Evaluation of Different Single-Walled Carbon Nanotube Surface Coatings for Single-Particle Tracking Applications in Biological Environments
Abstract
:1. Introduction
2. Results and Discussion
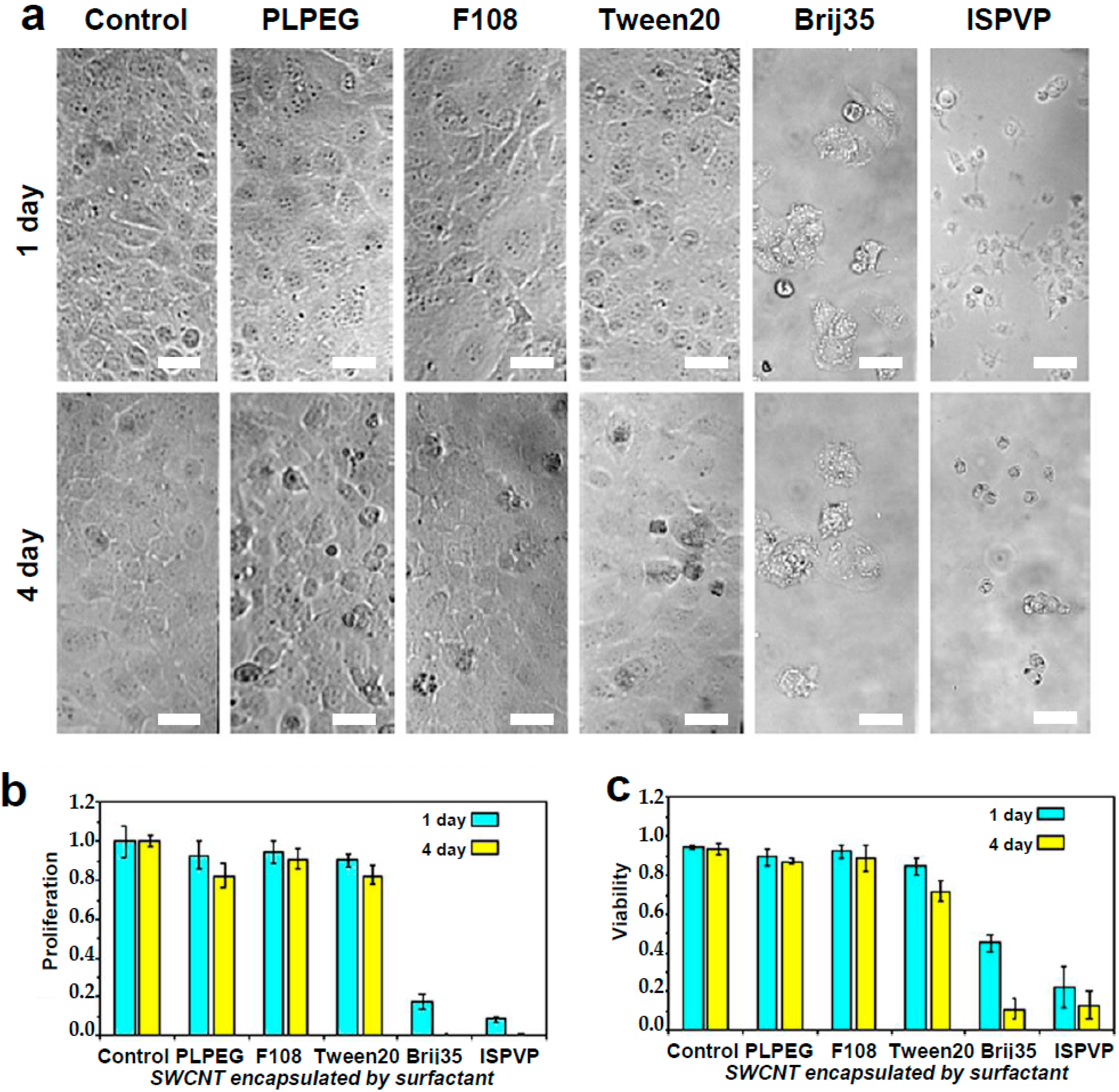
2.1. Cytotoxicity Experiments
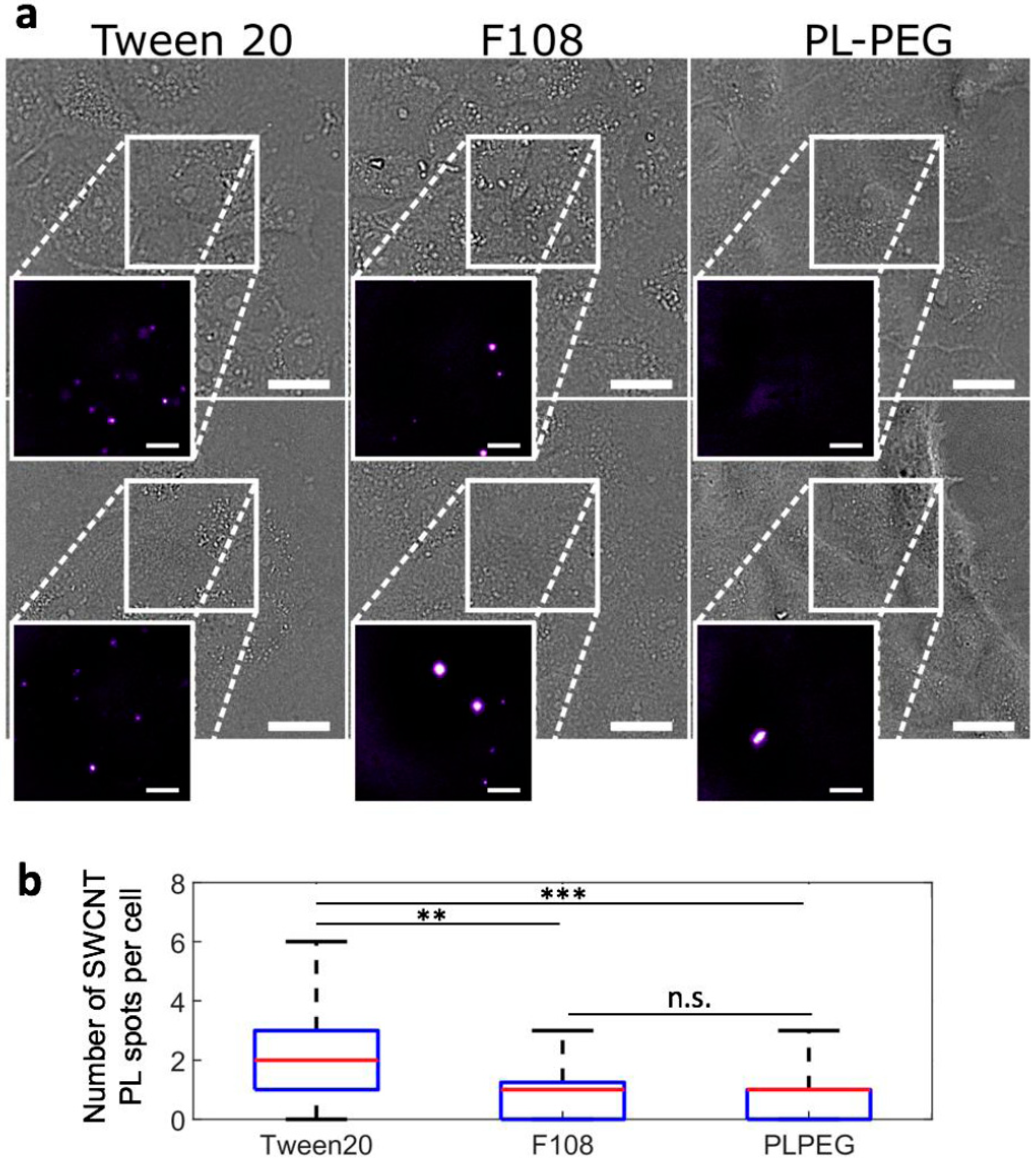
2.2. Photoluminescence Imaging of Biocompatible Nanotubes
2.2.1. Photoluminescence Imaging of Biocompatible Nanotubes in Biological Medium
2.2.2. Photoluminescence Imaging of Biocompatible PLPEG- or F108-Coated SWCNT in Thick Biocompatible Aqueous Gels
3. Materials and Methods
3.1. Preparation of SWCNT Dispersions
3.2. Cell Culture Studies
3.3. Single Nanotube Fluorescence Microscopy Setup
3.4. Agarose Sample Preparation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cognet, L.; Leduc, C.; Lounis, B. Advances in live-cell single-particle tracking and dynamic super-resolution imaging. Curr. Opin. Chem. Biol. 2014, 20, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Godin, A.G.; Lounis, B.; Cognet, L. Super-resolution Microscopy Approaches for Live Cell Imaging. Biophys. J. 2014, 107, 1777–1784. [Google Scholar] [CrossRef] [PubMed]
- Manzo, C.; Garcia-Parajo, M.F. A review of progress in single particle tracking: From methods to biophysical insights. Rep. Prog. Phys. 2015, 78, 124601. [Google Scholar] [CrossRef] [PubMed]
- Biermann, B.; Sokoll, S.; Klueva, J.; Missler, M.; Wiegert, J.S.; Sibarita, J.B.; Heine, M. Imaging of molecular surface dynamics in brain slices using single-particle tracking. Nat. Commun. 2014, 5, 3024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varela, J.A.; Dupuis, J.P.; Etchepare, L.; Espana, A.E.S.; Cognet, L.; Groc, L. Targeting neurotransmitter receptors with nanoparticles in vivo allows single-molecule tracking in acute brain slices. Nat. Commun. 2016, 7, 10947. [Google Scholar] [CrossRef] [PubMed]
- Al-Juboori, S.I.; Dondzillo, A.; Stubblefield, E.A.; Felsen, G.; Lei, T.C.; Klug, A. Light Scattering Properties Vary across Different Regions of the Adult Mouse Brain. PLoS ONE 2013, 8, e67626. [Google Scholar] [CrossRef] [PubMed]
