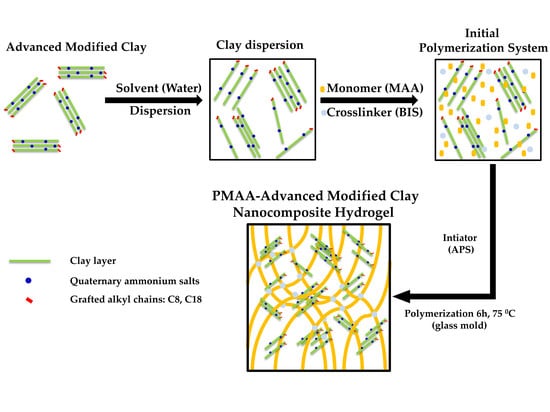
Novel Hydrogel-Advanced Modified Clay Nanocomposites as Possible Vehicles for Drug Delivery and Controlled Release
Abstract
:1. Introduction
2. Results
2.1. Hydrogel Nanocomposites Characterization
2.1.1. Swelling/De-Swelling Properties
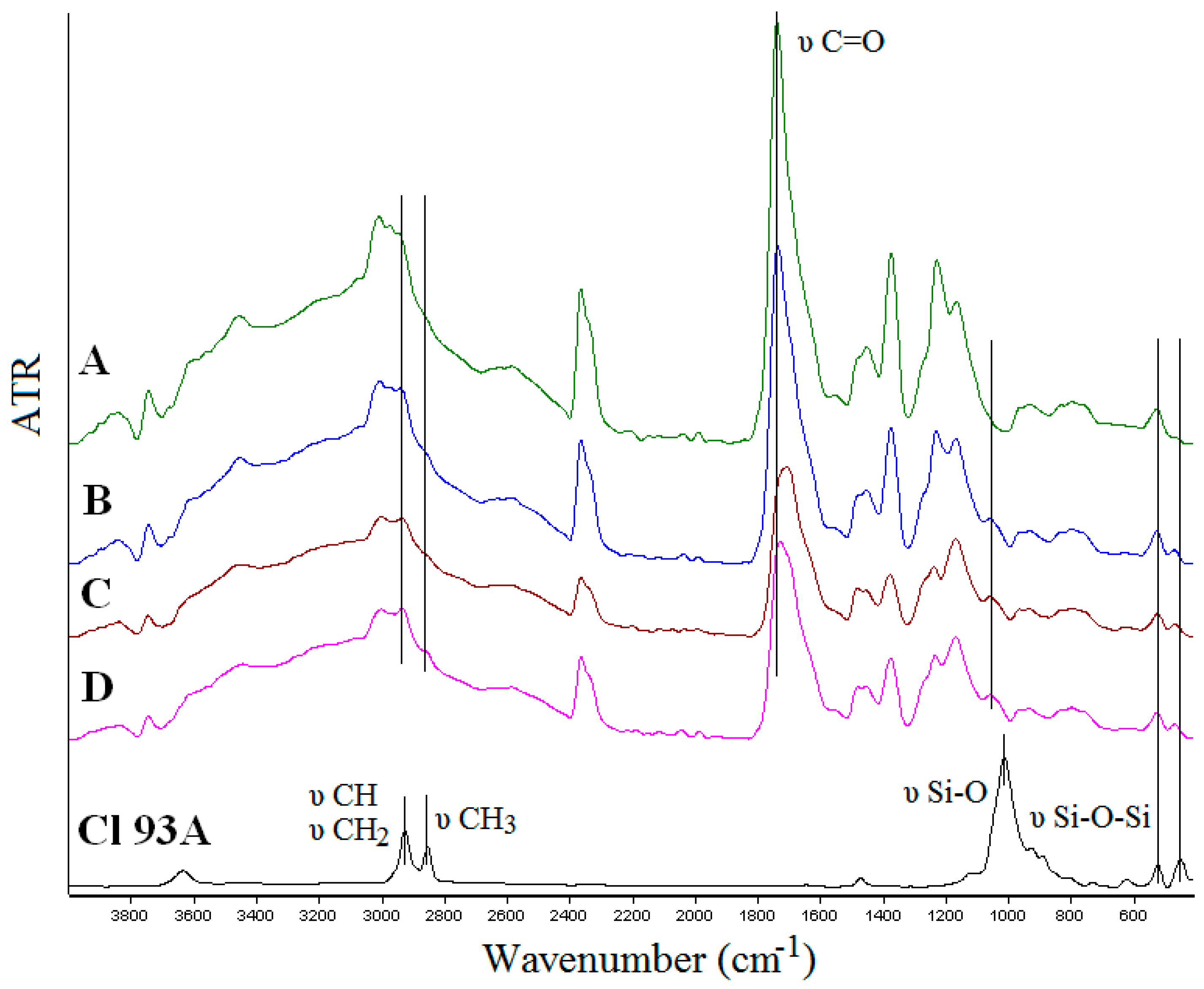
2.1.2. FTIR Spectroscopy
- -
- shifted wavenumbers values around 294 cm−1 due to CH2 stretching from PMAA and quaternary ammonium salts from clays;
- -
- shifted wavenumber values of C=O group (1736 cm−1) originated from PMAA toward lower values (Cl 93A—1733 cm−1; Cl 93A-C8—1723 cm−1; Cl 93A-C18—1707 cm−1);
- -
- the peak of Cl 93A clay from 1007 cm−1 corresponding to Si–O stretching vibration, shifted toward higher values with the inclusion of clay in the PMAA matrix (1036 cm−1);
- -
- clay peaks observed at 516 cm−1 and 441 cm−1 (Si–O–Si and Al–O–Si deformation) appeared in the nanocomposites FTIR spectra at higher values 518 cm−1 and 464 cm−1.
