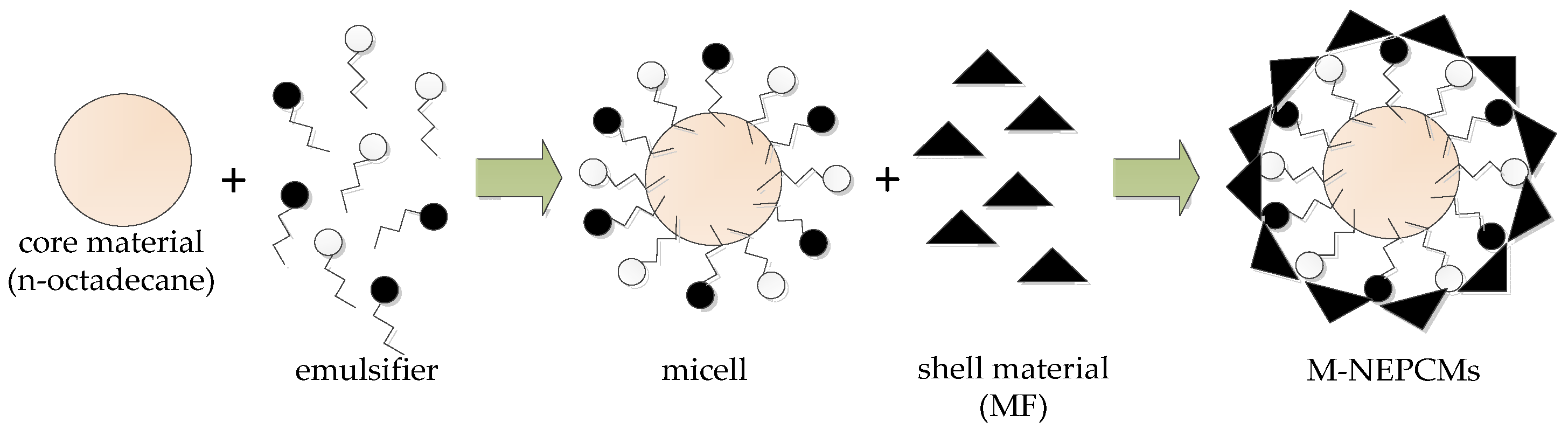
Stable and uniformly dispersed emulsion of the core material is critical for the preparation of M-NEPCMs, which has a significant impact on the capsule size and the encapsulation efficiency of capsules. Three factors affecting the stability and dispersity of the PCM emulsion, including the type and mixing ratio of compound emulsifiers, the emulsifying style as well as the amount of emulsifier, were studied and discussed as below.
3.1. Mixing Ratio of the Compound Emulsifiers
According to the views of hydrophilic–lipophilic balance (HLB) [
29,
30], each of the emulsifiers has a numerical value indicating its hydrophilic–lipophilic balance. A HLB value of the emulsifier that is closer to the required HLB value for the emulsion dispersing system indicates a more stable prepared emulsion.
Based on this principle, the three components of the compound emulsifiers used in this work, including SDS, Span80 and Tween80, were blended at four different ratios as listed in
Table 1. These ratios were based on the consideration that the HLB value of the compound emulsifier was controlled at 10 to 13. Typically, the HLB value required for oil-in-water emulsion is in the range of 8 to 18. The HLB value of the compound emulsifier can be obtained approximately by the following equation:
where,
HLB is the hydrophilic–lipophilic balance value;
ξ is the weight ratio of a single emulsifier in the compound emulsifiers; the subscript com, SD, SP and TW represents the compound emulsifiers, SDS, Span80 and Tween80, respectively.
The compound emulsifiers with different mixing ratios were tested respectively to prepare an emulsion of n-octadecane. The preparation conditions were all the same, except for the mixing ratios of the emulsifiers. The total amount of the compound emulsifier was 15% of the mass of n-octadecane. After emulsification at a constant temperature of 80 °C by the homogenizer, the four emulsion samples prepared from compound emulsifiers with four different mixing ratios were placed in beakers for 2 h. When the emulsification just finished, all the four emulsions were uniformly dispersed. The stability of them was continuously observed by the naked eye during 2 h standing.
The stability of the four emulsion samples after two hours standing are shown in the
Figure 2, with the codes from left to wright corresponding to the sample codes listed in the
Table 1. As can be seen from the figure, Sample 2 is dispersed most uniformly without significant phase separation. However, obvious delamination is observed in the other three samples. The phenomenon shown in
Figure 2 indicates that the stability of emulsion in sample two is the best among the four samples. In short, Sample 2 contains the optimal mixing ratios of compound emulsifiers used for emulsion.
