Evaluation of Fibrin-Based Interpenetrating Polymer Networks as Potential Biomaterials for Tissue Engineering
Abstract
:1. Introduction
2. Results
2.1. Synthesis of Fibrin-Based IPN Hydrogels
2.2. Cytocompatibility of IPN Hydrogels
2.3. Interactions of Organotypic-Derived Cells with IPN Hydrogels
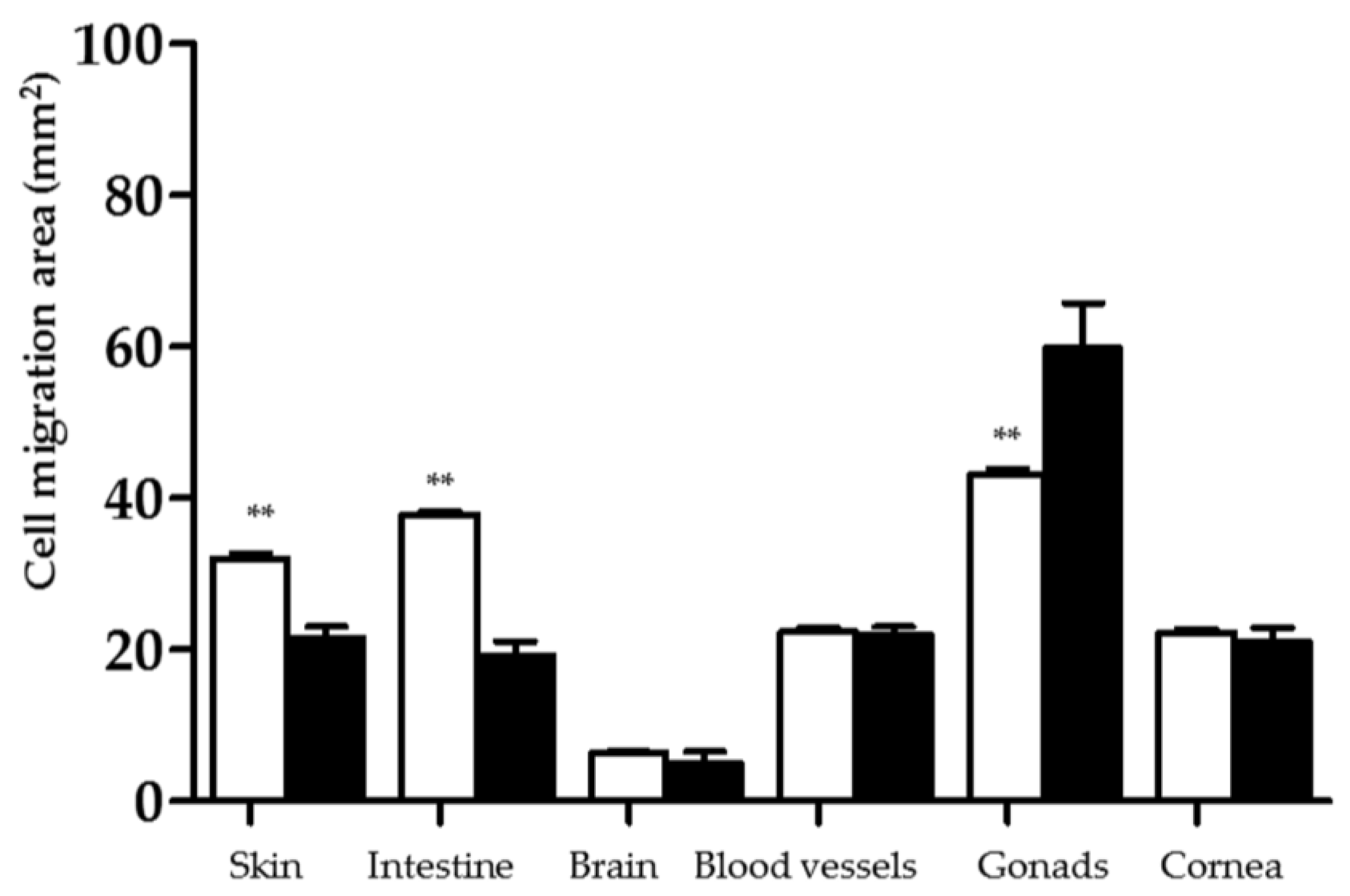
2.3.1. Cell Migration from Tissues to IPN Hydrogels
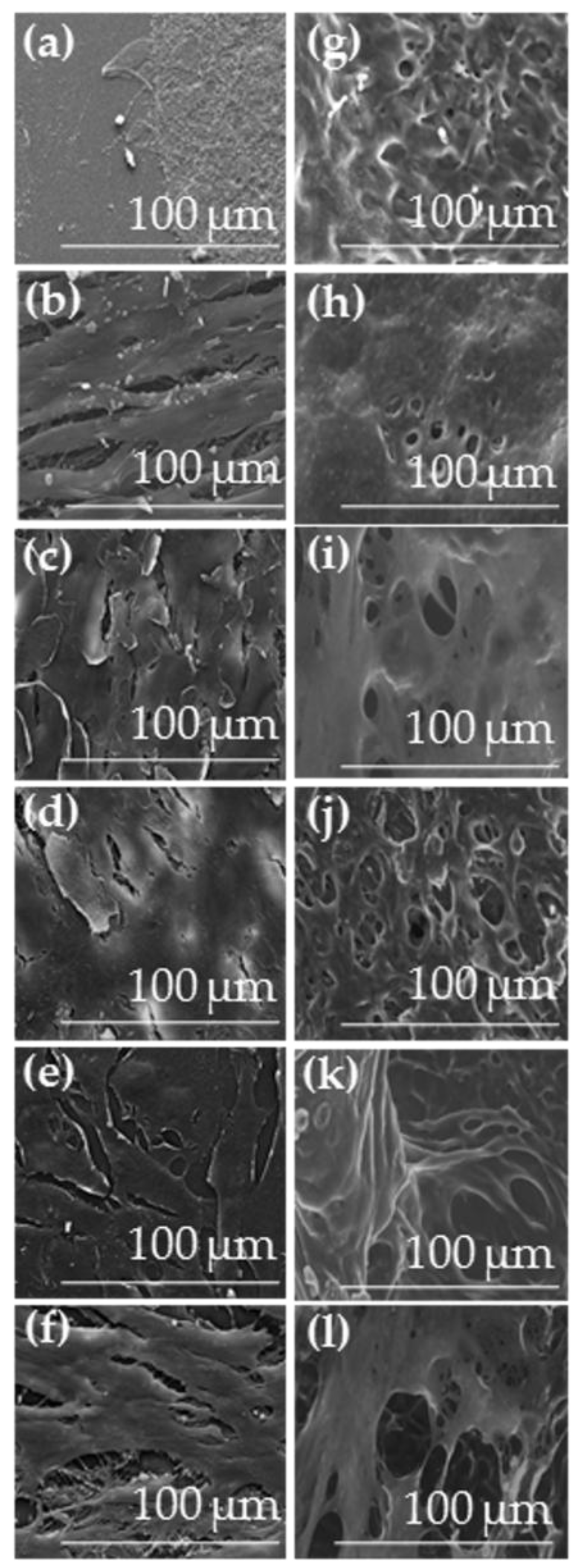
2.3.2. Cell Density Assessment on IPN Hydrogels
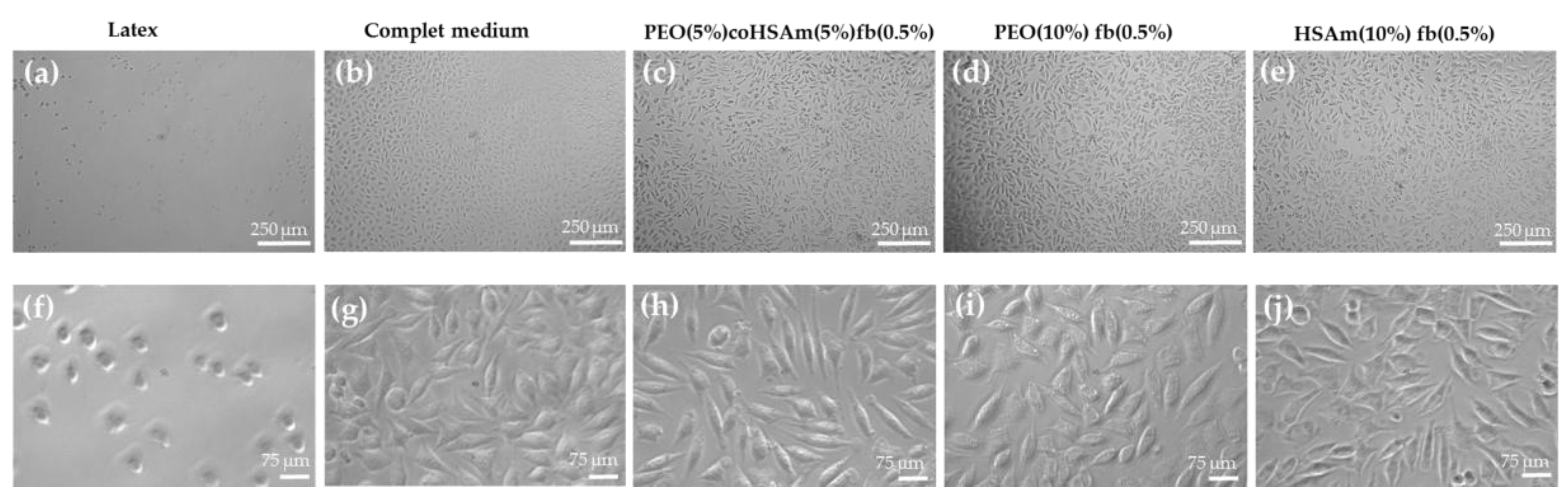
2.3.3. Cell Adhesion Assessment on IPN Hydrogels
2.3.4. Cell Morphology on IPN Hydrogels
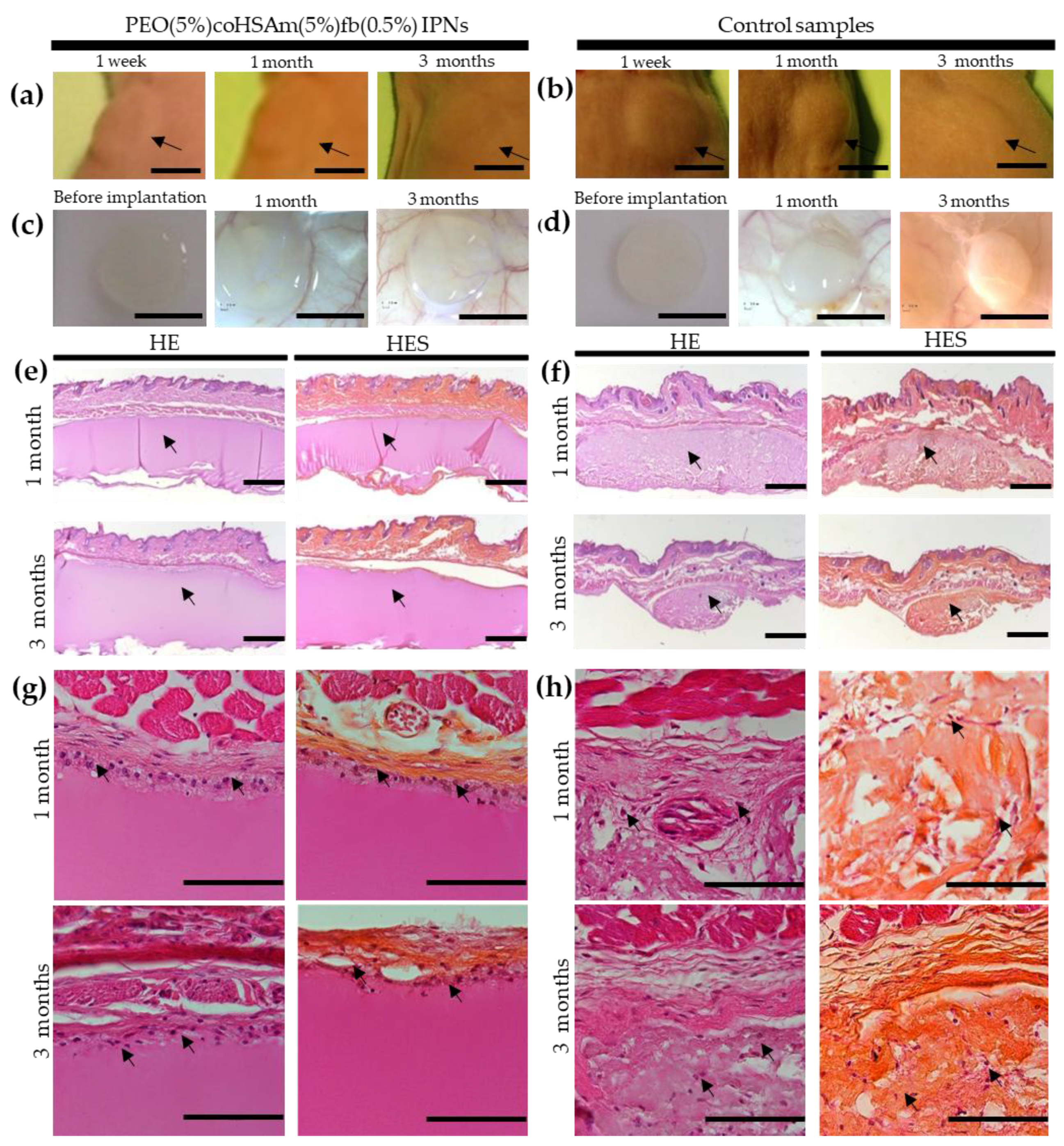
2.4. In Vivo Implantations
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Functionalization of Serum Albumin with Methacrylate Groups
4.3. Hydrogel Syntheses
4.4. Biological Characterization
4.4.1. MTS Cytotoxic Assay Following the ISO-10993 Guidelines
4.4.2. Organotypic Culture
4.4.3. Subcutaneous Implantations
4.5. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Bencherif, S.A.; Srinivasan, A.; Horkay, F.; Hollinger, J.O.; Matyjaszewski, K.; Washburn, N.R. Influence of the degree of methacrylation on hyaluronic acid hydrogels properties. Biomaterials 2008, 29, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Bencherif, S.A.; Srinivasan, A.; Sheehan, J.A.; Walker, L.M.; Gayathri, C.; Gil, R.; Hollinger, J.O.; Matyjaszewski, K.; Washburn, N.R. End-group effects on the properties of PEG-co-PGA hydrogels. Acta Biomater. 2009, 5, 1872–1883. [Google Scholar] [CrossRef] [PubMed]
- Bencherif, S.A.; Sheehan, J.A.; Hollinger, J.O.; Walker, L.M.; Matyjaszewski, K.; Washburn, N.R. Influence of cross-linker chemistry on release kinetics of PEG-co-PGA hydrogels. J. Biomed. Mater. Res. A 2009, 90A, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Lin-Gibson, S.; Bencherif, S.; Antonucci, J.M.; Jones, R.L.; Horkay, F. Synthesis and Characterization of Poly(ethylene glycol) Dimethacrylate Hydrogels. Macromol. Symp. 2005, 227, 243–254. [Google Scholar] [CrossRef]