- Pascu, A.; Romanitan, M.O.; Delgado, J.M.; Danaila, L.; Pascu, M.L. Laser-Induced Autofluorescence Measurements on Brain Tissues. Anat. Rec. 2009, 292, 2013–2022. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Sordillo, L.A.; Rodríguez-Contreras, A.; Alfano, R. Transmission in near-infrared optical windows for deep brain imaging. J. Biophotonics 2015, 9, 38–43. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, M.J.; Bachilo, S.M.; Huffman, C.B.; Moore, V.C.; Strano, M.S.; Haroz, E.H.; Rialon, K.L.; Boul, P.J.; Noon, W.H.; Kittrell, C.; et al. Band gap fluorescence from individual single-walled carbon nanotubes. Science 2002, 297, 593–596. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.; Diao, S.; Antaris, A.L.; Dai, H. Carbon Nanomaterials for Biological Imaging and Nanomedicinal Therapy. Chem. Rev. 2015, 115, 10816–10906. [Google Scholar] [CrossRef] [PubMed]
- Welsher, K.; Liu, Z.; Sherlock, S.P.; Robinson, J.T.; Chen, Z.; Daranciang, D.; Dai, H. A route to brightly fluorescent carbon nanotubes for near-infrared imaging in mice. Nat. Nanotechnol. 2009, 4, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.; Diao, S.; Chang, J.; Antaris, A.L.; Chen, C.; Zhang, B.; Zhao, S.; Atochin, D.N.; Huang, P.L.; Andreasson, K.I.; et al. Through-skull fluorescence imaging of the brain in a new near-infrared window. Nat. Photonics 2014, 8, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Reuel, N.F.; Dupont, A.; Thouvenin, O.; Lamb, D.C.; Strano, M.S. Three-Dimensional Tracking of Carbon Nanotubes within Living Cells. ACS Nano 2012, 6, 5420–5428. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, N.; Wessel, A.D.; Willms, C.; Pasquali, M.; Klopfenstein, D.R.; MacKintosh, F.C.; Schmidt, C.F. High-resolution mapping of intracellular fluctuations using carbon nanotubes. Science 2014, 344, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Cherukuri, P.; Gannon, C.J.; Leeuw, T.K.; Schmidt, H.K.; Smalley, R.E.; Curley, S.A.; Weisman, R.B. Mammalian pharmacokinetics of carbon nanotubes using intrinsic near-infrared fluorescence. Proc. Natl. Acad. Sci. USA 2006, 103, 18882–18886. [Google Scholar] [CrossRef] [PubMed]
- Jena, P.V.; Shamay, Y.; Shah, J.; Roxbury, D.; Paknejad, N.; Heller, D.A. Photoluminescent carbon nanotubes interrogate the permeability of multicellular tumor spheroids. Carbon 2016, 97, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Godin, A.G.; Varela, J.A.; Gao, Z.; Danné, N.; Dupuis, J.P.; Lounis, B.; Groc, L.; Cognet, L. Single-nanotube tracking reveals the nanoscale organization of the extracellular space in the live brain. Nat. Nanotechnol. 