Free-Standing and Self-Crosslinkable Hybrid Films by Core–Shell Particle Design and Processing
Abstract
:1. Introduction
2. Experimentation
2.1. Materials
2.2. Synthesis of PMMA-co-ALMA and PS-co-ALMA Core Particles
2.3. Particle Shell Formation by Copoylmerization of Alkoxysilane-Containing Methacrylates with Ethyl Acrylate
2.4. Melt-Shear Organization Procedure
2.5. Characterization
3. Results and Discussion
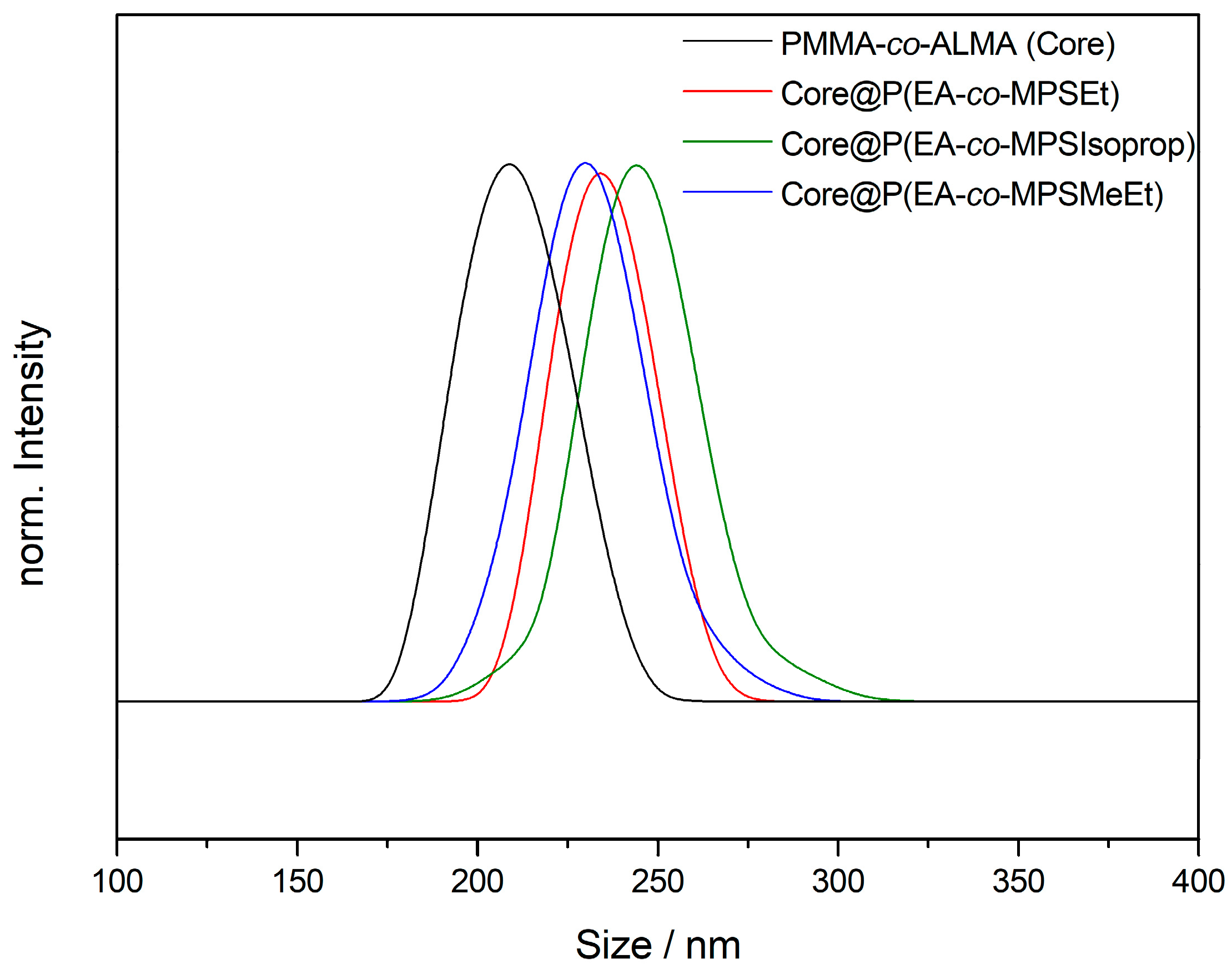
3.1. Synthesis Hybrid Particles with Alkoxysilane-Containing Methacrylates
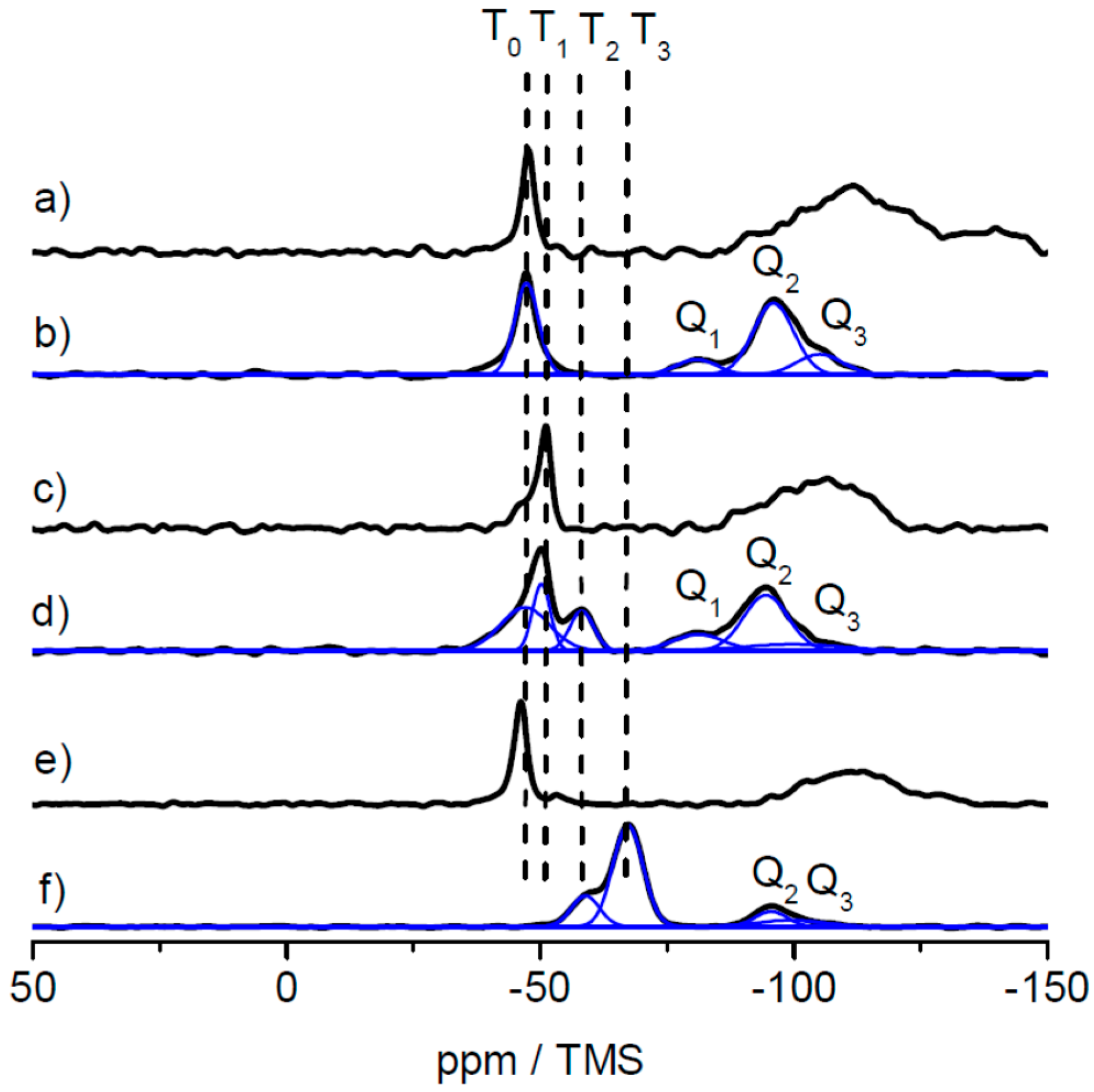