- Bencherif, S.A.; Braschler, T.M.; Renaud, P. Advances in the design of macroporous polymer scaffolds for potential applications in dentistry. J. Periodontal Implant Sci. 2013, 43, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.E.; Barth, T.A.; Bencherif, S.A.; Washburn, N.R. Complex Fluids Based on Methacrylated Hyaluronic Acid. Biomacromolecules 2010, 11, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Seliktar, D. Designing cell-compatible hydrogels for biomedical applications. Science 2012, 336, 1124–1128. [Google Scholar] [CrossRef] [PubMed]
- Bencherif, S.A.; Sands, R.W.; Ali, O.A.; Li, W.A.; Lewin, S.A.; Braschler, T.M.; Shih, T.-Y.S.; Verbeke, C.S.; Bhatta, D.; Dranoff, G.; et al. Injectable Cryogel-based Whole Cell Cancer Vaccines. Nat. Commun. 2015, 6, 7556. [Google Scholar] [CrossRef] [PubMed]
- Bencherif, S.A.; Sands, R.W.; Bhatta, D.; Arany, P.; Verbeke, C.S.; Edwards, D.A.; Mooney, D.J. Injectable preformed scaffolds with shape-memory properties. Proc. Natl. Acad. Sci. USA 2012, 109, 19590–19595. [Google Scholar] [CrossRef] [PubMed]
- Baier Leach, J.; Bivens, K.A.; Patrick, C.W.; Schmidt, C.E. Photocrosslinked hyaluronic acid hydrogels: Natural, biodegradable tissue engineering scaffolds. Biotechnol. Bioeng. 2003, 82, 578–589. [Google Scholar] [CrossRef] [PubMed]
- Tibbitt, M.W.; Anseth, K.S. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol. Bioeng. 2009, 103, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Marchant, R.E. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev. Med. Devices 2011, 8, 607–626. [Google Scholar] [CrossRef] [PubMed]
- Gsib, O.; Egles, C.; Bencherif, S.A. Fibrin: An underrated biopolymer for skin tissue engineering. J. Mol. Biol. Biotechnol. 2017, 2. [Google Scholar]
- Janmey, P.A.; Winer, J.P.; Weisel, J.W. Fibrin gels and their clinical and bioengineering applications. J. R. Soc. Interface 2009, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.A.E.; Dare, E.V.; Hincke, M. Fibrin: A versatile scaffold for tissue engineering applications. Tissue Eng. Part B Rev. 2008, 14, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Linnes, M.P.; Ratner, B.D.; Giachelli, C.M. A fibrinogen-based precision microporous scaffold for tissue engineering. Biomaterials 2007, 28, 5298–5306. [Google Scholar] [CrossRef] [PubMed]
- Laurens, N.; Koolwijk, P.; de Maat, M.P.M. Fibrin structure and wound healing. J. Thromb. Haemost. JTH 2006, 4, 932–939. [Google Scholar] [CrossRef] [PubMed]