2.1.3. X-ray Diffractograms
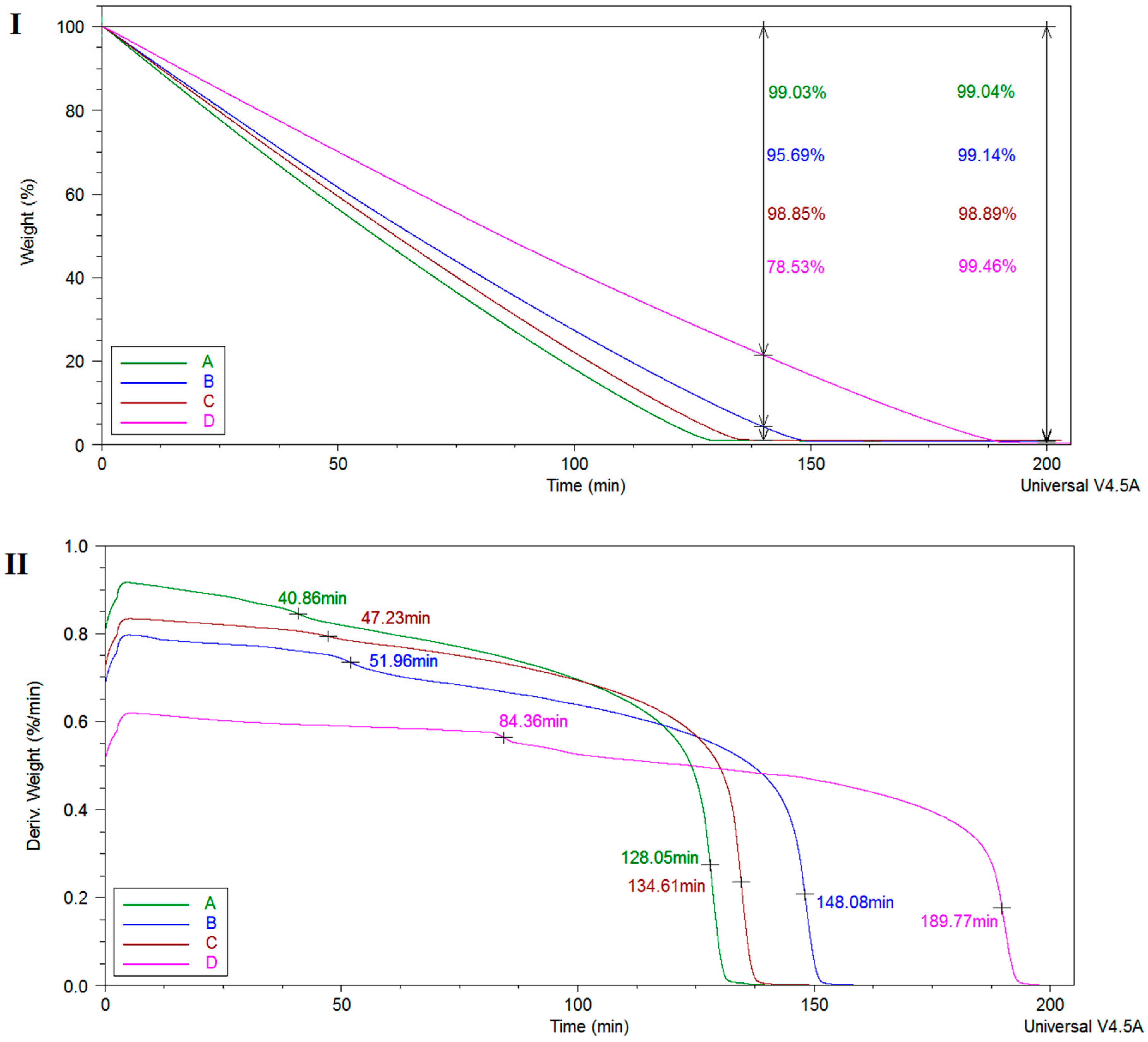
2.1.4. Thermo-Mechanical Characteristics
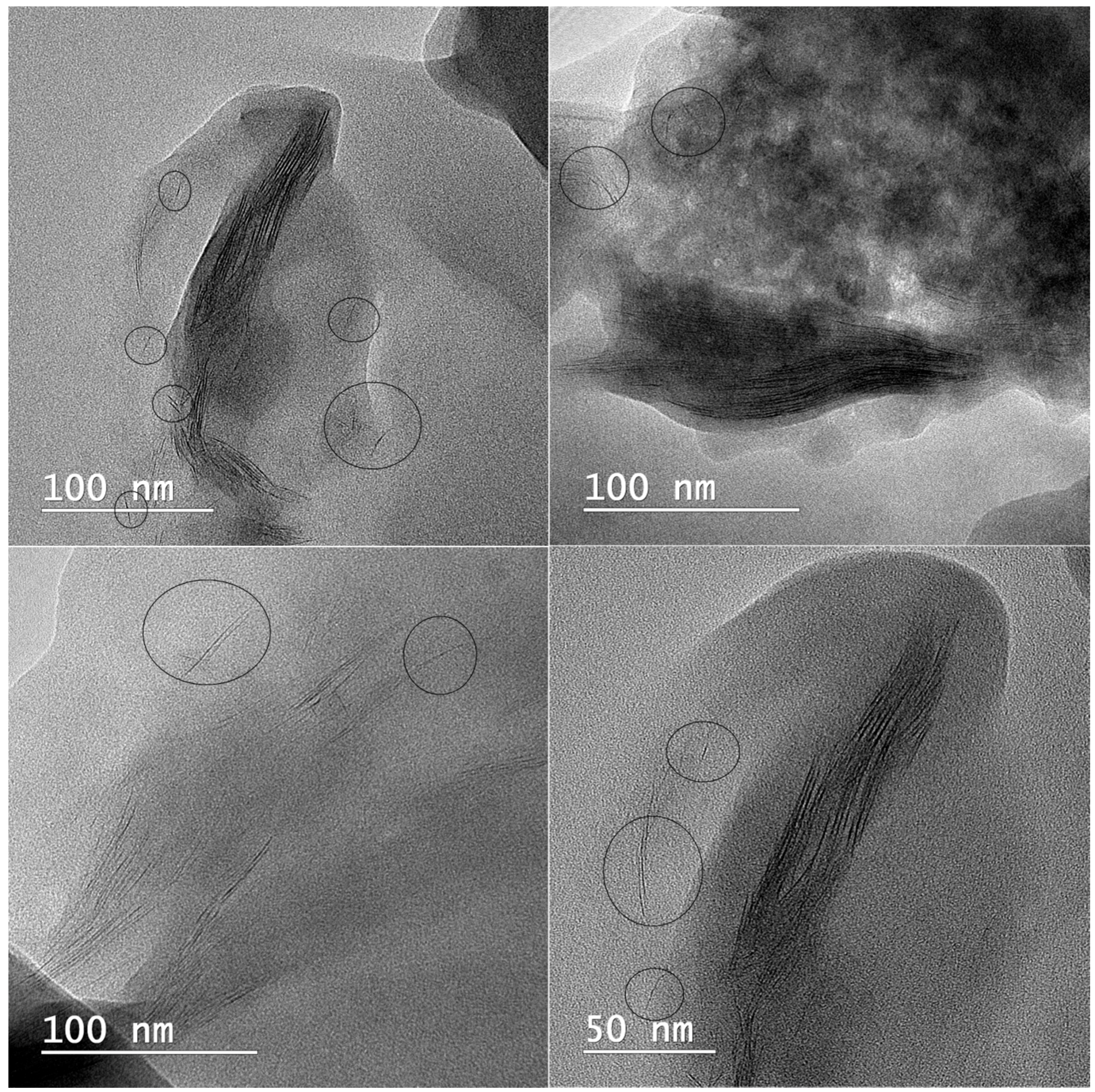
2.1.5. Microscopy Analyses
2.2. Cell Proliferation Assay
2.2.1. Cytotoxicity
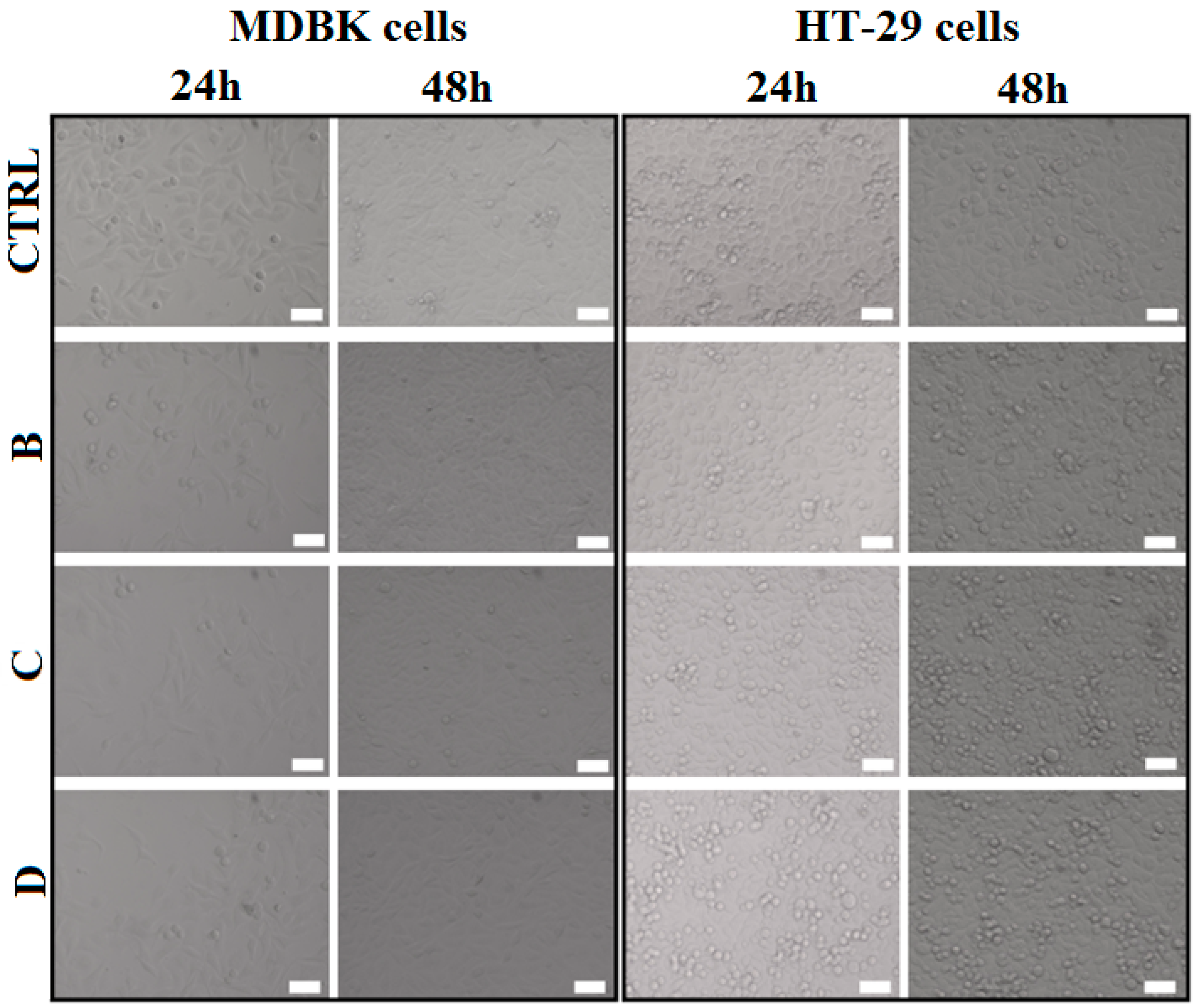
2.2.2. Cell Morphology
3. Experimental Section
3.1. Materials
3.2. The Synthesis of Composite Hydrogels
3.3. Hydrogel Nanocomposites Characterization
3.3.1. Physical-Chemical Characterization
3.3.2. In Vitro Cell Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hennink, W.E.; Nostrum, C.F. Novel crosslinking methods to design hydrogels. Adv. Drug Deliv. Rev. 2012, 64, 223–236. [Google Scholar] [CrossRef]
- Li, L.L.; Wang, Y.Q.; Pan, L.J.; Shi, Y.; Cheng, W.; Shi, Y.; Yu, G.H. A Nanostructured Conductive Hydrogels-Based Biosensor Platform for Human Metabolite Detection. Nano Lett. 2015, 15, 1146–1151. [Google Scholar] [CrossRef] [PubMed]
- Lau, H.K.; Kiick, K.L. Opportunities for multicomponent hybrid hydrogels in biomedical applications. Biomacromolecules 2015, 16, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Hood, M.A.; Mari, M.; Muñoz-Espí, R. Synthetic strategies in the preparation of polymer/ınorganic hybrid nanoparticles. Materials 2014, 7, 4057–4087. [Google Scholar] [CrossRef] [PubMed]
- Catauro, M.; Bollino, F.; Papale, F.; Gallicchio, M.; Pacifico, S. Synthesis and chemical characterization of new silica polyethylene glycol hybrid nanocompositem materials for controlled drug delivery. J. Drug Deliv. Sci. Technol. 2014, 24, 320–325. [Google Scholar] [CrossRef]
- Hickey, D.J.; Ercan, B.; Sun, L.; Webster, T.J. Adding MgO nanoparticles to hydroxyapatite PLLA nanocomposites for improved bone tissue engineering applications. Acta Biomater. 2015, 14, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Bounabi, L.; Mokhnachi, N.B.; Haddadine, N.; Ouazib, F.; Barille, R. Development of poly(2-hydroxyethyl methacrylate)/clay composites as drug delivery systems of paracetamol. J. Drug Deliv. Sci. Technol. 2016, 33, 58–65. [Google Scholar] [CrossRef]
- Haraguchi, K.; Li, H.J.; Matsuda, K.; Takehisa, T.; Elliott, E. Mechanism of Forming Organic/Inorganic Network Structures During in-Situ Free-Radical Polymerization in PNIPAM Clay Nanocomposite Hydrogels. Macromolecules 2005, 38, 3482–3490. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, Characterization and Applications: A Review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Lu, X.; Liu, K.; Wang, K.; Fang, L.; Weng, L.-T.; Zhang, H.; Tang, Y.; Ren, F.; Zhao, C.; et al. Mussel-Inspired Adhesive and Tough Hydrogel Based on Nanoclay Confined Dopamine Polymerization. ACS Nano 2017, 11, 2561–2574. [Google Scholar] [CrossRef] [PubMed]
