Bioactivity Studies on Titania Coatings and the Estimation of Their Usefulness in the Modification of Implant Surfaces
Abstract
:1. Introduction
2. Results
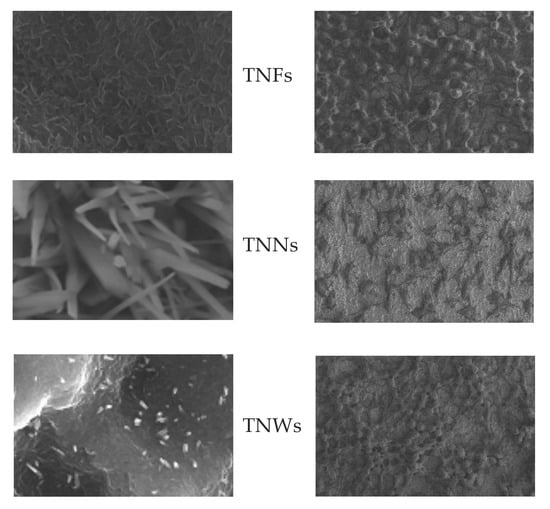
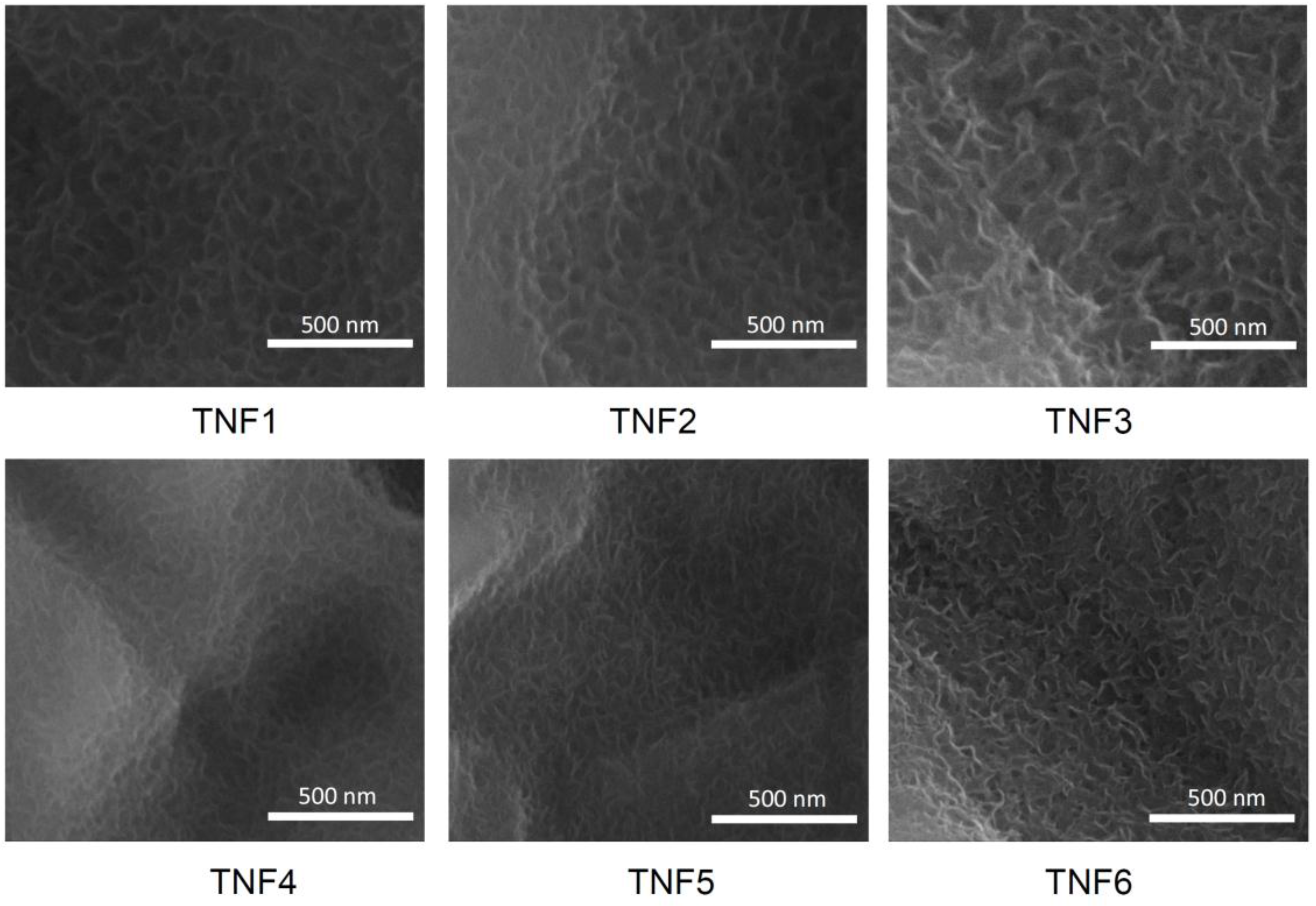
2.1. The Production of Titania Nanofibrous Coatings (TNFs) and Their Characterization
2.2. Titania Coatings Produced by Thermal Oxidation and Their Characterization
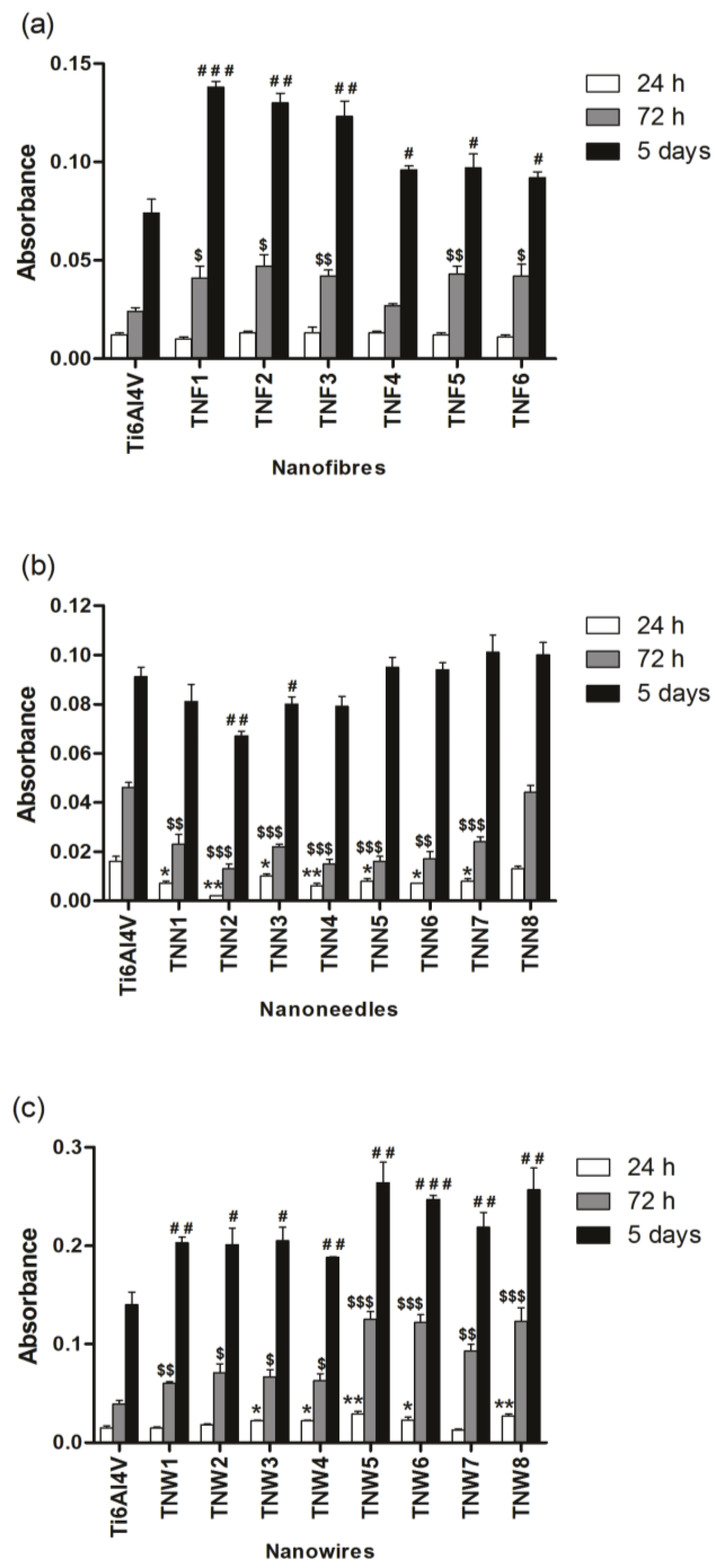
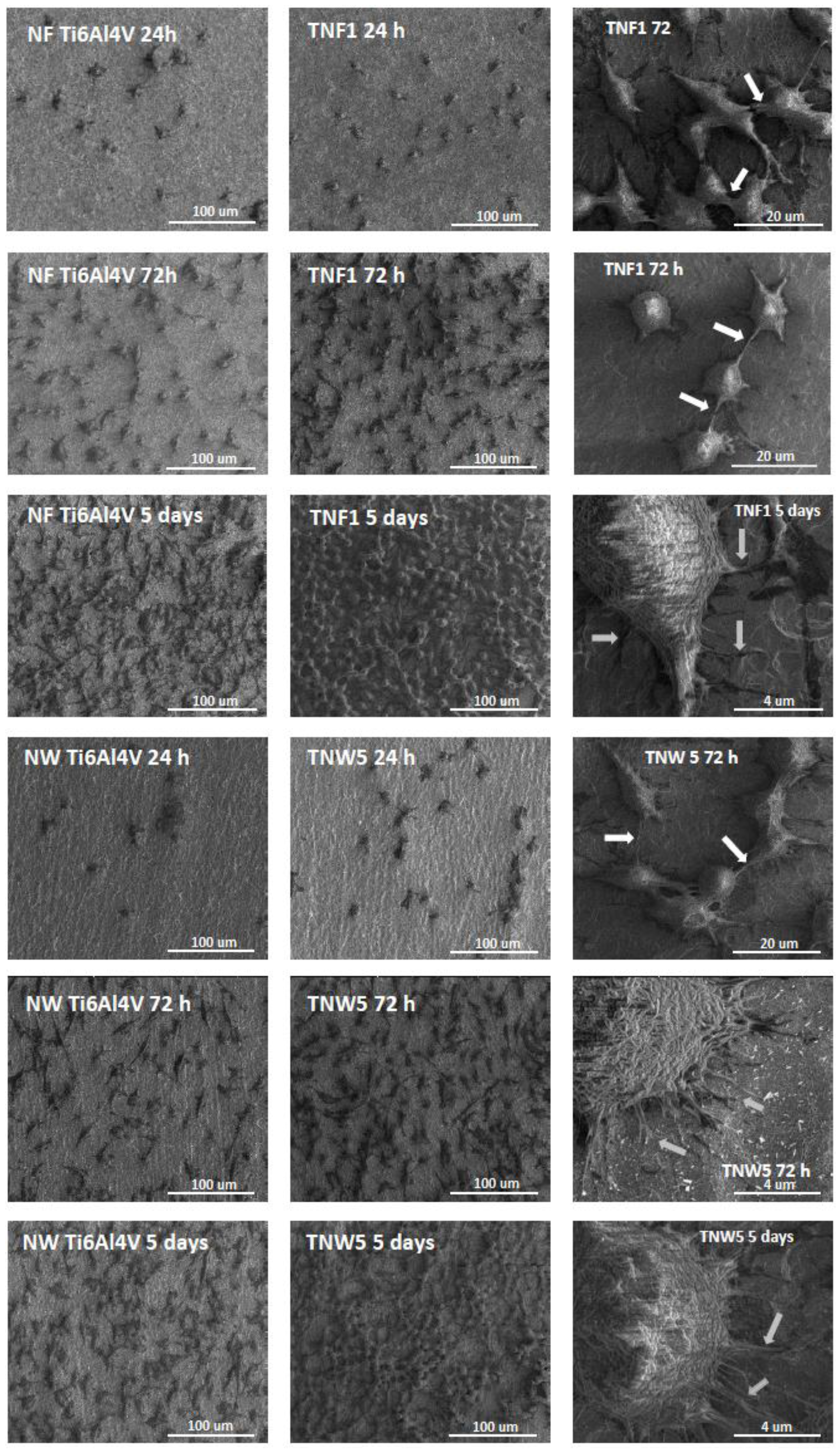
2.3. Fibroblasts’ Adhesion and Proliferation
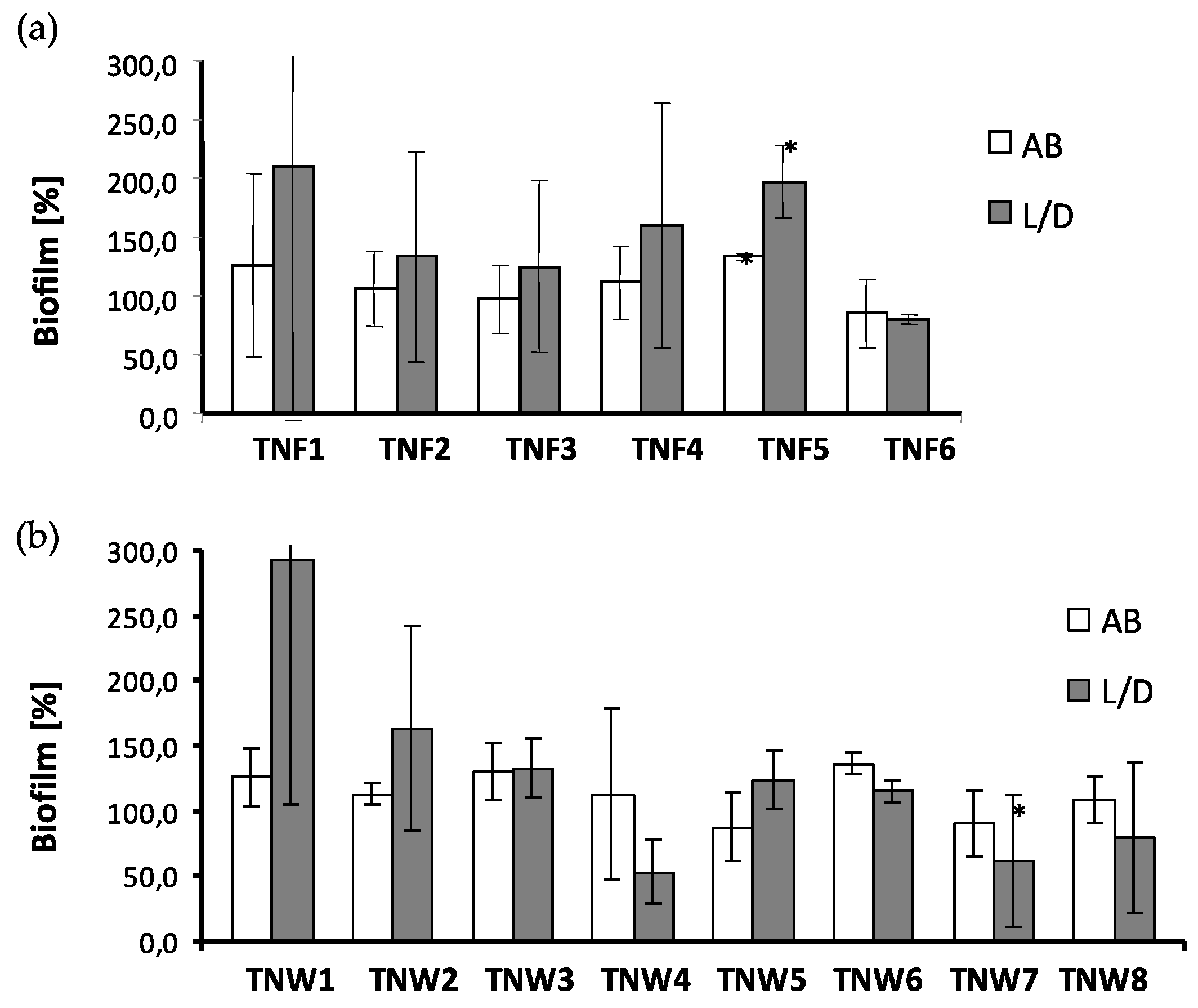
2.4. The Microbial Aggregates/Biofilm Formation on Titania Coatings
2.5. Wettability of TiO2 Nanostructures
2.6. Photocatalytic Activity
3. Discussion
4. Materials and Methods
4.1. Ti6Al4V Substrates
4.2. Chemical Oxidation
4.3. Thermal Oxidation
4.4. Characterization of Titania Coatings
4.5. Adhesion and Proliferation of L929 Cells on TNFs, TNNs, and TNWs Arrays
4.6. Cell Morphology Observed by Scanning Electron Microscopy
4.7. Statistical Analysis in the MTT Assay
4.8. Microbial Aggregates/Biofilm Formation on the Studied Nanocoatings
4.9. Statistical Analysis in the Microbiological Tests
4.10. Photocatalytic Activity Studies
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Parithimarkalaignan, S.; Padmanabhan, T.V. Osseointegration: An Update. J. Indian Prosthodont. Soc. 2013, 13, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Anil, S.; Anand, P.S.; Alghamdi, H.; Jansen, J.A. Dental Implant Surface Enhancement and Osseointegration. In Implant Dentistry—A Rapidly Evolving Practice; Ilser, T., Ed.; InTech: Rijeka, Croatia, 2011; ISBN 978-953-307-658-4. [Google Scholar]
- Novaes, A.B., Jr.; Souza, S.L.S.D.; Barros, R.R.M.D.; Pereira, K.K.Y.; Iezzi, G.; Piattelli, A. Influence of implant surface on osseointegration. Braz. Dent. J. 2010, 21, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Mavrogenis, A.F.; Dimitriou, R.; Parvizi, J.G.C. Babis, Biology of implant osseointegration. J. Musculoskelet. Neuronal Interact. 2009, 9, 61–71. [Google Scholar] [PubMed]