It can be deduced that the best stability of the emulsion in Sample 2 may be attributed to the HLB value of the used compound emulsifiers. By adjusting the mixing ratios of the three components in the compound emulsifiers to ξSD:ξSP:ξTW = 0.1:0.6:0.3, the HLB value of the compound emulsifiers (HLBcom = 11.08) can reach or come very close to the required value of the n-octadecane emulsion system prepared in this study. In comparison, the HLB values obtained from other compounding ratios are relatively large compared to that required for the emulsion system.
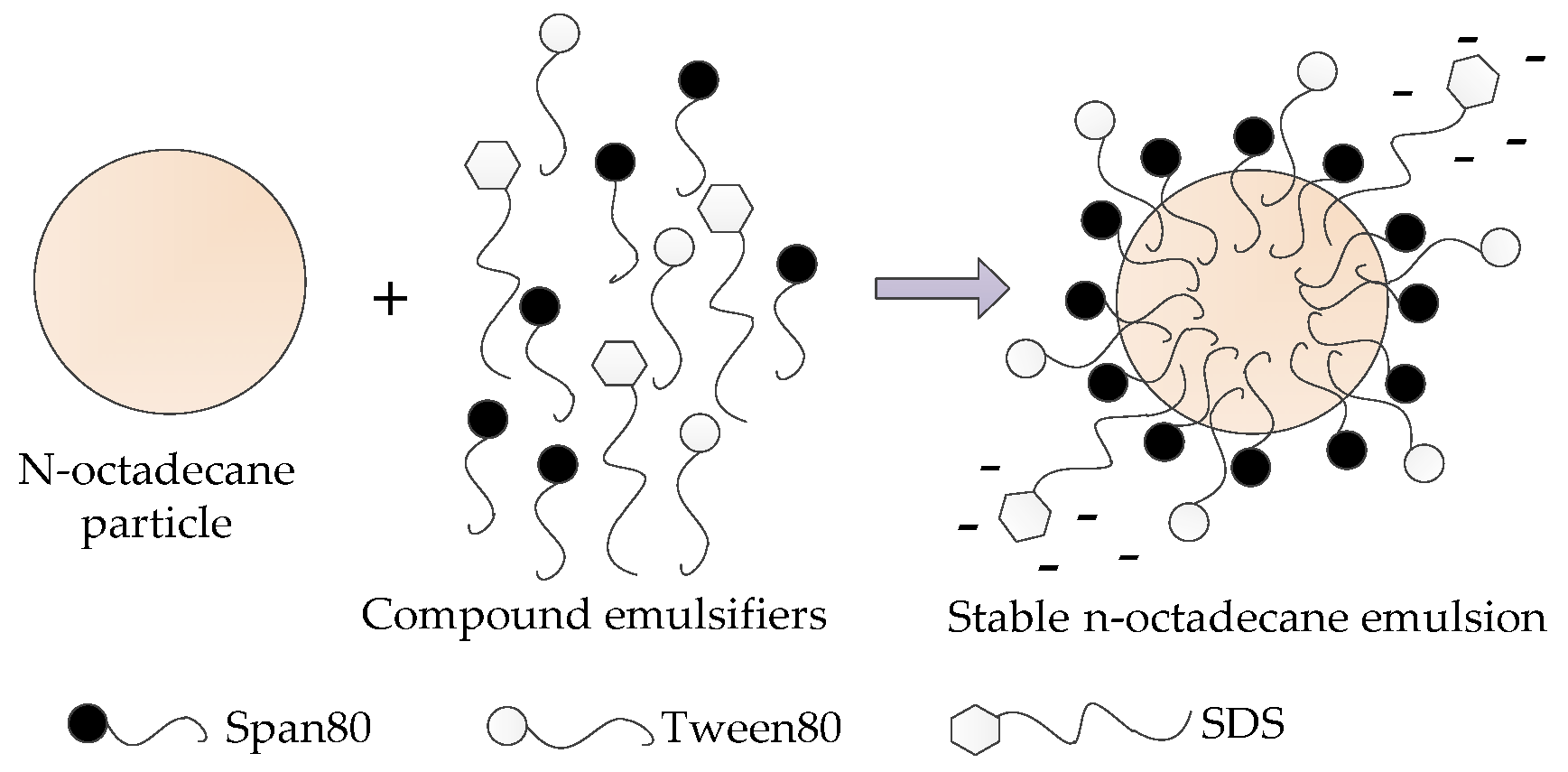
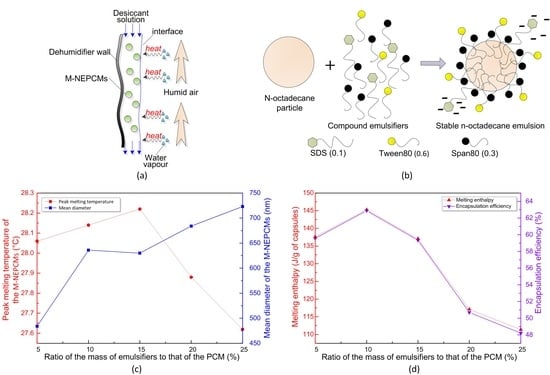
The emulsification mechanism of the composite emulsifiers used herein can be explicated with reference to
Figure 3. The HLB value of Span80 is relatively small, thus its lipophilicity is higher and results in the arrangement of Span80 molecules on the interfacial film that is closer to the oil phase. Essentially, this arrangement causes the Span80 molecules to be closer to the thin droplets of the core material
n-octadecane. In comparison, the HLB value of Tween80 is higher and its hydrophilicity is higher, thus resulting in its arrangement on the interface film being closer to the water phase. The molecules of Span80 and Tween80 are arranged at intervals on the liquid film surface, which makes the emulsifier molecules on the interface film more closely aligned and thereby enhances the stability of the emulsion. Moreover, the anionic emulsifier SDS has an HLB value of 40 and its high hydrophilicity can effectively reduce the surface tension. Furthermore, the surface-active anions form a negative electric field on the surface of the core particle. This electric repulsive force among the particles and the increase in zeta-potential of the particles results in the particles being more difficult to agglomerate, again improving the stability of the emulsion.