- Cadenea, A.; Durand-Vidala, S.; Turqa, P.; Brendle, J. Study of individual Na-montmorillonite particle size and morpholgy and aparent charge. J. Colloid Interface Sci. 2005, 285, 719–730. [Google Scholar] [CrossRef] [PubMed]
- Do Nascimento, G.M. Structure of Clays and Polymer-Clay CompositesStudied by X-ray Absorption Spectroscopies. Appl. Clay Sci. 2017, 146, 439–448. [Google Scholar] [CrossRef]
- Miotke, M.; Strankowska, J.; Kwela, J.; Strankowski, M.; Piszczyk, L.; Józefowicz, M.; Gazda, M. Nanosize effect of clay mineral nanoparticles on the drug diffusion processes in polyurethane nanocomposite hydrogels. Eur. Phys. J. Plus 2017, 132, 401. [Google Scholar] [CrossRef]
- Takeno, H.; Kimuri, Y.; Nakamura, W. Mechanical, Swelling and Structural Properties of Mechanically Tough Clay-Sodium Polyacrylate Blend Hydrogels. Gels 2017, 3, 10. [Google Scholar] [CrossRef]
- Guilherme, M.R.; Fajardo, A.R.; Moia, T.A.; Kunita, M.H.; Goncalves, M.D.; Rubira, A.F.; Tambourgi, E.B. Porous Nanocomposite Hydrogel of Vinyled Montmorillonite-Crosslinked Maltodextrin-Co-Dimethylacrylamide as a Highly Stable Polymer Carrier for Controlled Release Systems. Eur. Polym. J. 2010, 46, 1465–1474. [Google Scholar] [CrossRef]
- Mahdavinia, G.R.; Hasanpour, J.; Rahmani, Z.; Karami, S.; Etemadi, H. Nanocomposite Hydrogel from Grafting of Acrylamide onto Hpmc Using Sodium Montmorillonite Nanoclay and Removal of Crystal Violet Dye. Cellulose 2013, 20, 2591–2604. [Google Scholar] [CrossRef]
- Ferfera-Harrar, H.; Aiouaz, N.; Dairi, N.; Hadj-Hamou, A.S. Preparation of Chitosan-g-Poly(acrylamide)/Montmorillonite Superabsorbent Polymer Composites: Studies on Swelling, Thermal and Antibacterial Properties. J. Appl. Polym. Sci. 2014, 131, 39747–39761. [Google Scholar] [CrossRef]
- Gao, D.Y.; Heimann, R.B.; Williams, M.C.; Wardhaugh, L.T.; Muhammad, M. Rheological Properties of Poly(acrylamide)-Bentonite Composite Hydrogels. J. Mater. Sci. 1999, 34, 1543–1552. [Google Scholar] [CrossRef]
- Su, X.; Mahalingam, S.; Edirisinghe, M.; Chen, B. Highly stretchable and highly resilient polymerclay nanocomposite hydrogels with low hysteresis. ACS Appl. Mater. Interfaces 2017, 9, 22223–22234. [Google Scholar] [CrossRef]
- Asgari, M.; Abouelmagd, A.; Sundararaj, A. Silane functionalization of sodium montmorillonite nanoclay and its effect on rheological and mechanical properties of HDPE/clay nanocomposites. Appl. Clay Sci. 2017, 146, 439–448. [Google Scholar] [CrossRef]