- Javed, F.; Ahmed, H.B.; Crespi, R.; Romanos, G.E. Role of primary stability for successful osseointegration of dental implants: Factors of influence and evaluation. Interv. Med. Appl. Sci. 2013, 5, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Rupp, F.; Gittens, R.A.; Scheideler, L. A Review on the Wettability of Dental Implant Surfaces: Theoretical and Experimental Aspects. Acta Biomater. 2014, 10, 2894–2906. [Google Scholar] [CrossRef] [PubMed]
- Gittens, R.A.; Scheideler, L.; Rupp, F. A Review on the Wettability of Dental Implant Surfaces II: Biological and Clinical Aspects. Acta Biomater. 2014, 10, 2907–2918. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, K.M.; Reddy, G.B.; Hyzy, S.L.; Schwartz, Z.; Boyan, B.D.; Olivares-Navarette, R. Titanium surface characteristics, including topography and wettability, alter macrophage activation. Acta Biomater. 2016, 31, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Sartoretto, S.C.; Alves, A.T.N.N.; Zarranz, L.; Jorge, M.Z.; Granjeiro, J.M.; Calasans-Maia, M.D. Hydrophilic surface of Ti6Al4V-ELI alloy improves the early bone apposition of sheep tibia. Clin. Oral Impl. Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Elias, C.N.; Oshida, Y.; Lima, J.H.C.; Muller, C.A. Relationship between surface properties (roughness, wettability and morphology) of titanium and dental implant removal torque. J. Mech. Behav. Biomed. Mater. 2008, 1, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Sidambe, A.T. Biocompatibility of Advanced Manufactured Titanium Implants—A Review. Materials 2014, 7, 8168–8188. [Google Scholar] [CrossRef]
- Oldani, C.; Dominguez, A. Titanium as a Biomaterial for Implants. In Recent Advances in Arthroplasty; Samo, F., Ed.; InTech: Rijeka, Croatia, 2012; ISBN 978-953-307-990-5. [Google Scholar]
- Oshida, Y.; Tuna, E.B.; Aktören, O.; Gençay, K. Dental Implant Systems. Int. J. Mol. Sci. 2010, 11, 1580–1678. [Google Scholar] [CrossRef] [PubMed]
- Khan, W.; Muntimadugu, E.; Jaffe, M.; Domb, A.J. Implantable Medical Devices. In Focal Controlled Drug Delivery; Springer: New York, NY, USA, 2014. [Google Scholar]