3.2. Effect of Emulsification Method on M-NEPCMs Properties
According to the preparation procedures for M-NEPCMs described in
Section 2.1. and the aforementioned optimal mixing ratio of the compound emulsifiers (
ξSD:
ξSP:
ξTW = 0.1:0.6:0.3), 10 g of
n-octadecane, 1.5 g of the compound emulsifiers and 5 mL of the deionized water at 80 °C were first mixed, before two emulsification methods were tested respectively in the subsequent emulsification process. The details of the two emulsification methods are listed in
Table 2.
As shown in the
Table 2, the only difference between the two emulsification methods is a pre-emulsification in Method (B). All the other conditions, including the volume of deionized water added, the dropping rate of deionized water, the stirring speed as well as the temperature of emulsification, are all the same. In Method (A), the emulsification by the homogenizer and the dropping of deionized water are conducted simultaneously. In comparison, the mixture is first pre-emulsified for 5 min by the homogenizer in Method (B), before the deionized water is added dropwise to the mixture when the emulsification is continued.
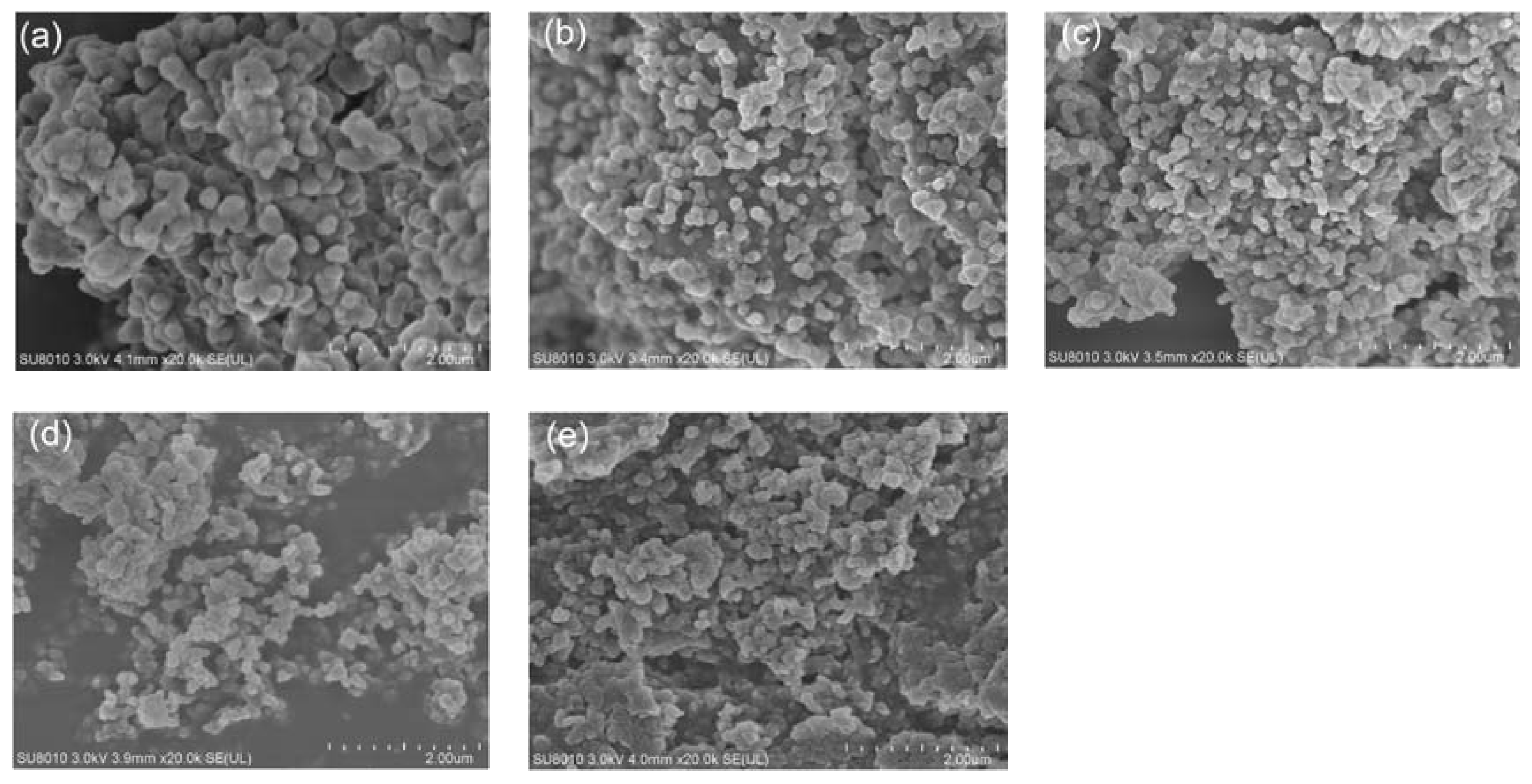
The morphology of M-NEPCMs prepared using the two emulsifier methods is shown in
Figure 4. As seen in the figure, compared with the M-NEPCMs prepared with Method (A), the M-NEPCMs in
Figure 4b have more regular spherical shape, smoother surfaces and better dispersibility, while the micro-nanocapsules in
Figure 4a show an obvious agglomeration. Therefore, the morphology of micro-nanocapsules prepared with Method (B) is better than that of Method (A).
