous Mater. 2009, 117, 193–200. [Google Scholar] [CrossRef]
- Harraz, F.A.; El-Hout, S.E.; Killa, H.M.; Ibrahim, I.A. Palladium nanoparticles stabilized by polyethylene glycol: Efficient, recyclable catalyst for hydrogenation of styrene and nitrobenzene. J. Catal. 2012, 286, 184–192. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, K.; Xu, D.; Yang, G.; Huang, H.; Nie, F.; Liu, C.; Yang, S. CuO nanostructures: Synthesis, characterization, growth mechanisms, fundamental properties, and applications. Prog. Mater. Sci. 2014, 60, 208–337. [Google Scholar] [CrossRef]
- Jodłowski, P.J.; Chlebda, D.; Piwowarczyk, E.; Chrzan, M.; Jędrzejczyk, R.J.; Sitarz, M.; Węgrzynowicz, A.; Kołodziej, A.; Łojewska, J. In situ and operando spectroscopic studies of sonically aided catalysts for biogas exhaust abatement. J. Mol. Struct. 2016, 1126, 132–140. [Google Scholar]
- Masjedi-Arani, M.; Salavati-Niasari, M. A simple sonochemical approach for synthesis and characterization of Zn2SiO4 nanostructures. Ultrason. Sonochem. 2016, 29, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Pokhrel, N.; Kiran Vabbina, P.; Pala, N. Sonochemistry: Science and Engineering. Ultrason. Sonochem. 2016, 29, 104–128. [Google Scholar] [CrossRef] [PubMed]
- Thokchom, B.; Pandit, A.B.; Qiu, P.; Park, B.; Choi, J.; Khim, J. A review on sonoelectrochemical technology as an upcoming alternative for pollutant degradation. Ultrason. Sonochem. 2015, 27, 210–234. [Google Scholar] [CrossRef] [PubMed]
- Bera, P.; Hegde, M.S. Noble metal ions in CeO2 and TiO2: Synthesis, structure and catalytic properties. RSC Adv. 2015, 5, 94949–94979. [Google Scholar] [CrossRef]
- Chen, B.; Yang, Z.; Zhu, Y.; Xia, Y. Zeolitic imidazolate framework materials: Recent progress in synthesis and applications. J. Mater. Chem. A 2014, 2, 16811–16831. [Google Scholar] [CrossRef]
- Abbas, M.; Torati, S.R.; Kim, C. A novel approach for the synthesis of ultrathin silica-coated iron oxide nanocubes decorated with silver nanodots (Fe3O4/SiO2/Ag) and their superior catalytic reduction of 4-nitroaniline. Nanoscale 2015, 7, 12192–12204. [Google Scholar] [CrossRef] [PubMed]
- Pirola, C.; Bianchi, C.L.; Di Michele, A.; Diodati, P.; Boffito, D.; Ragaini, V. Ultrasound and microwave assisted synthesis of high loading Fe-supported Fischer-Tropsch catalysts. Ultrason. Sonochem. 2010, 17, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zeiger, B.W.; Suslick, K.S. Sonochemical synthesis of nanomaterials. Chem. Soc. Rev. 2013, 42, 2555–2567. [Google Scholar] [CrossRef] [PubMed]
- Jodłowski, P.J.; Jędrzejczyk, R.J.; Chlebda, D.; Dziedzicka, A. Method of preparation of metal oxide catalyst. 2017; submitted. [Google Scholar]
- Kolaczkowski, S.T. Catalytic stationary gas turbine combustors: A review of the challenges faced to clear the next set of hurdles. Chem. Eng. Res. Des. 1995, 73, 168–190. [Google Scholar]