- Lei, P.; Padmashali, R.M.; Andreadis, S.T. Cell-controlled and spatially arrayed gene delivery from fibrin hydrogels. Biomaterials 2009, 30, 3790–3799. [Google Scholar] [CrossRef] [PubMed]
- Eyrich, D.; Brandl, F.; Appel, B.; Wiese, H.; Maier, G.; Wenzel, M.; Staudenmaier, R.; Goepferich, A.; Blunk, T. Long-term stable fibrin gels for cartilage engineering. Biomaterials 2007, 28, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.; Tawil, B.; Dunn, J.C.Y.; Wu, B.M. The behavior of human mesenchymal stem cells in 3D fibrin clots: Dependence on fibrinogen concentration and clot structure. Tissue Eng. 2006, 12, 1587–1595. [Google Scholar] [CrossRef] [PubMed]
- Rowe, S.L.; Lee, S.; Stegemann, J.P. Influence of thrombin concentration on the mechanical and morphological properties of cell-seeded fibrin hydrogels. Acta Biomater. 2007, 3, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Bidault, L.; Deneufchatel, M.; Vancaeyzeele, C.; Fichet, O.; Larreta-Garde, V. Self-supported fibrin-polyvinyl alcohol interpenetrating polymer networks: An easily handled and rehydratable biomaterial. Biomacromolecules 2013, 14, 3870–3879. [Google Scholar] [CrossRef] [PubMed]
- Dragan, E.S. Design and applications of interpenetrating polymer network hydrogels. A review. Chem. Eng. J. 2014, 243, 572–590. [Google Scholar] [CrossRef]
- Sperling, L.H. Interpenetrating Polymer Networks: An Overview. In Interpenetrating Polymer Networks; Advances in Chemistry; American Chemical Society: Washington, DC, USA, 1994; Volume 239, pp. 3–38. ISBN 978-0-8412-2528-2. [Google Scholar]
- Sperling, L.H.; Mishra, V. The current status of interpenetrating polymer networks. Polym. Adv. Technol. 1996, 7, 197–208. [Google Scholar] [CrossRef]
- Jenkins, A.D.; Kratochvíl, P.; Stepto, R.F.T.; Suter, U.W. Glossary of basic terms in polymer science (IUPAC Recommendations 1996). Pure Appl. Chem. 1996, 68, 2287–2311. [Google Scholar] [CrossRef]
- Lee, F.; Kurisawa, M. Formation and stability of interpenetrating polymer network hydrogels consisting of fibrin and hyaluronic acid for tissue engineering. Acta Biomater. 2013, 9, 5143–5152. [Google Scholar] [CrossRef] [PubMed]