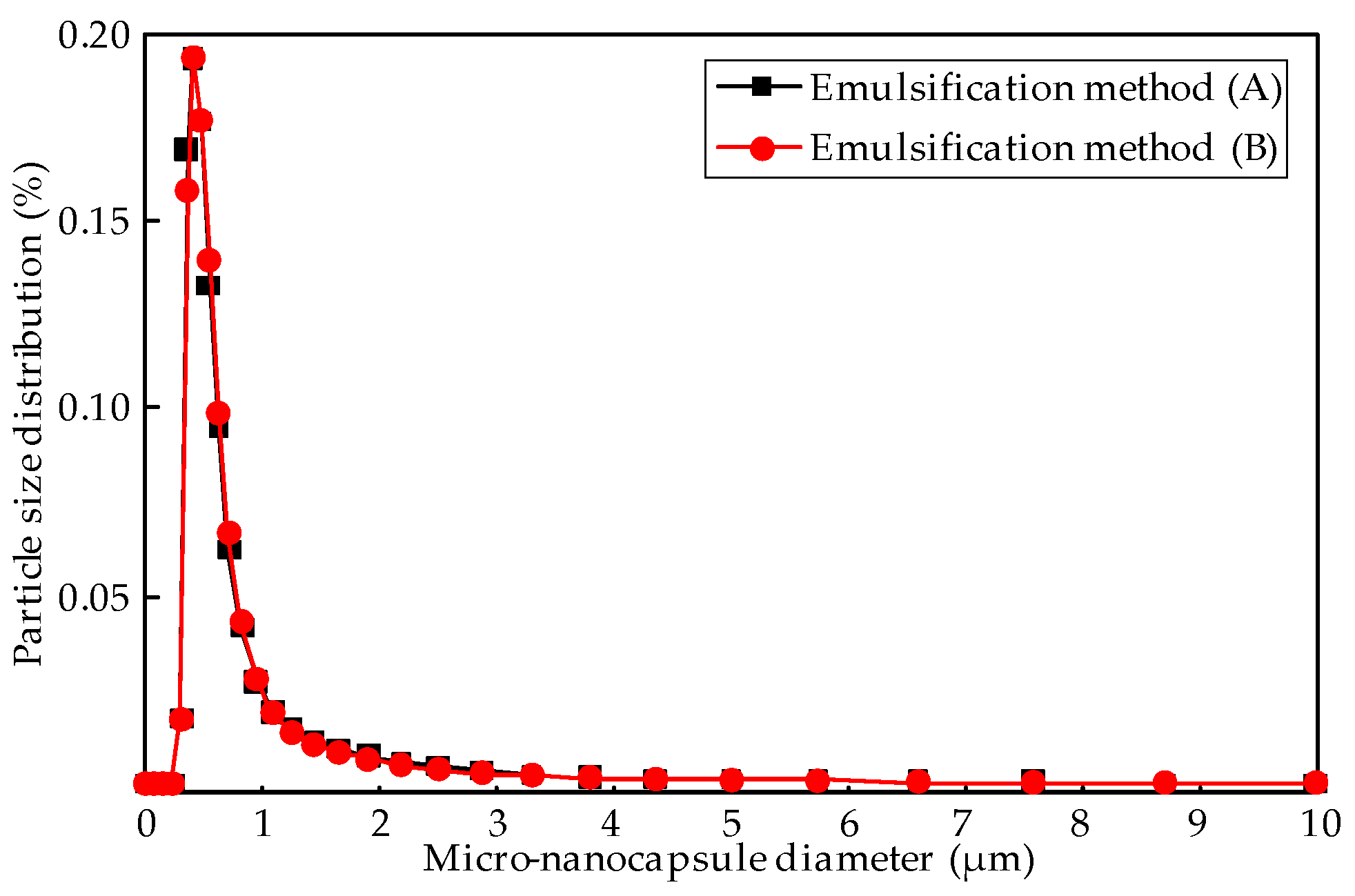
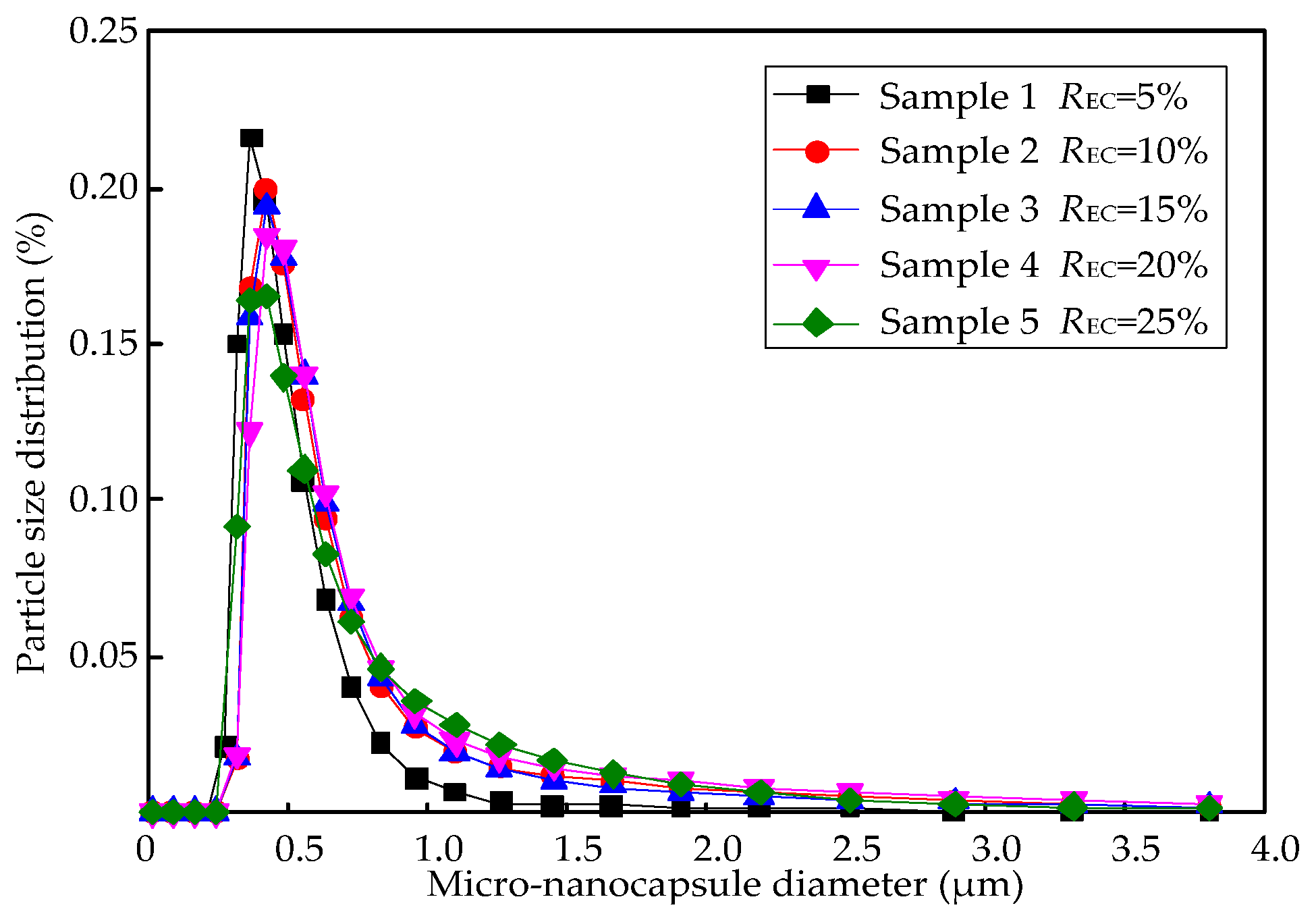
Figure 4 also shows that the M-NEPCMs have a similar size when prepared with either of the two methods, which is further proved by the particle distribution of the micro-nanocapsules shown in
Figure 5. There was almost no difference in the distributions of the particles with the same diameters obtained by different emulsification methods. Furthermore, the mean diameters obtained from Equation (1) shows that the mean diameter of the micro-nanocapsules prepared with emulsification Method (A) is 630 nm, while that of the micro-nanocapsules prepared by Method (B) is 632 nm. Therefore, the two emulsification methods tested in this paper have little difference with regards to the size distribution of micro-nanocapsules.