- Okal, J.; Zawadzki, M.; Baranowska, K. Methane combustion over bimetallic Ru-Re/γ-Al2O3 catalysts: Effect of Re and pretreatments. Appl. Catal. B Environ. 2016, 194, 22–31. [Google Scholar] [CrossRef]
- Ercolino, G.; Grzybek, G.; Stelmachowski, P.; Specchia, S.; Kotarba, A.; Specchia, V. Pd/Co3O4-based catalysts prepared by solution combustion synthesis for residual methane oxidation in lean conditions. Catal. Today 2015, 257, 66–71. [Google Scholar] [CrossRef]
- Paredes, J.R.; Díaz, E.; Díez, F.V.; Ordóñe, S. Combustion of Methane in Lean Mixtures over Bulk Transition-Metal Oxides: Evaluation of the Activity and Self-Deactivation. Energy Fuels 2009, 23, 86–93. [Google Scholar] [CrossRef]
- Maione, A.; André, F.; Ruiz, P. The effect of Rh addition on Pd/γ-Al2O3 catalysts deposited on FeCrAlloy fibers for total combustion of methane. Appl. Catal. A Gen. 2007, 333, 1–10. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, X.; Arandiyan, H.; Peng, Y.; Chang, H.; Li, J. Low temperature complete combustion of methane over cobalt chromium oxides catalysts. Catal. Today 2013, 201, 12–18. [Google Scholar] [CrossRef]
- Dobber, D.; Kiessling, D.; Shmitz, W.; Wendt, G. MnOx/ZrO2 catalysts for the total oxidation of methane and chloromethane. Appl. Catal. B Environ. 2004, 52, 135–143. [Google Scholar] [CrossRef]
- Zavyalova, U.; Scholz, P.; Ondruschka, B. Influence of cobalt precursor and fuels on the performance of combustion synthesized Co3O4/γ-Al2O3 catalysts for total oxidation of methane. Appl. Catal. A Gen. 2007, 323, 226–233. [Google Scholar] [CrossRef]
- Pérez, A.; Lamonier, J.F.; Giraudon, J.M.; Molina, R.; Moreno, S. Catalytic activity of Co-Mg mixed oxides in the VOC oxidation: Effects of ultrasonic assisted in the synthesis. Catal. Today 2011, 176, 286–291. [Google Scholar] [CrossRef]
- Genty, E.; Brunet, J.; Poupin, C.; Casale, S.; Capelle, S.; Massiani, P.; Siffert, S.; Cousin, R. Co-Al Mixed Oxides Prepared via LDH Route Using Microwaves or Ultrasound: Application for Catalytic Toluene Total Oxidation. Catalysts 2015, 5, 851–867. [Google Scholar] [CrossRef]
- Jodłowski, P.J.; Jędrzejczyk, R.J.; Rogulska, A.; Wach, A.; Kuśtrowski, P.; Sitarz, M.; Łojewski, T.; Kołodziej, A.; Łojewska, J. Spectroscopic characterization of Co3O4 catalyst doped with CeO2 and PdO for methane catalytic combustion. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 131, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Ercolino, G.; Grodzka, A.; Grzybek, G.; Stelmachowski, P.; Specchia, S.; Kotarba, A. The Effect of the Preparation Method of Pd-Doped Cobalt Spinel on the Catalytic Activity in Methane Oxidation Under Lean Fuel Conditions. Top. Catal. 2016, 60, 333. [Google Scholar] [CrossRef]
- Lee, J.H.; Trimm, D.L. Catalytic combustion of methane. Fuel Process. Technol. 1995, 42, 339–359. [Google Scholar] [CrossRef]
- Xiao, T. Methane combustion over supported cobalt catalysts. J. Mol. Catal. A Chem. 2001, 175, 111–123. [Google Scholar] [CrossRef]