- Piscitelli, F.; Posocco, P.; Toth, R.; Fermeglia, M.; Pricl, S.; Mensitieri, G.; Lavorgna, M. Sodium montmorillonite silylation: Unexpected effect of the aminosilane chain length. J. Colloid Interface Sci. 2010, 351, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kima, B.; Seo, D.; Rhee, K.; Lyu, Y. Effects of a silane treatment on the mechanical interfacial properties of montmorillonite/epoxy nanocomposites. Mater. Sci. Eng. A 2009, 526, 74–78. [Google Scholar] [CrossRef]
- Piscitelli, F.; Scamardella, A.M.; Romeo, V.; Lavorgna, M.; Barra, G.; Amendola, E. Epoxy composites based on amino-silylated Mt: The role of interfaces and clay morphology. J. Appl. Polym. Sci. 2012, 124, 616–628. [Google Scholar] [CrossRef]
- Haragouchi, K.; Takehisa, H.; Fan, S. Effects of Clay Content on the Properties of Nanocomposite Hydrogels Composed of Poly(N-isopropylacrylamide) and Clay. Macromolecules 2002, 35, 10162–10171. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, M.; Liu, X.; Zhang, W.; Sun, B.; Chen, Y.; Adler, H.-J.P. High clay content nanocomposite hydrogels with surprising mechanical strength and interesting de-swelling kinetics. Polymer 2006, 47, 1–5. [Google Scholar] [CrossRef]
- Fukasawa, M.; Sakai, T.; Chung, U.; Haraguchi, K. Synthesis and Mechanical Properties of a Nanocomposite Gel Consisting of a Tetra-PEG/Clay Network. Macromolecules 2010, 43, 4370–4378. [Google Scholar] [CrossRef]
- Chang, C.-W.; Van Spreeuwel, A.; Zhang, C.; Varghese, S. PEG/clay nanocomposite hydrogel: A mechanically robust tissue engineering scaffold. Soft Matter 2010, 6, 5157–5164. [Google Scholar] [CrossRef]
- Chen, Y.; Pen, Y.; Liu, W.; Zeng, G.; Li, X.G.; Yan, X.H. Effect of AMPS and Clay on the Acrylic Acid Based Superabsorbent Hydrogels. Appl. Mech. Mater. 2013, 427–429, 364–367. [Google Scholar] [CrossRef]
- Shen, M.; Li, L.; Xu, J.; Guo, X.; Prud’homme, R. Rheology and Adhesion of Poly(acrylic acid)/Laponite Nanocomposite Hydrogels as Biocompatible Adhesives. Langmuir 2014, 30, 1636–1642. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Gersappe, D. Structure formation in nanocomposite hydrogels. Soft Matter 2017, 13, 1850–1861. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.H.; Pawar, K.C.; Jog, J.P.; Badiger, M.V. Swelling and mechanical behavior of modified poly(vinyl alcohol)/laponite nanocomposite membranes. J. Appl. Polym. Sci. 2006, 5, 2869–2903. [Google Scholar] [CrossRef]
- Gocalves, M.; Figueira, P.; Maciel, D.; Rodrigues, J.; Qu, X.; Liu, C.; Toams, H.; Li, Y. pH-sensitive Laponite/doxorubicin/alginate nanohybrids with improved anticancer efficacy. Acta Biomater. 2014, 10, 300–307. [Google Scholar] [CrossRef]