As shown in
Table 3, the phase change temperatures of the M-NEPCMs prepared by the two emulsification methods are very close. However, the phase change enthalpy of the M-NEPCMs obtained by using the emulsification Method (A) was relatively lower, with its melting enthalpy and crystallization enthalpy being 61.27 J/g of capsules and 70.56 J/g of capsules, respectively. The corresponding encapsulation efficiency of micro-nanocapsules is 26.52%. In comparison, the phase change enthalpy of the micro-nanocapsules prepared by using the emulsification Method (B) is higher, with the melting enthalpy and the crystallization enthalpy reaching 137.07 J/g of capsules and 137.86 J/g of capsules, respectively. Its corresponding encapsulation efficiency is 59.34%. The melting enthalpy of emulsification Method (B) is 123.7% higher than that of Method (A). Therefore, the two emulsification methods have different influences on the thermal properties of the prepared M-NEPCMs. Compared with the emulsification Method (A), the phase change enthalpy of M-NEPCMs can be improved significantly by Method (B), which may be attributed to the high stability and dispersity of the emulsion prepared using Method (B). As previously mentioned, the only difference between the two emulsification methods is the 5 minutes of pre-emulsification prior to the addition of deionized water in emulsification process (B), which may have an impact on the thermal properties of the M-NEPCMs as follows:
For the emulsification Method (A), deionized water is added simultaneously at the beginning of emulsification. Under the shearing force by the stirring of the homogenizer, the continuous addition of deionized water will reduce the effective shearing forces acting on the capsule core material n-octadecane, which results in the deterioration of the emulsification of the core material. Thus, the encapsulation efficiency will be decreased in the subsequent microencapsulation process. Correspondingly, the phase change enthalpy of the prepared M-NEPCMs will be relatively low.
In the case of using emulsification Method (B) in the emulsification, the deionized water is not added until the emulsification is carried out for 5 minutes. In the 5 minutes of pre-emulsification, the core material was sufficiently emulsified. Thus, the deionized water added afterwards will not affect the stability of the emulsion, which leads to a higher encapsulation efficiency during microencapsulation and a higher phase change enthalpy of the prepared MEPCM.
In addition, as a result of the pre-emulsification in emulsification Method (B), the emulsifier molecules can be fully and uniformly dispersed on the surface of the water droplets, thus resulting in the fine aqueous droplets being covered with the emulsifier molecules to form a stable interface film. When the deionized water is added gradually thereafter, the continuous phase will be aqueous under the shearing action and a complete phase inversion will occur, with the dispersed phase (core material) being completely coated by the interfacial film to form an emulsion with high stability. For emulsification Method (A), simultaneous addition of deionized water at the beginning of the emulsification results in a partial coverage of the emulsifier with the fine aqueous droplets. Consequently, the incomplete phase inversion from the oil continuous phase to the aqueous continuous phase will occur, which finally leads to the lower stability of the n-octadecane emulsion. Therefore, the stability of the n-octadecane emulsion prepared by emulsification Method (B) is better, with the micro-nanocapsules prepared by emulsion Method (B) having a higher enthalpy of phase change. The stability of the core material emulsion prepared using emulsion Method (A) is relatively poor and hence, the phase change enthalpy of micro-nanocapsule obtained from Method (A) is also low.
3.4. Thermal Cycling Test of the Prepared M-NEPCMs
When the
n-octadecane M-NEPCMs is used in the liquid desiccant system, it will be cycled at a temperature range between 20 and 70 °C. The M-NEPCMs should maintain stable thermal properties for long-term cycling at the working condition of fluctuating temperatures. Therefore, a thermal cycling test was performed to evaluate the thermal reliability of the prepared M-NEPCMs in this study. The M-NEPCMs of Sample 4 in
Table 6 was chosen as the tested sample. Two grams of the sample was loaded in a glass vial, before being heated at 80 °C and cooled at 10 °C using two water-baths alternately. The procedure was performed consecutively for 300 times. The changes in the thermal properties and morphology of the sample after thermal cycling were measured by DSC and SEM, respectively.