- Águila, G.; Gracia, F.; Cortés, J.; Araya, P. Effect of copper species and the presence of reaction products on the activity of methane oxidation on supported CuO catalysts. Appl. Catal. B Environ. 2008, 77, 325–338. [Google Scholar] [CrossRef]
- Reddy, B.M.; Khan, A.; Lakshmanan, P.; Aouine, M.; Loridant, S.; Volta, J.C. Structural characterization of nanosized CeO2-SiO2, CeO2-TiO2, and CeO2-ZrO2 catalysts by XRD, Raman, and HREM techniques. J. Phys. Chem. B 2005, 109, 3355–3363. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Jin, Y.; Xing, C.; Zeng, C.; Kawabata, T.; Imai, K.; Matsuda, K.; Tan, Y.; Tsubaki, N. Studies on surface impregnation combustion method to prepare supported Co/SiO2 catalysts and its application for Fischer–Tropsch synthesis. Appl. Catal. A Gen. 2012, 435–436, 217–224. [Google Scholar] [CrossRef]
- Pabisch, S.; Feichtenschlager, B.; Kickelbick, G.; Peterlik, H. Effect of interparticle interactions on size determination of zirconia and silica based systems—A comparison of SAXS, DLS, BET, XRD and TEM. Chem. Phys. Lett. 2012, 521, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewska, E.; Soliwoda, K.; Kadziola, K.; Tkacz-Szczesna, B.; Celichowski, G.; Cichomski, M.; Szmaja, W.; Grobelny, J.; Tomaszewska, E.; Soliwoda, K.; et al. Detection Limits of DLS and UV-Vis Spectroscopy in Characterization of Polydisperse Nanoparticles Colloids. J. Nanomater. 2013, 2013, 313081. [Google Scholar] [CrossRef]
- Pankaj; Ashokkumar, M. Theoretical and Experimental Sonochemistry Involving Inorganic Systems; Springer Science+Business Media B.V.: Dordrecht, The Netherlands, 2011. [Google Scholar]
- Camp, W.K.; Diaz, E.; Wawak, B. Electron Microscopy of Shale Hydrocarbon Reservoirs: AAPG Memoir 102; AAPG Memoir; American Association of Petroleum Geologists: Bath, UK, 2013. [Google Scholar]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Meth. 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Downs, R.T.; Hall-Wallace, M. The American Mineralogist crystal structure database. Am. Mineral. 2003, 88, 247–250. [Google Scholar]
- Malolepszy, A.; Mazurkiewicz, M.; Stobinski, L.; Lesiak, B.; Kövér, L.; Tóth, J.; Mierzwa, B.; Borodzinski, A.; Nitze, F.; Wågberg, T. Deactivation resistant Pd-ZrO2 supported on multiwall carbon nanotubes catalyst for direct formic acid fuel cells. Int. J. Hydrog. Energy 2015, 40, 16724–16733. [Google Scholar] [CrossRef]
- Zhao, L.; Ji, S.; Yin, F.; Lu, Z.; Liu, H.; Li, C. Catalytic Combustion of Methane over Col-,Mg,O/A12O3/FeCrAl Monolithic Catalysts. J. Nat. Gas Chem. 2006, 15, 287–296. [Google Scholar] [CrossRef]
- Sidhu, G.K.; Kumar, R. Role of anionic and cationic surfactants on the structural and dielectric properties of ZrO2 nanoparticles. Appl. Surf. Sci. 2017, 392, 598–607. [Google Scholar] [CrossRef]