- Shikanov, A.; Xu, M.; Woodruff, T.K.; Shea, L.D. Interpenetrating fibrin-alginate matrices for in vitro ovarian follicle development. Biomaterials 2009, 30, 5476–5485. [Google Scholar] [CrossRef] [PubMed]
- Rowe, S.L.; Stegemann, J.P. Interpenetrating collagen-fibrin composite matrices with varying protein contents and ratios. Biomacromolecules 2006, 7, 2942–2948. [Google Scholar] [CrossRef] [PubMed]
- Benavides, O.M.; Quinn, J.P.; Pok, S.; Petsche Connell, J.; Ruano, R.; Jacot, J.G. Capillary-like network formation by human amniotic fluid-derived stem cells within fibrin/poly(ethylene glycol) hydrogels. Tissue Eng. Part A 2015, 21, 1185–1194. [Google Scholar] [CrossRef] [PubMed]
- Almany, L.; Seliktar, D. Biosynthetic hydrogel scaffolds made from fibrinogen and polyethylene glycol for 3D cell cultures. Biomaterials 2005, 26, 2467–2477. [Google Scholar] [CrossRef] [PubMed]
- Krsko, P.; Libera, M. Biointeractive hydrogels. Mater. Today 2005, 8, 36–44. [Google Scholar] [CrossRef]
- Vrana, N.E.; Cahill, P.A.; McGuinness, G.B. Endothelialization of PVA/gelatin cryogels for vascular tissue engineering: Effect of disturbed shear stress conditions. J. Biomed. Mater. Res. A 2010, 94, 1080–1090. [Google Scholar] [CrossRef] [PubMed]
- Kamoun, E.A.; Chen, X.; Mohy Eldin, M.S.; Kenawy, E.-R.S. Crosslinked poly(vinyl alcohol) hydrogels for wound dressing applications: A review of remarkably blended polymers. Arab. J. Chem. 2015, 8, 1–14. [Google Scholar] [CrossRef]
- Islam, A.; Yasin, T.; Rehman, I.U. Synthesis of hybrid polymer networks of irradiated chitosan/poly(vinyl alcohol) for biomedical applications. Radiat. Phys. Chem. 2014, 96, 115–119. [Google Scholar] [CrossRef]
- Paradossi, G.; Cavalieri, F.; Chiessi, E.; Spagnoli, C.; Cowman, M.K. Poly(vinyl alcohol) as versatile biomaterial for potential biomedical applications. J. Mater. Sci. Mater. Med. 2003, 14, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Akpalo, E.; Bidault, L.; Boissière, M.; Vancaeyzeele, C.; Fichet, O.; Larreta-Garde, V. Fibrin-polyethylene oxide interpenetrating polymer networks: New self-supported biomaterials combining the properties of both protein gel and synthetic polymer. Acta Biomater. 2011, 7, 2418–2427. [Google Scholar] [CrossRef] [PubMed]