- Chia, H.N.; Wu, B.M. Recent advances in 3D printing of biomaterials. J. Biol. Eng. 2015, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Goel, M.; Mehta, R.; Kumar, A.; Kumar, V.; Bhayana, G.; Wadhwa, S. Implant Surface Modification and Osseointegration-Past, Present and Future. J. Oral Health Community Dent. 2014, 8, 113–118. [Google Scholar]
- Mandracci, P.; Mussano, F.; Rivolo, P.; Carossa, S. Surface Treatments and Functional Coatings for Biocompatibility Improvement and Bacterial Adhesion Reduction in Dental Implantology. Coatings 2016, 6, 7. [Google Scholar] [CrossRef]
- Rani, V.D.; Vinoth-Kumar, L.; Anitha, V.C.; Manzoor, K.; Deepthy, M.; Shantikumar, V.N. Osteointegration of titanium implant is sensitive to specific nanostructure morphology. Acta Biomater. 2012, 8, 1976–1989. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Wang, J.; Guo, H.; Liu, S.; He, W. The Effects of Surface Properties of Nanostructured Bone Repair Materials on Their Performances. J. Nanomater. 2015, 2015, 11. [Google Scholar] [CrossRef]
- Vandrovcova, M.; Bacakova, L. Adhesion, Growth and Differentiation of Osteoblasts on Surface-Modified Materials Developed for Bone Implants. Physiol. Res. 2011, 60, 403–417. [Google Scholar] [PubMed]
- Sobieszczyk, S. Surface Modifications of Ti and its Alloys. Adv. Mater. Sci. 2010, 10, 29–42. [Google Scholar] [CrossRef]
- Jemat, A.; Ghazali, M.J.; Razali, M.; Otsuka, Y. Surface Modifications and Their Effects on Titanium Dental Implants. BioMed. Res. Int. 2015, 2015, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chu, P.K.; Ding, C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. R Rep. 2004, 47, 49–121. [Google Scholar] [CrossRef]
- Tan, A.W.; Pingguan-Murphy, B.; Ahmad, R.; Akbar, S.A. Review of titania nanotubes: Fabrication and cellular response. Ceram. Int. 2012, 38, 4421–4435. [Google Scholar] [CrossRef]
- Brammer, K.S.; Frandsen, C.J.; Jin, S. TiO2 nanotubes for bone regeneration. Trends Biotechnol. 2012, 30, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Mazare, A.; Schmuki, P. One-dimensional titanium dioxide nanomaterials: Nanotubes. Chem. Rev. 2014, 114, 9385–9454. [Google Scholar] [CrossRef] [PubMed]