- Nagahama, K.; Kawano, D.; Oyama, N.; Takemoto, A.; Kumano, T.; Kawami, J. Self-Assembling Polymer Micelle/Clay Nanodisk/Doxorubicin Hybrid Injectable Gels for Safe and Efficient Focal Treatment of Cancer. Biomacromolecules 2015, 16, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Castro, R.; Maciel, D.; Goncalves, M.; Chi, X.; Rodrigues, J.; Toams, H. Fine tuning of the pH-sensitivity of laponite-doxorubicin nanohybrids by polyelectrolyte multilayer coating. Mater. Sci. Eng. C 2016, 60, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.J.A.; Lugao, A.B.; Parra, D.F. Nanocomposite Polymer Clay to Support the Release of Drug. Mater. Sci. Forum 2017, 899, 335–340. [Google Scholar] [CrossRef]
- Ianchis, R.; Rosca, I.D.; Ghiurea, M.; Spataru, C.I.; Nicolae, C.A.; Gabor, R.; Raditoiu, V.; Preda, S.; Fierascu, R.C.; Donescu, D. Synthesis and properties of new epoxy-organolayered silicates nanocomposites. Appl. Clay Sci. 2015, 103, 28–33. [Google Scholar] [CrossRef]
- Qi, X.; Wei, W.; Li, J.; Liu, Y.; Hu, X.; Zhang, J.; Bi, L.; Dong, W. Fabrication and Characterization of a Novel Anticancer Drug Delivery System: Salecan/Poly(methacrylic acid) Semi-Interpenetrating Polymer Network Hydrogel. ACS Biomater. Sci. Eng. 2015, 1, 1287–1299. [Google Scholar] [CrossRef]
- Liew, C.-W.; Ng, H.-M.; Numan, A.; Ramesh, S. Poly(Acrylic acid)-Based Hybrid Inorganic-Organic Electrolytes Membrane for Electrical Double Layer Capacitors Application. Polymer 2016, 8, 179. [Google Scholar] [CrossRef]
- Mohd, S.S.; Abdullah, M.A.A.; Amin, K.A.M. Gellan gum/clay hydrogels for tissue engineering application: Mechanical, thermal behavior, cell viability and antibacterial properties. J. Bioact. Compat. Polym. 2016, 31, 648–666. [Google Scholar] [CrossRef]
- Garcia, D.M.; Escobar, J.L.; Bada, N.; Casquero, J.; Hernaez, E.; Katime, I. Synthesis and characterization of poly(methacrylic acid) hydrogels for metoclopramide delivery. Eur. Polym. J. 2004, 40, 1637–1643. [Google Scholar] [CrossRef]
- Amin, A.; Sarkar, R.; Moorefield, C.N.; Newkome, G.R. Synthesis of polymer-clay nanocomposites of some vinyl monomers by surface-initiated atom transfer radical polymerization. Des. Monomers Polym. 2013, 16, 528–536. [Google Scholar] [CrossRef]
- Nayak, P.J.; Singh, B.K. Instrumental characterization of clay by XRF, XRD and FTIR. Bull. Mater. Sci. 2007, 30, 235. [Google Scholar] [CrossRef]