- Bidault, L.; Deneufchatel, M.; Hindié, M.; Vancaeyzeele, C.; Fichet, O.; Larreta-Garde, V. Fibrin-based interpenetrating polymer network biomaterials with tunable biodegradability. Polymer 2015, 62, 19–27. [Google Scholar] [CrossRef]
- Oss-Ronen, L.; Seliktar, D. Polymer-conjugated albumin and fibrinogen composite hydrogels as cell scaffolds designed for affinity-based drug delivery. Acta Biomater. 2011, 7, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Kratz, F. Albumin as a drug carrier: Design of prodrugs, drug conjugates and nanoparticles. J. Control. Release 2008, 132, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Nair, C.H.; Dhall, D.P. Studies on fibrin network structure: The effect of some plasma proteins. Thromb. Res. 1991, 61, 315–325. [Google Scholar] [CrossRef]
- Galanakis, D.K.; Lane, B.P.; Simon, S.R. Albumin modulates lateral assembly of fibrin polymers: Evidence of enhanced fine fibril formation and of unique synergism with fibrinogen. Biochemistry 1987, 26, 2389–2400. [Google Scholar] [CrossRef]
- Pretorius, E.; Lipinski, B.; Bester, J.; Vermeulen, N.; Soma, P. Albumin stabilizes fibrin fiber ultrastructure in low serum albumin type 2 diabetes. Ultrastruct. Pathol. 2013, 37, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Serrano, M.C.; Pagani, R.; Vallet-Regı́, M.; Peña, J.; Rámila, A.; Izquierdo, I.; Portolés, M.T. In vitro biocompatibility assessment of poly(ε-caprolactone) films using L929 mouse fibroblasts. Biomaterials 2004, 25, 5603–5611. [Google Scholar] [CrossRef] [PubMed]
- Verrier, S.; Blaker, J.J.; Maquet, V.; Hench, L.L.; Boccaccini, A.R. PDLLA/Bioglass composites for soft-tissue and hard-tissue engineering: An in vitro cell biology assessment. Biomaterials 2004, 25, 3013–3021. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, J.; Montesin, F.E.; Di Silvio, L.; Pitt Ford, T.R. The chemical constitution and biocompatibility of accelerated Portland cement for endodontic use. Int. Endod. J. 2005, 38, 834–842. [Google Scholar] [CrossRef] [PubMed]
- A Practical Guide to ISO 10993-5: Cytotoxicity | MDDI Medical Device and Diagnostic Industry News Products and Suppliers. Available online: http://www.mddionline.com/article/practical-guide-iso-10993-5-cytotoxicity (accessed on 12 June 2017).