- Brammer, K.S.; Oh, S.; Cobb, C.J.; Bjursten, L.M.; van der Heyde, H.; Jin, S. Improved bone-forming functionality on diameter-controlled TiO2 nanotube surface. Acta Biomater. 2009, 5, 3215–3223. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xia, Y. Fabrication of Titania Nanofibers by Electrospinning. Nano Lett. 2003, 3, 555–560. [Google Scholar] [CrossRef]
- Liang, D.; Hsiao, B.S. Functional electrospun nanofibrous scaffolds for biomedical applications. Adv. Drug Deliv. Rev. 2007, 59, 1392–1412. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhu, J.; Yin, L.; Liu, S.; Zhang, X.; Ao, Y.; Chen, H. Evaluation of the morphology and osteogenic potential of titania-based electrospun nanofibers. J. Nanomater. 2012, 2012, 1–7. [Google Scholar] [CrossRef]
- Kiran, A.S.K.; Balu, R.; Kumar, T.S.S.J. Vero cell viability and human osteoblast cell response to electrospun phase controlled titania nanofibers. J. Biomater. Tissue Eng. 2012, 2, 292–298. [Google Scholar] [CrossRef]
- Venugopal, J.; Vadgama, P.; Kumar, T.S.S.; Ramakrishna, S. Biocomposite nanofibres and osteoblasts for bone tissue engineering. Nanotechnology 2007, 18, 55101. [Google Scholar] [CrossRef]
- Park, Y.-J.; Song, H.-J.; Kim, I.; Yang, H.-S. Surface characteristics and bioactivity of oxide film on titanium metal formed by thermal oxidation. J. Mater. Sci. Mater. Med. 2007, 18, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Dregia, S.; Akbar, S.; Alhoshan, M. Growth of 1-D TiO2 Nanowires on Ti and Ti alloys by Oxidation. J. Nanomater. 2010, 2010, 7. [Google Scholar] [CrossRef]
- Smith, B.S.; Yoriya, S.; Johnson, T.; Popat, K.C. Dermal fibroblast and epidermal keratinocyte functionality on titania nanotube arrays. Acta Biomater. 2011, 7, 2686–2696. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Bose, S.; Bandyopadhyay, A. TiO2 nanotubes on Ti: Influence of nanoscale morphology on bone cell-materials interaction. J. Biomed. Mater. Res. 2009, 90, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Brammer, K.S.; Oh, S.; Frandsen, C.J.; Varghese, S.; Jin, S. Nanotube surface triggers increased chondrocyte extracellular matrix production. Mater. Sci. Eng. C 2010, 30, 518–525. [Google Scholar] [CrossRef]