2017, 12, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Moghimi, S.M.; Andersen, A.J.; Hashemi, S.H.; Lettiero, B.; Ahmadvand, D.; Hunter, A.C.; Andresen, T.L.; Hamad, I.; Szebeni, J. Complement activation cascade triggered by PEG-PL engineered nanomedicines and carbon nanotubes: The challenges ahead. J. Control. Release 2010, 146, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Cherukuri, P.; Bachilo, S.M.; Litovsky, S.H.; Weisman, R.B. Near-Infrared Fluorescence Microscopy of Single-Walled Carbon Nanotubes in Phagocytic Cells. J. Am. Chem. Soc. 2004, 126, 15638–15639. [Google Scholar] [CrossRef] [PubMed]
- Holt, B.D.; Short, P.A.; Rape, A.D.; Wang, Y.-L.; Islam, M.F.; Dahl, K.N. Carbon Nanotubes Reorganize Actin Structures in Cells and ex Vivo. ACS Nano 2010, 4, 4872–4878. [Google Scholar] [CrossRef] [PubMed]
- Huczko, A.; Lange, H.; Całko, E.; Grubek-Jaworska, H.; Droszcz, P. Physiological testing of carbon nanotubes: Are they asbestos-like? Fuller. Sci. Technol. 2001, 9, 251–254. [Google Scholar] [CrossRef]
- Pauwels, J.; Hoogmartens, J.; Van Schepdael, A. Application of carbon nanotubes for in-capillary incubations with cytochrome P450 enzymes. Electrophoresis 2010, 31, 3867–3873. [Google Scholar] [CrossRef] [PubMed]
- Duque, J.G.; Cognet, L.; Parra-Vasquez, A.N.G.; Nicholas, N.; Schmidt, H.K.; Pasquali, M. Stable Luminescence from Individual Carbon Nanotubes in Acidic, Basic, and Biological Environments. J. Am. Chem. Soc. 2008, 130, 2626–2633. [Google Scholar] [CrossRef] [PubMed]
- Wenseleers, W.; Vlasov, I.I.; Goovaerts, E.; Obraztsova, E.D.; Lobach, A.S.; Bouwen, A. Efficient Isolation and Solubilization of Pristine Single-Walled Nanotubes in Bile Salt Micelles. Adv. Funct. Mater. 2004, 14, 1105–1112. [Google Scholar] [CrossRef]
- Cognet, L.; Tsyboulski, D.A.; Rocha, J.-D.R.; Doyle, C.D.; Tour, J.M.; Weisman, R.B. Stepwise quenching of exciton fluorescence in carbon nanotubes by single-molecule reactions. Science 2007, 316, 1465–1468. [Google Scholar] [CrossRef] [PubMed]