- Guidoin, R.; Sigot, M.; King, M.; Sigot-Luizard, M.F. Biocompatibility of the Vascugraft: Evaluation of a novel polyester urethane vascular substitute by an organotypic culture technique. Biomaterials 1992, 13, 281–288. [Google Scholar] [CrossRef]
- Lampin, M.; Warocquier-Clérout; Legris, C.; Degrange, M.; Sigot-Luizard, M.F. Correlation between substratum roughness and wettability, cell adhesion, and cell migration. J. Biomed. Mater. Res. 1997, 36, 99–108. [Google Scholar] [CrossRef]
- Petite, H.; Duval, J.L.; Frei, V.; Abdul-Malak, N.; Sigot-Luizard, M.F.; Herbage, D. Cytocompatibility of calf pericardium treated by glutaraldehyde and by the acyl azide methods in an organotypic culture model. Biomaterials 1995, 16, 1003–1008. [Google Scholar] [CrossRef]
- Williams, D.F. On the mechanisms of biocompatibility. Biomaterials 2008, 29, 2941–2953. [Google Scholar] [CrossRef] [PubMed]
- Hallab, N.J.; Bundy, K.J.; O’Connor, K.; Clark, R.; Moses, R.L. Cell adhesion to biomaterials: Correlations between surface charge, surface roughness, adsorbed protein, and cell morphology. J. Long-Term Eff. Med. Implants 1995, 5, 209–231. [Google Scholar] [PubMed]
- Polyzois, G.L.; Hensten-Pettersen, A.; Kullmann, A. An assessment of the physical properties and biocompatibility of three silicone elastomers. J. Prosthet. Dent. 1994, 71, 500–504. [Google Scholar] [CrossRef]
- Ozdemir, K.G.; Yilmaz, H.; Yilmaz, S. In vitro evaluation of cytotoxicity of soft lining materials on L929 cells by MTT assay. J. Biomed. Mater. Res. B 2009, 90, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Pera, P.; Conserva, E.; Pin, D.; Acquaviva, A.; Riboldi, A.; Mariottini, G.L.; Pane, L. Cytotoxicity in vitro analysis of ceramic materials for “metal free” prosthetic substructures. Minerva Stomatol. 2005, 54, 363–371. [Google Scholar] [PubMed]
- Tang, A.T.; Li, J.; Ekstrand, J.; Liu, Y. Cytotoxicity tests of in situ polymerized resins: Methodological comparisons and introduction of a tissue culture insert as a testing device. J. Biomed. Mater. Res. A 1999, 45, 214–222. [Google Scholar] [CrossRef]
- Dahl, J.E.; Frangou-Polyzois, M.J.; Polyzois, G.L. In vitro biocompatibility of denture relining materials. Gerodontology 2006, 23, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Duval, J.-L.; Dinis, T.; Vidal, G.; Vigneron, P.; Kaplan, D.L.; Egles, C. Organotypic culture to assess cell adhesion, growth and alignment of different organs on silk fibroin. J. Tissue Eng. Regen. Med. 2017, 11, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Leong, K.F.; Du, Z.; Chua, C.K. The design of scaffolds for use in tissue engineering. Part I. Traditional factors. Tissue Eng. 2001, 7, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Hojo, M.; Inokuchi, S.; Kidokoro, M.; Fukuyama, N.; Tanaka, E.; Tsuji, C.; Miyasaka, M.; Tanino, R.; Nakazawa, H. Induction of vascular endothelial growth factor by fibrin as a dermal substrate for cultured skin substitute. Plast. Reconstr. Surg. 2003, 111, 1638–1645. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.R.; Longo, F.M.; Powell, H.C.; Lundborg, G.; Varon, S. Spatial-temporal progress of peripheral nerve regeneration within a silicone chamber: Parameters for a bioassay. J. Comp. Neurol. 1983, 218, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.F.; Lanir, N.; McDonagh, J.; Tognazzi, K.; Dvorak, A.M.; Dvorak, H.F. Fibroblast migration in fibrin gel matrices. Am. J. Pathol. 1993, 142, 273–283. [Google Scholar] [PubMed]
- Nomura, H.; Naito, M.; Iguchi, A.; Thompson, W.D.; Smith, E.B. Fibrin gel induces the migration of smooth muscle cells from rabbit aortic explants. Thromb. Haemost. 1999, 82, 1347–1352. [Google Scholar] [PubMed]
- Turner, R.M. Moving to the beat: A review of mammalian sperm motility regulation. Reprod. Fertil. Dev. 2006, 18, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Jockenhoevel, S.; Zund, G.; Hoerstrup, S.P.; Chalabi, K.; Sachweh, J.S.; Demircan, L.; Messmer, B.J.; Turina, M. Fibrin gel—Advantages of a new scaffold in cardiovascular tissue engineering. Eur. J. Cardio-Thorac. Surg. 2001, 19, 424–430. [Google Scholar] [CrossRef]
- Ye, Q.; Zünd, G.; Benedikt, P.; Jockenhoevel, S.; Hoerstrup, S.P.; Sakyama, S.; Hubbell, J.A.; Turina, M. Fibrin gel as a three dimensional matrix in cardiovascular tissue engineering. Eur. J. Cardio-Thorac. Surg. 2000, 17, 587–591. [Google Scholar] [CrossRef]
- Mol, A.; van Lieshout, M.I.; Dam-de Veen, C.G.; Neuenschwander, S.; Hoerstrup, S.P.; Baaijens, F.P.T.; Bouten, C.V.C. Fibrin as a cell carrier in cardiovascular tissue engineering applications. Biomaterials 2005, 26, 3113–3121. [Google Scholar] [CrossRef] [PubMed]