- Sharan, R.; Dutta, A. Structural analysis of Zr4+ doped ceria, a possible material for ammonia detection in ppm level. J. Alloys Compd. 2017, 693, 936–944. [Google Scholar] [CrossRef]
- Hadjiev, V.G.; Iliev, M.N.; Vergilov, I.V. The Raman spectra of Co3O4. J. Phys. Chem. C Solid State Phys. 1988, 21, L199–L202. [Google Scholar] [CrossRef]
- Sousa, P.M.; Silvestre, A.J.; Popovici, N.; Conde, O. Morphological and structural characterization of CrO2/Cr2O3 films grown by Laser-CVD. Appl. Surf. Sci. 2005, 247, 423–428. [Google Scholar] [CrossRef]
- Dines, T.J.; Inglis, S. Raman spectroscopic study of supported chromium(vi) oxide catalysts. Phys. Chem. Chem. Phys. 2003, 5, 1320–1328. [Google Scholar] [CrossRef]
- Kim, D.S.; Wachs, I.E. Surface chemistry supported chromium oxide catalysts. J. Catal. 1993, 142, 166–171. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, Q.; Fei, X.; He, Y.; Zhang, P.; Zhang, G.; Peng, L.; Xie, W. Synthesis of CuO nano- and micro-structures and their Raman spectroscopic studies. CrystEngComm 2010, 12, 2232–2237. [Google Scholar] [CrossRef]
- Demoulin, O.; Navez, M.; Gaigneaux, E.M.; Ruiz, P.; Mamede, A.-S.; Granger, P.; Payen, E. Operando resonance Raman spectroscopic characterisation of the oxidation state of palladium in Pd/γ-Al2O3 catalysts during the combustion of methane. Phys. Chem. Chem. Phys. 2003, 5, 4394–4401. [Google Scholar] [CrossRef]
- Fujimoto, K.; Ribeiro, F.H.; Avalos-Borja, M.; Iglesia, E. Structure and Reactivity of PdOx/ZrO2 Catalysts for Methane Oxidation at Low Temperatures. J. Catal. 1998, 179, 431–442. [Google Scholar] [CrossRef]
- Gélin, P. Complete oxidation of methane at low temperature over noble metal based catalysts: A review. Appl. Catal. B Environ. 2002, 39, 1–37. [Google Scholar] [CrossRef]
- Baldwin, T.R.; Burch, R. Catalytic combustion of methane over supported palladium catalysts I. Alumina supported catalysts. Appl. Catal. 1990, 66, 337–358. [Google Scholar] [CrossRef]
- Choudhary, T.V.; Banerjee, S.; Choudhary, V.R. Catalysts for combustion of methane and lower alkanes. Appl. Catal. A Gen. 2002, 234, 1–23. [Google Scholar] [CrossRef]
- Morales-Torres, S.; Maldonado-Hódar, F.J.; Pérez-Cadenas, A.F.; Carrasco-Marín, F. Design of low-temperature Pt-carbon combustion catalysts for VOC’s treatments. J. Hazard. Mater. 2010, 183, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Jodłowski, P.J.; Jędrzejczyk, R.J.; Chlebda, D.; Tyczkowski, J.; Kryca, J.; Kołodziej, A.; Łojewska, J. Structure effects on activity of plasma deposited cobalt oxide catalysts for VOC combustion. Top. Catal. 2016, 60, 318–325. [Google Scholar] [CrossRef]
- Cao, S.; Tao, F.; Tang, Y.; Li, Y.; Yu, J. Size- and shape-dependent catalytic performances of oxidation and reduction reactions on nanocatalysts. Chem. Soc. Rev. 2016, 100, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, V.R.; Uphade, B.S.; Pataskar, S.G.; Keshavaraja, A. Low-Temperature Complete Combustion of Methane over Mn-, Co-, and Fe-Stabilized ZrO2. Angew. Chem. Int. Ed. Engl. 1996, 35, 2393–2395. [Google Scholar] [CrossRef]