- Duque, J.G.; Pasquali, M.; Cognet, L.; Lounis, B. Environmental and Synthesis-Dependent Luminescence Properties of Individual Single-Walled Carbon Nanotubes. ACS Nano 2009, 3, 2153–2156. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Cai, W.; He, L.; Nakayama, N.; Chen, K.; Sun, X.; Chen, X.; Dai, H. In vivo biodistribution and highly efficient tumour targeting of carbon nanotubes in mice. Nat. Nanotechnol. 2006, 2, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.J.; Bangsaruntip, S.; Drouvalakis, K.A.; Kam, N.W.S.; Shim, M.; Li, Y.; Kim, W.; Utz, P.J.; Dai, H. Noncovalent functionalization of carbon nanotubes for highly specific electronic biosensors. Proc. Natl. Acad. Sci. USA 2003, 100, 4984–4989. [Google Scholar] [CrossRef] [PubMed]
- Moore, V.C.; Strano, M.S.; Haroz, E.H.; Hauge, R.H.; Smalley, R.E.; Schmidt, J.; Talmon, Y. Individually Suspended Single-Walled Carbon Nanotubes in Various Surfactants. Nano Lett. 2003, 3, 1379–1382. [Google Scholar] [CrossRef]
- Santos, S.M.; Yuma, B.; Berciaud, S.; Shaver, J.; Gallart, M.; Gilliot, P.; Cognet, L.; Lounis, B. All-Optical Trion Generation in Single-Walled Carbon Nanotubes. Phys. Rev. Lett. 2011, 107, 187401. [Google Scholar] [CrossRef] [PubMed]
- Budhathoki-Uprety, J.; Langenbacher, R.E.; Jena, P.V.; Roxbury, D.; Heller, D.A. A Carbon Nanotube Optical Sensor Reports Nuclear Entry via a Noncanonical Pathway. ACS Nano 2017, 11, 3875–3882. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Bachilo, S.M.; Simonette, R.A.; Beckingham, K.M.; Weisman, R.B. Oxygen Doping Modifies Near-Infrared Band Gaps in Fluorescent Single-Walled Carbon Nanotubes. Science 2010, 330, 1656–1659. [Google Scholar] [CrossRef] [PubMed]
- Monopoli, M.P.; Åberg, C.; Salvati, A.; Dawson, K.A. Biomolecular coronas provide the biological identity of nanosized materials. Nat. Nanotechnol. 2012, 7, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Varela, J.A.; Groc, L.; Lounis, B.; Cognet, L. Toward the suppression of cellular toxicity from single-walled carbon nanotubes. Biomater. Sci. 2016, 4, 230–244. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, N.; MacKintosh, F.C.; Lounis, B.; Cognet, L.; Pasquali, M. Brownian motion of stiff filaments in a crowded environment. Science 2010, 330, 1804–1807. [Google Scholar] [CrossRef] [PubMed]

| Nanotubes | Surfactant | Biological System | Dose | Exposure Time | Assay Method | Conclusion | Reference |
|---|---|---|---|---|---|---|---|