- Barsotti, M.C.; Felice, F.; Balbarini, A.; Di Stefano, R. Fibrin as a scaffold for cardiac tissue engineering. Biotechnol. Appl. Biochem. 2011, 58, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Rajangam, T.; An, S.S.A. Fibrinogen and fibrin based micro and nano scaffolds incorporated with drugs, proteins, cells and genes for therapeutic biomedical applications. Int. J. Nanomed. 2013, 8, 3641–3662. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Schwab, I.R.; Madsen, T.K.; Isseroff, R.R. A fibrin-based bioengineered ocular surface with human corneal epithelial stem cells. Cornea 2002, 21, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Rama, P.; Bonini, S.; Lambiase, A.; Golisano, O.; Paterna, P.; De Luca, M.; Pellegrini, G. Autologous fibrin-cultured limbal stem cells permanently restore the corneal surface of patients with total limbal stem cell deficiency. Transplantation 2001, 72, 1478–1485. [Google Scholar] [CrossRef] [PubMed]
- Alaminos, M.; Del Carmen Sánchez-Quevedo, M.; Muñoz-Avila, J.I.; Serrano, D.; Medialdea, S.; Carreras, I.; Campos, A. Construction of a complete rabbit cornea substitute using a fibrin-agarose scaffold. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3311–3317. [Google Scholar] [CrossRef] [PubMed]
- Rosso, F.; Marino, G.; Giordano, A.; Barbarisi, M.; Parmeggiani, D.; Barbarisi, A. Smart materials as scaffolds for tissue engineering. J. Cell. Physiol. 2005, 203, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.T.; Jankharia, B.B. Imaging of common breast implants and implant-related complications: A pictorial essay. Indian J. Radiol. Imaging 2016, 26, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Wolfram, D.; Dolores, W.; Rainer, C.; Christian, R.; Niederegger, H.; Harald, N.; Piza, H.; Hildegunde, P.; Wick, G.; Georg, W. Cellular and molecular composition of fibrous capsules formed around silicone breast implants with special focus on local immune reactions. J. Autoimmun. 2004, 23, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Hester, T.R.; Nahai, F.; Bostwick, J.; Cukic, J. A 5-year experience with polyurethane-covered mammary prostheses for treatment of capsular contracture, primary augmentation mammoplasty, and breast reconstruction. Clin. Plast. Surg. 1988, 15, 569–585. [Google Scholar] [PubMed]
- Li, D.J.; Ohsaki, K.; Ii, K.; Cui, P.C.; Ye, Q.; Baba, K.; Wang, Q.C.; Tenshin, S.; Takano-Yamamoto, T. Thickness of fibrous capsule after implantation of hydroxyapatite in subcutaneous tissue in rats. J. Biomed. Mater. Res. A 1999, 45, 322–326. [Google Scholar] [CrossRef]
- Ramires, P.A.; Miccoli, M.A.; Panzarini, E.; Dini, L.; Protopapa, C. In vitro and in vivo biocompatibility evaluation of a polyalkylimide hydrogel for soft tissue augmentation. J. Biomed. Mater. Res. B 2005, 72, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Wang, D.; Shen, J. Fabrication of porous collagen/chitosan scaffolds with controlling microstructure for dermal equivalent. Polym. Adv. Technol. 2003, 14, 373–379. [Google Scholar] [CrossRef]
- Rnjak-Kovacina, J.; Wise, S.G.; Li, Z.; Maitz, P.K.M.; Young, C.J.; Wang, Y.; Weiss, A.S. Tailoring the porosity and pore size of electrospun synthetic human elastin scaffolds for dermal tissue engineering. Biomaterials 2011, 32, 6729–6736. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Gao, C.; Mao, Z.; Zhou, J.; Shen, J.; Hu, X.; Han, C. Collagen/chitosan porous scaffolds with improved biostability for skin tissue engineering. Biomaterials 2003, 24, 4833–4841. [Google Scholar] [CrossRef]
- Vrana, N.E.; Builles, N.; Kocak, H.; Gulay, P.; Justin, V.; Malbouyres, M.; Ruggiero, F.; Damour, O.; Hasirci, V. EDC/NHS cross-linked collagen foams as scaffolds for artificial corneal stroma. J. Biomater. Sci. Polym. Ed. 2007, 18, 1527–1545. [Google Scholar] [PubMed]
- Yang, F.; Murugan, R.; Ramakrishna, S.; Wang, X.; Ma, Y.-X.; Wang, S. Fabrication of nano-structured porous PLLA scaffold intended for nerve tissue engineering. Biomaterials 2004, 25, 1891–1900. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Lee, G.Y.; Wong, J.Y.; Desai, T.A. Development and characterization of a porous micro-patterned scaffold for vascular tissue engineering applications. Biomaterials 2006, 27, 4775–4782. [Google Scholar] [CrossRef] [PubMed]
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Boccaccini, A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef] [PubMed]
- Hollister, S.J. Porous scaffold design for tissue engineering. Nat. Mater. 2005, 4, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Malich, G.; Markovic, B.; Winder, C. The sensitivity and specificity of the MTS tetrazolium assay for detecting the in vitro cytotoxicity of 20 chemicals using human cell lines. Toxicology 1997, 124, 179–192. [Google Scholar] [CrossRef]
- O’Toole, S.A.; Sheppard, B.L.; McGuinness, E.P.J.; Gleeson, N.C.; Yoneda, M.; Bonnar, J. The MTS assay as an indicator of chemosensitivity/resistance in malignant gynaecological tumours. Cancer Detect. Prev. 2003, 27, 47–54. [Google Scholar] [CrossRef]
- Riss, T.L.; Moravec, R.A. Comparison of MTT, XTT, and a Novel Tetrazolium Compound MTS for In Vitro Proliferation and Chemosensitivity Assays. Available online: https://eurekamag.com/research/030/631/030631215.php (accessed on 12 June 2017).