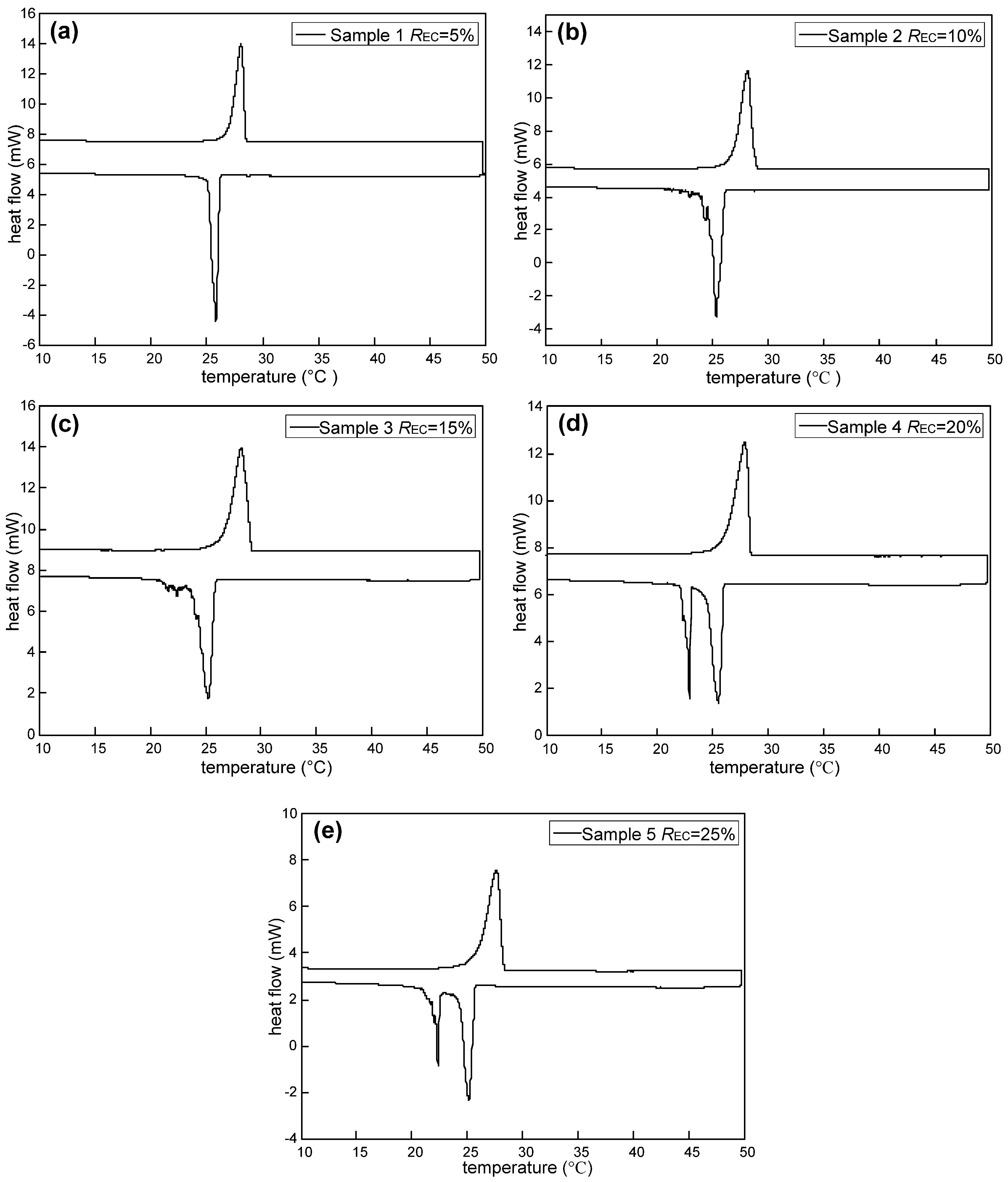
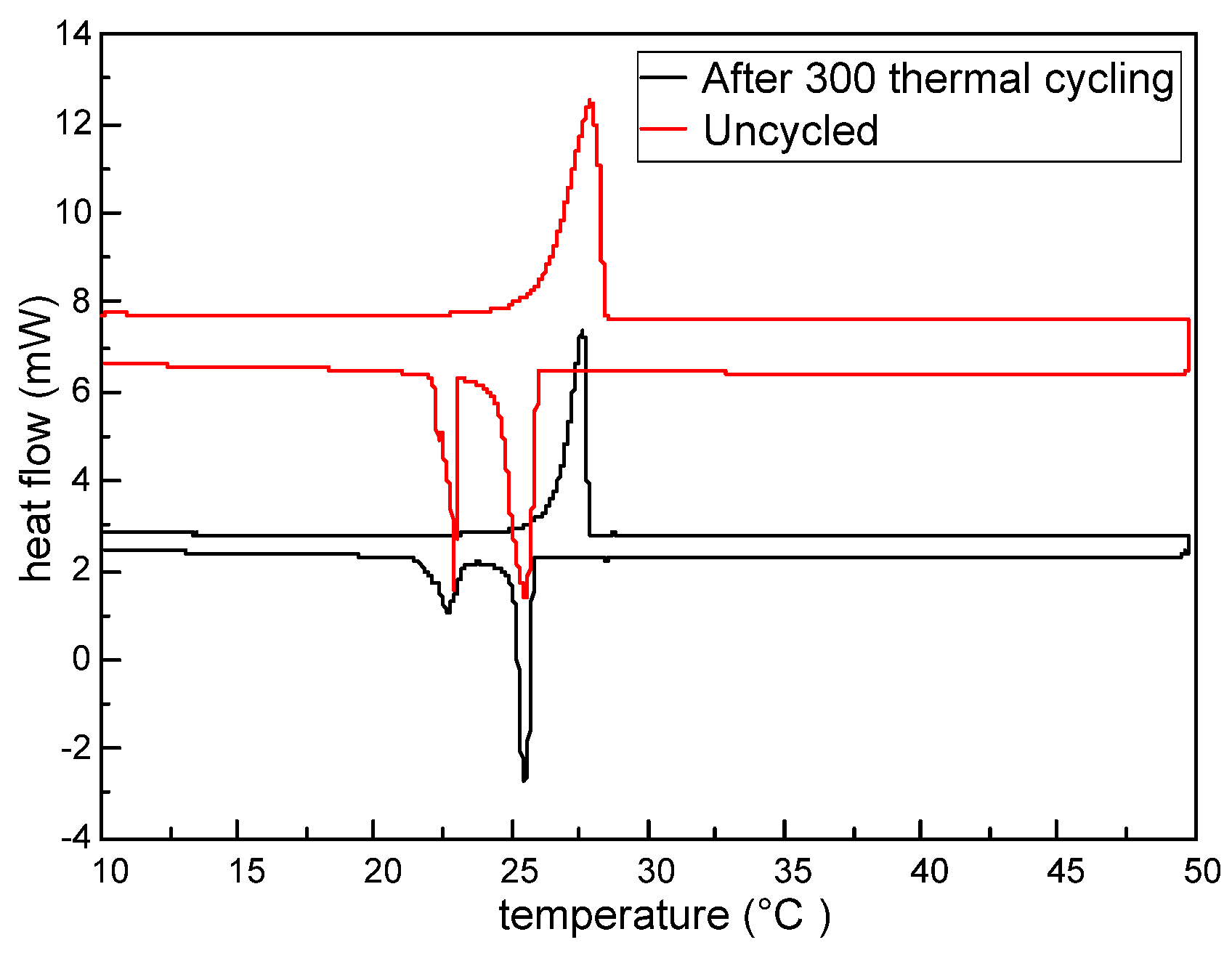
The DSC thermograms of the sample before and after thermal cycling 300 times are shown in
Figure 9, with its thermal properties given in
Table 8. As observed from
Figure 9, after thermal cycling 300 times, the DSC heating and cooling curves are basically consistent with those before the thermal cycling. The detailed thermal properties listed in
Table 8 show that the peak melting temperature of the tested M-NEPCMs changes by 0.27 °C, while the two peak crystallization temperatures change by 0.03 and 0.22 °C. As for the enthalpy of phase change after thermal cycling 300 times, the melting enthalpy of the tested M-NEPCMs changes by 2.09 J/g of capsules and the crystallization enthalpy changes by 1.95 J/g of capsules. These changes in phase change temperatures and phase change enthalpy of the M-NEPCMs with repeated thermal cycling are acceptable for its application in the liquid desiccant system. Thus, the test results prove the high thermal reliability of the M-NEPCMs prepared by using the compound emulsifiers in this study.
In addition, the SEM image of the M-NEPCMs after the thermal cycling test is shown in
Figure 10. Compared with its SEM image before the thermal cycling (see
Figure 6d), it can be observed that the changes in the morphology and shape of the capsule particles after thermal cycling 300 times are not significant. Moreover, no obvious rupture of the capsules and leakage of
n-octadecane are observed in the figure, which indicates that repeated heating and cooling cycles has little influence on the structure and mechanical stability of the prepared M-NEPCMs.