- Peng, L.; Elgroth, M.L.; La Tempa, T.J.; Grimes, C.A.; Desai, T.A. The effect of TiO2 nanotubes on endothelial function and smooth muscle proliferation. Biomaterials 2009, 30, 1268–1272. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Bauer, S.; von der Mark, K.; Schmuki, P. Nanosize and vitality: TiO2 nanotube diameter directs cell fate. Nano Lett. 2007, 7, 1686–1691. [Google Scholar] [CrossRef] [PubMed]
- Azadmanjiri, J.; Wang, P.-Y.; Pingle, H.; Kingshott, P.; Wang, J.; Srivastava, V.K.; Kapoor, A. Enhanced attachment of human mesenchymal stem cells on nanograined titania surfaces. RSC Adv. 2016, 6, 55825–55833. [Google Scholar]
- Tavangar, A.; Tan, B.; Venkatakrishnan, K. Synthesis of Bio-Functionalized Three-Dimensional Titania Nanofibrous Structures Using Femtosecond Laser Ablation. Acta Biomater. 2011, 7, 2726–2732. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, S.; Pingguan-Murphy, B.; Osman, N.A.A. Progress of key strategies in development of electrospun scaffolds: Bone tissue. Sci. Technol. Adv. Mater. 2012, 13, 043002. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.S.; Kim, T.G.; Park, T.G. Surface-functionalized electrospun nanofibers for tissue engineering and drug delivery. Adv. Drug Deliv. Rev. 2009, 61, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Christenson, E.M.; Anseth, K.S.; van den Beucken, L. Nanobiomaterial applications in orthopedics. J. Orthop. Res. 2007, 25, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Ding, B. Applications of electrospun fibers. Recent Pat. Nanotechnol. 2008, 2, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Kumbar, S.G.; James, R.; Nukavarapu, S.P.; Laurencin, C.T. Electrospun nanofiber scaffolds: Engineering soft tissues. Biomed. Mater. 2008, 3, 034002. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, R.; Suzuki, Y.; Yoshikawa, S. Syntheses of TiO2 (B) nanowires and TiO2 anatase nanowires by hydrothermal and post-heat treatments. J. Solid State Chem. 2008, 178, 2179–2185. [Google Scholar] [CrossRef]
- Azad, A.M.; Hershey, R.; Ali, S. Goel, Bactericidal efficacy of electrospun pure and Fe-doped titania nanofibers. J. Mater. Res. 2010, 25, 1761–1770. [Google Scholar] [CrossRef]