| Catalyst | Solution | Sonication Time, h | Metal Content, (Pd, Co, Cu, Cr), wt % | SBET, (m2/g) | Vp Total (cm3/g) | Nanoparticle Size (DLS), nm | Specific Activity **, (mmol/gs) |
|---|---|---|---|---|---|---|---|
| Pd/ZrO2 | 0.001 M Pd(NO3)2 | 0.33 | 3.43 ± 0.89 | 24.23 | 0.14 | 199 ± 121 | 2.43 × 10−2 |
| Co/ZrO2 | 0.1 M Co(NO3)2 | 0.33 | 0.020 ± 0.001 | 22.91 | 0.15 | 241 ± 52 | 2.30 |
| Cu/ZrO2 | 0.1 M Cu(NO3)2 | 0.33 | 0.32 ± 0.02 | 24.76 | 0.15 | 356 ± 71 | 1.06 × 10−1 |
| Cr/ZrO2 | 0.1 M Cr(NO3)2 | 0.33 | 0.23 ± 0.01 | 22.93 | 0.15 | 172 ± 22 | 1.71 × 10−1 |
| Pd/ZrO2/SDS | 0.001 M Pd(NO3)2 | 0.33 | 0.050 ± 0.002 | 25.10 | 0.12 | 324 ± 120 | 1.67 |
| Co/ZrO2/SDS | 0.1 M Co(NO3)2 | 0.33 | 2.06 ± 0.07 | 24.97 | 0.11 | 400 ± 131 | 2.69 × 10−2 |
| Cu/ZrO2/SDS | 0.1 M Cu(NO3)2 | 0.33 | 0.86 ± 0.01 | 22.28 | 0.13 | 201 ± 80 | 4.55 × −2 |
| Cr/ZrO2/SDS | 0.1 M Cr(NO3)2 | 0.33 | 1.44 ± 0.07 | 23.04 | 0.14 | 188 ± 34 | 2.97 × 10−2 |
| ZrO2 | - | - | - | 29.6, 20–30 * | 0.16 | - |
| Catalyst | Solution | Impregnation Time, h | Metal Content *, (Pd, Co, Cu, Cr), wt % | SBET, (m2/g) | Vp Total (cm3/g) | Specific Activity **, (mmol/gs) |
|---|---|---|---|---|---|---|
| Pd/ZrO2/ref | 0.001 M Pd(NO3)2 | 0.33 | 3.5 | 48.57 | 0.23 | 2.38 × 10−2 |
| Co/ZrO2/ref | 0.1 M Co(NO3)2 | 0.33 | 0.02 | 25.77 | 0.20 | 1.66 |
| Cu/ZrO2/ref | 0.1 M Cu(NO3)2 | 0.33 | 0.3 | 39.77 | 0.23 | 8.85 × 10−2 |
| Cr/ZrO2/ref | 0.1 M Cr(NO3)2 | 0.33 | 0.2 | 27.03 | 0.22 | 2.37 × 10−1 |
| Pd/ZrO2/SDS/ref | 0.001 M Pd(NO3)2 | 0.33 | 0.05 | 32.26 | 0.25 | 5.98 × 10−1 |
| Co/ZrO2/SDS/ref | 0.1 M Co(NO3)2 | 0.33 | 2.0 | 26.37 | 0.22 | 2.12 × 10−2 |
| Cu/ZrO2/SDS/ref | 0.1 M Cu(NO3)2 | 0.33 | 0.9 | 29.79 | 0.22 | 5.16 × 10−2 |
| Cr/ZrO2/SDS/ref | 0.1 M Cr(NO3)2 | 0.33 | 1.5 | 32.04 | 0.22 | 2.73 × 10−2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jodłowski, P.J.; Jędrzejczyk, R.J.; Chlebda, D.K.; Dziedzicka, A.; Kuterasiński, Ł.; Gancarczyk, A.; Sitarz, M. Non-Noble Metal Oxide Catalysts for Methane Catalytic Combustion: Sonochemical Synthesis and Characterisation. Nanomaterials 2017, 7, 174. https://doi.org/10.3390/nano7070174
Jodłowski PJ, Jędrzejczyk RJ, Chlebda DK, Dziedzicka A, Kuterasiński Ł, Gancarczyk A, Sitarz M. Non-Noble Metal Oxide Catalysts for Methane Catalytic Combustion: Sonochemical Synthesis and Characterisation. Nanomaterials. 2017; 7(7):174. https://doi.org/10.3390/nano7070174
Chicago/Turabian StyleJodłowski, Przemysław J., Roman J. Jędrzejczyk, Damian K. Chlebda, Anna Dziedzicka, Łukasz Kuterasiński, Anna Gancarczyk, and Maciej Sitarz. 2017. "Non-Noble Metal Oxide Catalysts for Methane Catalytic Combustion: Sonochemical Synthesis and Characterisation" Nanomaterials 7, no. 7: 174. https://doi.org/10.3390/nano7070174