| HiPco SWCNTs | PLPEG | Human serum and intravenous injection in rats | 60 μg/mL | 0.5 h | ELISA | Activation of the complement system by SWCNTs in undiluted normal human serum and in vivo rats. | [18] |
| HiPco SWCNTs | PLPEG | Intravenous/brain injection in rats | 60 μg/mL | 0.5 h to days | Fluorescence | In vivo SWCNT circulation (vascular system, brain). Stable imaging in vivo and in tissues. | [11,17] |
| HiPco SWCNTs | Pluronic F108 | J774.1A mouse peritoneal macrophage | 11 ng/mL | 0, 8, 18 and 24 h | Fluorescence | Macrophages can ingest significant quantities of SWCNTs without showing toxic effects. Stable imaging in cells. | [19] |
| HiPco SWCNTs | Pluoronic F127 | HeLa cells | 200 μg/mL | 2 days | Fluorescence imaging | Induction of actin bundling in cells, reduced cellular proliferation. | [20] |
| Arc Discharge SWCNTs | Tween20 | Pathgen free guinea pigs | 50 mg/mL | 4 weeks | Lung function, bronchoalveolart lavega | No abnormalities of pulmonary function or measurable inflammation in guinea pigs. | [21] |
| MWCNTs | Sterile saline + Brij 35 | Incubation with cytochrome P450 enzymes (CYP3A4) | 0.067 mg/mL | 5 min at 37 °C | Capillary electrochromatography, enzyme activity monitoring | No effect on CYP3A4 activity. Substantial improvement of migration time and peak shape repeatability in capillary electrochromatography. | [22] |
| HiPco SWCNTs | ISPVP | Human embryonic kidney cells (HEK cells) | 1/30 μg/mL | 5 min at RT or 12 h at 37 °C | Fluorescence | Stable imaging in cells. | [23] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Z.; Danné, N.; Godin, A.G.; Lounis, B.; Cognet, L. Evaluation of Different Single-Walled Carbon Nanotube Surface Coatings for Single-Particle Tracking Applications in Biological Environments. Nanomaterials 2017, 7, 393. https://doi.org/10.3390/nano7110393
Gao Z, Danné N, Godin AG, Lounis B, Cognet L. Evaluation of Different Single-Walled Carbon Nanotube Surface Coatings for Single-Particle Tracking Applications in Biological Environments. Nanomaterials. 2017; 7(11):393. https://doi.org/10.3390/nano7110393
Chicago/Turabian StyleGao, Zhenghong, Noémie Danné, Antoine Guillaume Godin, Brahim Lounis, and Laurent Cognet. 2017. "Evaluation of Different Single-Walled Carbon Nanotube Surface Coatings for Single-Particle Tracking Applications in Biological Environments" Nanomaterials 7, no. 11: 393. https://doi.org/10.3390/nano7110393