- Tan, A.W.; Pingguan-Marphy, B.; Ahmad, R.; Akbar, S.A. Advanced in fabrication of TiO2 nanofiber/nanowire arrays toward the cellular response in biomedical implantations: A review. J. Mater. Sci. 2013, 48, 8337–8353. [Google Scholar] [CrossRef]
- Deal, B.E.; Grove, A.S. General Relationship for the Thermal Oxidation of Silicon. J. Appl. Phys. 1965, 36, 3770–3778. [Google Scholar] [CrossRef]
- Yang, K.; Zhu, J.; Zhu, J.; Huang, S.; Zhu, X.; Ma, G. Sonochemical synthesis and microstructure investigation of rod-like nanocrystalline rutile titania. Mater. Lett. 2003, 57, 4639–4642. [Google Scholar] [CrossRef]
- Song, Y.; Dhar, S.; Feldman, L.C. Modified Deal Grove model for the thermal oxidation of silicon carbide. J. Appl. Phys. 2004, 95, 4953–4957. [Google Scholar] [CrossRef]
- Kumar, A.; Madaria, A.R.; Zhou, C. Growth of Aligned Single-Crystalline Rutile TiO2 Nanowires on Arbitrary Substrates and Their Application in Dye-Sensitized Solar Cells. J. Phys. Chem. C 2010, 114, 7787–7792. [Google Scholar] [CrossRef]
- Cevc, G.; Vierl, U. Nanotechnology and the transdermal route: A state of the art review and critical appraisal. J. Control. Release 2010, 141, 277–299. [Google Scholar] [CrossRef] [PubMed]
- Lincks, J.; Boyan, B.D.; Blanchard, C.R.; Lohmann, C.H.; Liu, Y.; Cochran, D.L.; Dean, D.D.; Schwartz, Z. Response of MG63 osteoblast-like cells to titanium and titanium alloy is dependent on surface roughness and composition. Biomaterials 1998, 19, 2219–2232. [Google Scholar] [CrossRef]
- Bacakova, L.; Stary, V.; Kofronova, O.; Lisa, V. Polishing and coating carbon fiber-reinforced carbon composites with a carbon-titanium layer enhances adhesion and growth of osteoblast-like MG63 cells and vascular smooth muscle cells in vitro. J. Biomed. Mater. Res. 2001, 54, 567–578. [Google Scholar] [CrossRef]
- Khang, D.; Lu, J.; Yao, C.H.; Haberstroh, K.M.; Webster, T.J. The role of nanometer and sub-micron surface features on vascular and bone cell adhesion on titanium. Biomaterials 2008, 29, 970–983. [Google Scholar] [CrossRef] [PubMed]