- Ianchis, R.; Donescu, D.; Cinteza, L.O.; Purcar, V.; Nistor, C.L.; Petcu, C.; Nicolae, C.A.; Gabor, R.; Preda, S. Polymer-clay nanocomposites obtained by solution polymerization of vinyl benzyl triammonium chloride in the presence of advanced functionalized clay. J. Chem. Sci. 2014, 126, 609–616. [Google Scholar] [CrossRef]
- Kamei, D.; Ajiro, H.; Hongo, C.; Akashi, M. Solvent Effects on Isotactic Poly(methyl methacrylate) Crystallization and Syndiotactic Poly(methacrylic acid) Incorporation in Porous Thin Films Prepared by Stepwise Stereocomplex Assembly. Langmuir 2009, 25, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Azhar, F.F.; Olad, A. A study on sustained release formulations for oral delivery of 5-fluorouracil based on alginate-chitosan/montmorillonite nanocomposite systems. Appl. Clay Sci. 2014, 101, 288–296. [Google Scholar] [CrossRef]
- Sanglimsuwan, A.; Seeponkai, N.; Wootthikanokkhan, J. Effects of Concentration of Organically Modified Nanoclay on Properties of Sulfonated Poly(vinyl alcohol)Nanocomposite Membranes. Int. J. Electrochem. 2011, 2011, 785282. [Google Scholar] [CrossRef]
- Martinez, H.; Rodriguez-Lazcano, Y.; Castillo, F. Comparative study on the decomposition process of N-isopropylacrylamide in He, N2 and air plasmas. Plasma Sources Sci. Technol. 2007, 16, 427. [Google Scholar] [CrossRef]
- Jawaid, M.; Mohammad, F. Nanocellulose and Nanohydrogel Matrices: Biotechnological and Biomedical Applications; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2017. [Google Scholar] [CrossRef]
- Shinde, V.S.; Girme, M.R.; Pawar, V.U. Thermoresponsive polystyrene-b-poly(N-isopropylacrylamide) copolymers by atom transfer radical polymerization. Indian J. Chem. 2011, 50, 781–787. [Google Scholar]
- Nakan, U.; Rakhmetullayeva, R.K.; Urkimbaeva, P.I.; Shaikhutdinov, E.M.; Mun, G.A.; Yeligbayeva, G.Z.; Negim, E.S.M. The Effect of Nanoparticle Silver on the Thermal Stability of N-isopropylacrylamide (NIPAAm). World Appl. Sci. J. 2014, 29, 359–364. [Google Scholar] [CrossRef]
- Harun, N.A.; Kassim, S.; Muhammad, S.T.; Rohi, F.E.; Norzam, N.N.; Tahier, N.S.M. The effect of nonionic surfactants on emulsion polymerization of poly(methacrylic acid) nanoparticles. AIP Conf. Proc. 2017, 1885, 020032. [Google Scholar] [CrossRef]
- Caminos-Peruelo, D.; Wang, W.C.; Chin, T.S.; So, C.R.; Fabicon, R.M.; Hsieh, M.F. Preparation, characterization of chitosan/bamboo charcoal/poly(methacrylate) composite beads. Bull. Mater. Sci. 2017, 40, 1179–1187. [Google Scholar] [CrossRef]