- A Practical Guide to ISO 10993-12: Sample Preparation and Reference Materials | MDDI Medical Device and Diagnostic Industry News Products and Suppliers. Available online: http://www.mddionline.com/article/practical-guide-iso-10993-12-sample-preparation-and-reference-materials (accessed on 12 June 2017).
- ISO 10993-12:2012-Biological Evaluation of Medical Devices—Part 12: Sample Preparation and Reference Materials. Available online: https://www.iso.org/standard/53468.html (accessed on 12 June 2017).
- Duval, J.L.; Letort, M.; Sigot-Luizard, M.F. Comparative assessment of cell/substratum static adhesion using an in vitro organ culture method and computerized analysis system. Biomaterials 1988, 9, 155–161. [Google Scholar] [CrossRef]
- Debels, H.; Hamdi, M.; Abberton, K.; Morrison, W. Dermal matrices and bioengineered skin substitutes: A critical review of current options. Plast. Reconstr. Surg. Glob. Open 2015, 3, e284. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, E.; Duval, J.-L.; Egles, C.; Ihida, S.; Toshiyoshi, H.; Tixier-Mita, A. In vitro cyto-biocompatibility study of thin-film transistors substrates using an organotypic culture method. J. Mater. Sci. Mater. Med. 2017, 28, 4. [Google Scholar] [CrossRef] [PubMed]
- Van Bilsen, P.H.J.; Krenning, G.; Billy, D.; Duval, J.-L.; Huurdeman-Vincent, J.; van Luyn, M.J.A. Heparin coating of poly(ethylene terephthalate) decreases hydrophobicity, monocyte/leukocyte interaction and tissue interaction. Colloids Surf. B Biointerfaces 2008, 67, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Brunot-Gohin, C.; Duval, J.-L.; Azogui, E.-E.; Jannetta, R.; Pezron, I.; Laurent-Maquin, D.; Gangloff, S.C.; Egles, C. Soft tissue adhesion of polished versus glazed lithium disilicate ceramic for dental applications. Dent. Mater. 2013, 29, e205–e212. [Google Scholar] [CrossRef] [PubMed]
- Villars, F.; Conrad, V.; Rouais, F.; Lefebvre, F.; Amédée, J.; Bordenave, L. Ability of various inserts to promote endothelium cell culture for the establishment of coculture models. Cell Biol. Toxicol. 1996, 12, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Hazen, S.A.; Rowe, W.A.; Lynch, C.J. Monolayer cell culture of freshly isolated adipocytes using extracellular basement membrane components. J. Lipid Res. 1995, 36, 868–875. [Google Scholar] [PubMed]
- Levesque, M.J.; Nerem, R.M.; Sprague, E.A. Vascular endothelial cell proliferation in culture and the influence of flow. Biomaterials 1990, 11, 702–707. [Google Scholar] [CrossRef]
- Etienne, O.; Schneider, A.; Kluge, J.A.; Bellemin-Laponnaz, C.; Polidori, C.; Leisk, G.G.; Kaplan, D.L.; Garlick, J.A.; Egles, C. Soft tissue augmentation using silk gels: An in vitro and in vivo study. J. Periodontol. 2009, 80, 1852–1858. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.-H.; Liu, P.; Xie, J.-L.; Shu, B.; Xu, Y.-B.; Ke, C.-N.; Liu, X.-S.; Li, T.-Z. Experimental study on repairing of nude mice skin defects with composite skin consisting of xenogeneic dermis and epidermal stem cells and hair follicle dermal papilla cells. Burns 2008, 34, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Pabst, A.M.; Happe, A.; Callaway, A.; Ziebart, T.; Stratul, S.I.; Ackermann, M.; Konerding, M.A.; Willershausen, B.; Kasaj, A. In vitro and in vivo characterization of porcine acellular dermal matrix for gingival augmentation procedures. J. Periodontal Res. 2014, 49, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Lee, M.C.; Roh, H.; Lee, W.J. Multilayered implantation using acellular dermal matrix into nude mice. J. Mater. Sci. Mater. Med. 2014, 25, 2669–2676. [Google Scholar] [CrossRef] [PubMed]





© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gsib, O.; Duval, J.-L.; Goczkowski, M.; Deneufchatel, M.; Fichet, O.; Larreta-Garde, V.; Bencherif, S.A.; Egles, C. Evaluation of Fibrin-Based Interpenetrating Polymer Networks as Potential Biomaterials for Tissue Engineering. Nanomaterials 2017, 7, 436. https://doi.org/10.3390/nano7120436
Gsib O, Duval J-L, Goczkowski M, Deneufchatel M, Fichet O, Larreta-Garde V, Bencherif SA, Egles C. Evaluation of Fibrin-Based Interpenetrating Polymer Networks as Potential Biomaterials for Tissue Engineering. Nanomaterials. 2017; 7(12):436. https://doi.org/10.3390/nano7120436
Chicago/Turabian StyleGsib, Olfat, Jean-Luc Duval, Mathieu Goczkowski, Marie Deneufchatel, Odile Fichet, Véronique Larreta-Garde, Sidi Ahmed Bencherif, and Christophe Egles. 2017. "Evaluation of Fibrin-Based Interpenetrating Polymer Networks as Potential Biomaterials for Tissue Engineering" Nanomaterials 7, no. 12: 436. https://doi.org/10.3390/nano7120436