- Mendoca, G.; Mendoca, D.B.S.; Aragao, F.J.L.; Cooper, L.F. Advancing dental implant surface technology—From micron-to nanotopography. Biomaterials 2008, 29, 3822–3835. [Google Scholar] [CrossRef] [PubMed]
- Stary, V.; Bacakova, L.; Hornik, J.; Chmelik, V. Bio-compatibility of the surface layer of pyrolytic graphite. Thin Solid Films 2003, 433, 191–198. [Google Scholar] [CrossRef]
- Rosa, A.L.; Beloti, M.M. Effect of cpTi surface roughness on human bone marrow cell attachment, proliferation, and differentiation. Braz. Dent. J. 2003, 14, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Dalby, M.J.; Riehle, M.O.; Johnstone, H.; Affrossman, S.; Curtis, A.S. Investigating the limits of filopodial sensing: A brief report using SEM to image the interaction between 10 nm high nano-topography and fibroblast filopodia. Cell Biol. Int. 2004, 28, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Riehle, M.; Dalby, M.J.; Johnstone, H.; McIntosh, A.; Affrossman, S. Cell behaviour of rat calvaria bone cells on surfaces with random nanometric features. Mater. Sci. Eng. C 2003, 23, 337–340. [Google Scholar] [CrossRef]
- Lee, J.W.; Lee, K.B.; Jeon, H.S.; Park, H.K. Effects of surface nano-topography on human osteoblast filopodia. Anal. Sci. 2011, 27, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Furuhashi, A.; Ayukawa, Y.; Atsuta, I.; Okawachi, H.; Koyano, K. The difference of fibroblast behavior on titanium substrats with different surface characteristics. Odontology 2012, 100, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Mathew, D.; Bhardwaj, G.; Wang, Q.; Sun, L.; Ercan, B.; Geetha, M.; Webster, T.J. Decreased Staphylococcus aureus and increased osteoblast density on nanostructured electrophoretic-deposited hydroxyapatite on titanium without the use of pharmaceuticals. Int. J. Nanomed. 2014, 9, 1775–1781. [Google Scholar]
- Patel, S.S.; Aruni, W.; Inceoglu, S.; Akpolat, Y.T.; Botimer, G.D.; Cheng, W.K.; Danisa, O.A. A comparison of Staphylococcus aureus biofilm formation on cobalt-chrome and titanium-alloy spinal implants. J. Clin. Neurosci. 2016, 31, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Memarzadeh, K.; Chang, B.; Zhang, Y.; Ma, Z.; Allaker, R.P.; Ren, L.; Yang, K. Antibacterial effect of copper-bearing titanium alloy (Ti-Cu) against Streptococcus mutans and Porphyromonas gingivalis. Sci. Rep. 2016, 6, 29985. [Google Scholar] [CrossRef] [PubMed]