- Meirelles, L.A.; Raffin, F.V. Clay and Polymer-Based Composites Applied to Drug Release: A Scientific and Technological Prospection. J. Pharm. Pharm. Sci. 2017, 20, 115–134. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Yao, D.; Deng, L.; Dong, A.; Zhang, J. Composites of Polymer Hydrogels and Nanoparticulate Systems for Biomedical and Pharmaceutical Applications. Nanomaterials 2015, 5, 2054–2130. [Google Scholar] [CrossRef] [PubMed]
- Guler, M.A.; Gok, M.K.; Figen, A.K.; Ozgumus, S. Swelling, mechanical and mucoadhesion properties of Mt/starch-g-PMAA nanocomposite hydrogels. Appl. Clay Sci. 2015, 112–113, 44–52. [Google Scholar] [CrossRef]
- Okay, O.; Oppermann, W. Polyacrylamide−Clay Nanocomposite Hydrogels: Rheological and Light Scattering Characterization. Macromolecules 2007, 40, 3378. [Google Scholar] [CrossRef]
- Abdurrahmanoglu, S.; Can, V.; Okay, O. Equilibrium swelling behavior and elastic properties of polymer-clay nanocomposite hydrogels. J. Appl. Polym. Sci. 2008, 109, 3714–3724. [Google Scholar] [CrossRef]
- Abdurrahmanoglu, S.; Can, V.; Okay, O. Design of high-toughness polyacrylamide hydrogels by hydrophobic modification. Polymer 2009, 50, 5449–5455. [Google Scholar] [CrossRef]
- Nesrinne, S.; Djamel, A. Synthesis, characterization and rheological behavior of pH sensitive poly(acrylamide-co-acrylic acid) hydrogels. Arabian J. Chem. 2017, 10, 539–547. [Google Scholar] [CrossRef]
- Garcia-Hernandez, A.; Lobatos-Calleros, C.; Vernon-Carter, E.J.; Sosa-Hernandez, S.; Alvarez-Ramirez, J. Effects of clay concentration on the morphology and rheological properties of xanthan gum-based hydrogels reinforced with montmorillonite particles. J. Appl. Polym. Sci. 2017, 134, 8. [Google Scholar] [CrossRef]
- Zaman, I.; Le, Q.H.; Kuan, H.C.; Kawashima, N.; Luonag, L.; Gerson, A.; Ma, J. Interfacetuned epoxy/clay nanocomposites. Polymer 2011, 52, 497–504. [Google Scholar] [CrossRef]
- Mangal, R.; Srivastava, S.; Archer, L.A. Phase stability and dynamics of entangled polymer-nanoparticle composites. Nat. Commun. 2015, 6, 7198. [Google Scholar] [CrossRef] [PubMed]
- Caprarescu, S.; Ianchis, R.; Radu, A.-L.; Sarbu, A.; Somoghi, R.; Trica, B.; Alexandrescu, E.; Spataru, C.-I.; Fierascau, R.-C.; Ion-Ebrasu, D.; et al. Synthesis, characterization and efficiency of new organically modified montmorillonite polyethersulfone membranes for removal of zinc ions from wastewasters. Appl. Clay Sci. 2017, 137, 135–142. [Google Scholar] [CrossRef]
- Longo, S.; Mauro, M.; Daniel, C.; Galimberti, M.; Guerra, G. Clay exfoliation and polymer/clay aerogels by supercritical carbon dioxide. Front. Chem. 2013, 1, 28. [Google Scholar] [CrossRef] [PubMed]
- Decker, J.J.; Chvalun, S.N.; Nazarenko, S. Intercalation behavior of hydroxylated dendritic polyesters in polymer clay nanocomposites prepared from aqueous solution. Polymer 2011, 52, 3943–3955. [Google Scholar] [CrossRef]