3.2. Investigation of the Cross-Linking Capabilities of the Poly(alkoxysilane-methacrylate)s
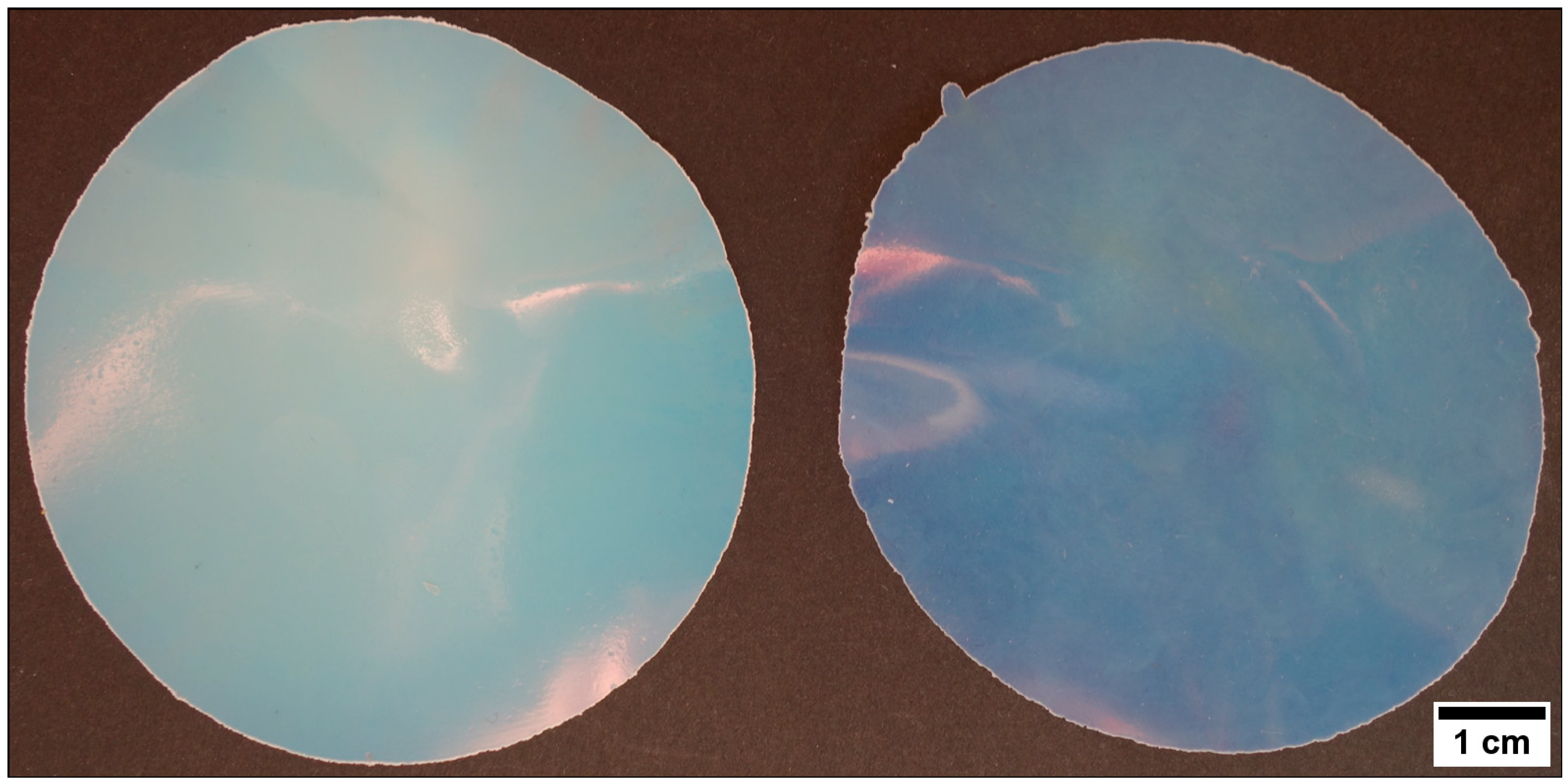
3.3. Hybrid Film Formation by Melt-Shear Organization