- Ercan, B.; Kummer, K.M.; Tarquinio, K.M.; Webster, T.J. Decreased Staphylococcus aureus biofilm growth on anodized nanotubular titanium and the effect of electrical stimulation. Acta Biomater. 2011, 7, 3003–3012. [Google Scholar] [CrossRef] [PubMed]
- Puckett, S.D.; Taylor, E.; Raimondo, T.; Webster, T.J. The relationship between the nanostructure of titanium surfaces and bacterial attachement. Biomater. 2010, 31, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, Z.; Piszczek, P.; Radtke, A.; Jędrzejewski, T.; Kozak, W.; Sadowska, B. The evaluation of the impact of titania nanotube covers morphology and crystal phase on their biological properties. J. Mater. Sci. Mater. Med. 2015, 26, 163. [Google Scholar] [CrossRef] [PubMed]
- Radtke, A.; Piszczek, P.; Topolski, A.; Lewandowska, Ż.; Talik, E.; Andersen, I.H.; Nielsen, L.P.; Heikkilä, M.; Leskelä, M. The structure and the photocatalytic activity of titania based nanotube and nanofiber coatings. Appl. Surf. Sci. 2016, 368, 165–172. [Google Scholar] [CrossRef]




© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radtke, A.; Topolski, A.; Jędrzejewski, T.; Kozak, W.; Sadowska, B.; Więckowska-Szakiel, M.; Piszczek, P. Bioactivity Studies on Titania Coatings and the Estimation of Their Usefulness in the Modification of Implant Surfaces. Nanomaterials 2017, 7, 90. https://doi.org/10.3390/nano7040090
Radtke A, Topolski A, Jędrzejewski T, Kozak W, Sadowska B, Więckowska-Szakiel M, Piszczek P. Bioactivity Studies on Titania Coatings and the Estimation of Their Usefulness in the Modification of Implant Surfaces. Nanomaterials. 2017; 7(4):90. https://doi.org/10.3390/nano7040090
Chicago/Turabian StyleRadtke, Aleksandra, Adrian Topolski, Tomasz Jędrzejewski, Wiesław Kozak, Beata Sadowska, Marzena Więckowska-Szakiel, and Piotr Piszczek. 2017. "Bioactivity Studies on Titania Coatings and the Estimation of Their Usefulness in the Modification of Implant Surfaces" Nanomaterials 7, no. 4: 90. https://doi.org/10.3390/nano7040090