- Lordan, S.; Kennedy, J.E.; Higginbotham, C.L. Cytotoxic effects induced by unmodified and organically modified nanoclays in the human hepatic HepG2 cell line. J. Appl. Toxicol. 2011, 31, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Bragg, W. The Diffraction of Short Electromagnetic Waves by a Crystal. Proc. Camb. Philos. Soc. 1913, 17, 43–57. [Google Scholar]
- Roseanu, A.; Florian, P.E.; Moisei, M.; Sima, L.E.; Evans, R.W.; Trif, M. Liposomalization of lactoferrin enhanced its anti-tumoral effects on melanoma cells. Biometals 2010, 23, 485–492. [Google Scholar] [CrossRef] [PubMed]






| Sample | 2 Theta (Å) | d-Spacing (nm) | ||
|---|---|---|---|---|
| (001) | (002) | (001) | (002) | |
| Cl 93A | 3.28 | 6.55 | 26.88 | 13.48 |
| PMAA | - | - | - | - |
| PMAA-Cl 93A | 2.99 | 6.39 | 29.48 | 13.83 |
| PMAA-Cl 93A-C8 | 3.12 | 6.40 | 28.22 | 13.80 |
| PMAA-Cl 93A-C18 | 2.99 | 6.43 | 29.50 | 13.74 |
| Sample | TGA/DTG | DMA | ||||||
|---|---|---|---|---|---|---|---|---|
| Weight Loss Intervals (%) | Decomposition Temperatures (°C) | Residue 700 °C (%) | Tg1 (°C) | Tg2 (°C) | ||||
| 0–120 °C | 120 °C–300 °C | 300 °C–700 °C | T1 | T2 | ||||
| A | 8.28 | 10.63 | 71.43 | 230 | 420 | 9.59 | 85.15 | 226.30 |
| B | 6.46 | 10.88 | 70.88 | 229 | 421 | 11.43 | 96.52 | 227.46 |
| C | 6.96 | 10.30 | 71.26 | 231 | 380/419 | 11.48 | 94.36 | 233.27 |
| D | 6.71 | 10.10 | 70.47 | 230 | 381/410 | 12.73 | 110.81 | 231.56 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ianchis, R.; Ninciuleanu, C.M.; Gifu, I.C.; Alexandrescu, E.; Somoghi, R.; Gabor, A.R.; Preda, S.; Nistor, C.L.; Nitu, S.; Petcu, C.; et al. Novel Hydrogel-Advanced Modified Clay Nanocomposites as Possible Vehicles for Drug Delivery and Controlled Release. Nanomaterials 2017, 7, 443. https://doi.org/10.3390/nano7120443
Ianchis R, Ninciuleanu CM, Gifu IC, Alexandrescu E, Somoghi R, Gabor AR, Preda S, Nistor CL, Nitu S, Petcu C, et al. Novel Hydrogel-Advanced Modified Clay Nanocomposites as Possible Vehicles for Drug Delivery and Controlled Release. Nanomaterials. 2017; 7(12):443. https://doi.org/10.3390/nano7120443
Chicago/Turabian StyleIanchis, Raluca, Claudia M. Ninciuleanu, Ioana C. Gifu, Elvira Alexandrescu, Raluca Somoghi, Augusta R. Gabor, Silviu Preda, Cristina L. Nistor, Sabina Nitu, Cristian Petcu, and et al. 2017. "Novel Hydrogel-Advanced Modified Clay Nanocomposites as Possible Vehicles for Drug Delivery and Controlled Release" Nanomaterials 7, no. 12: 443. https://doi.org/10.3390/nano7120443