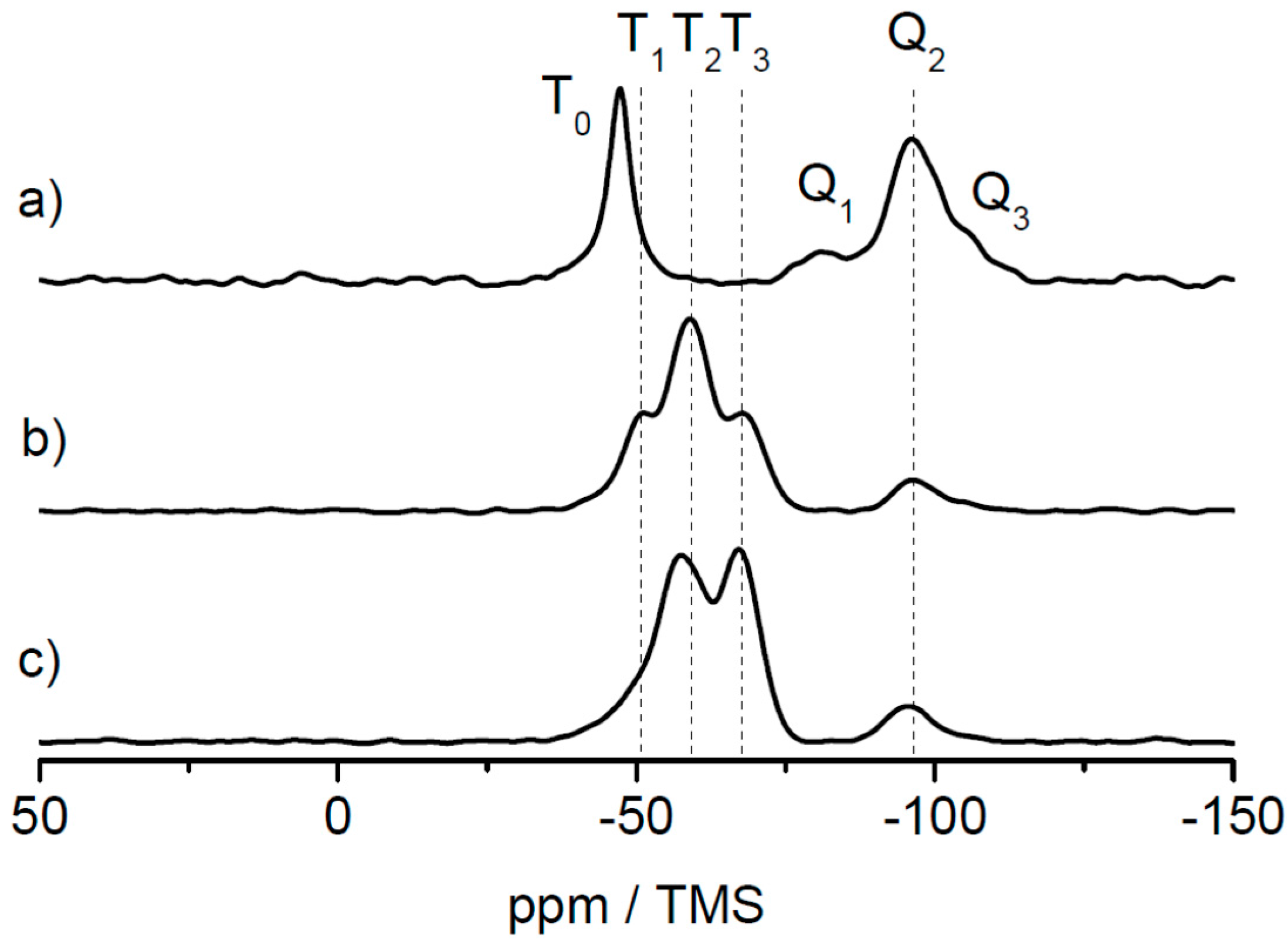
3.4. Investigation of the Cross-Linked Siloxane Moieties after Film Formation
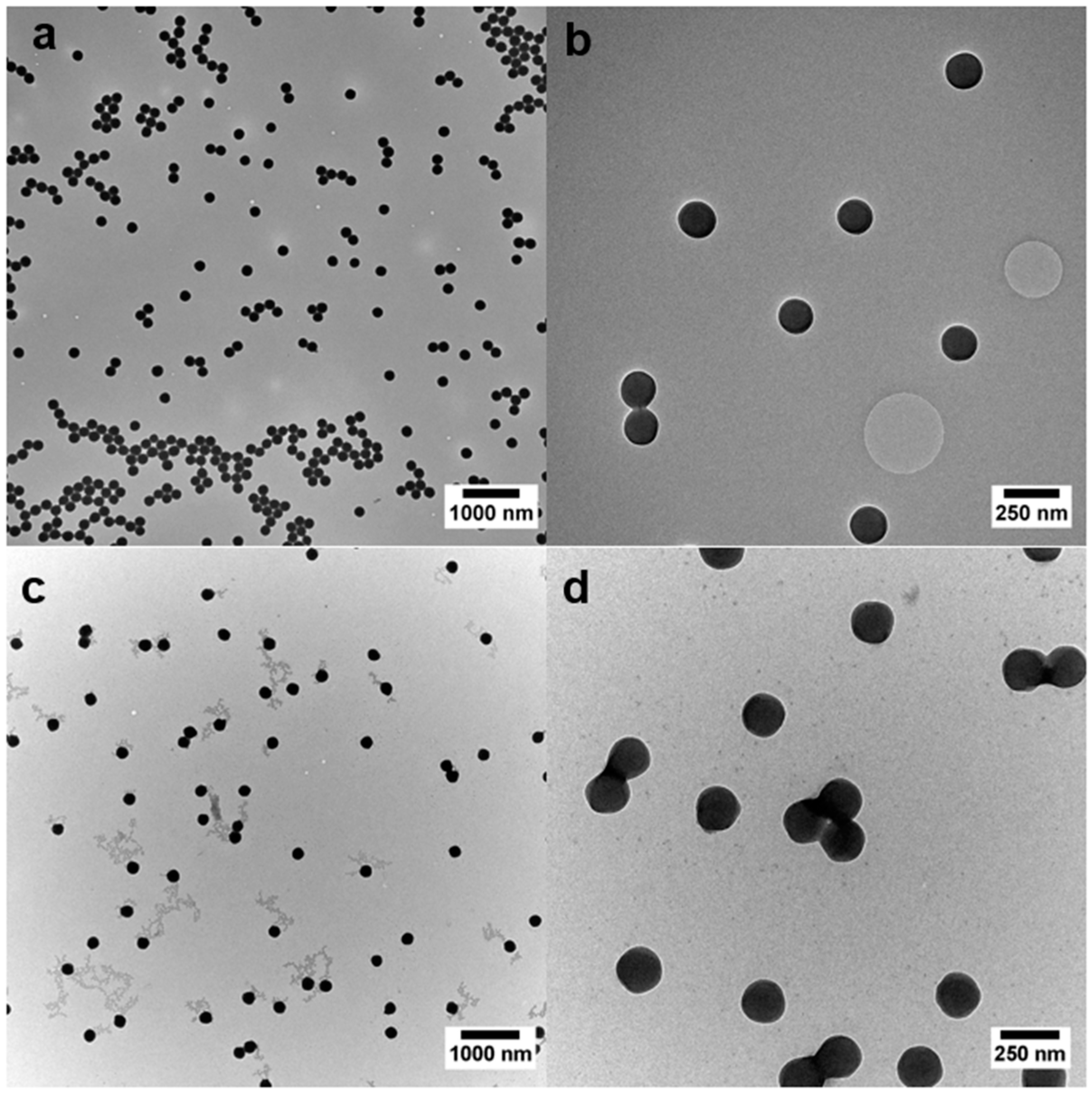
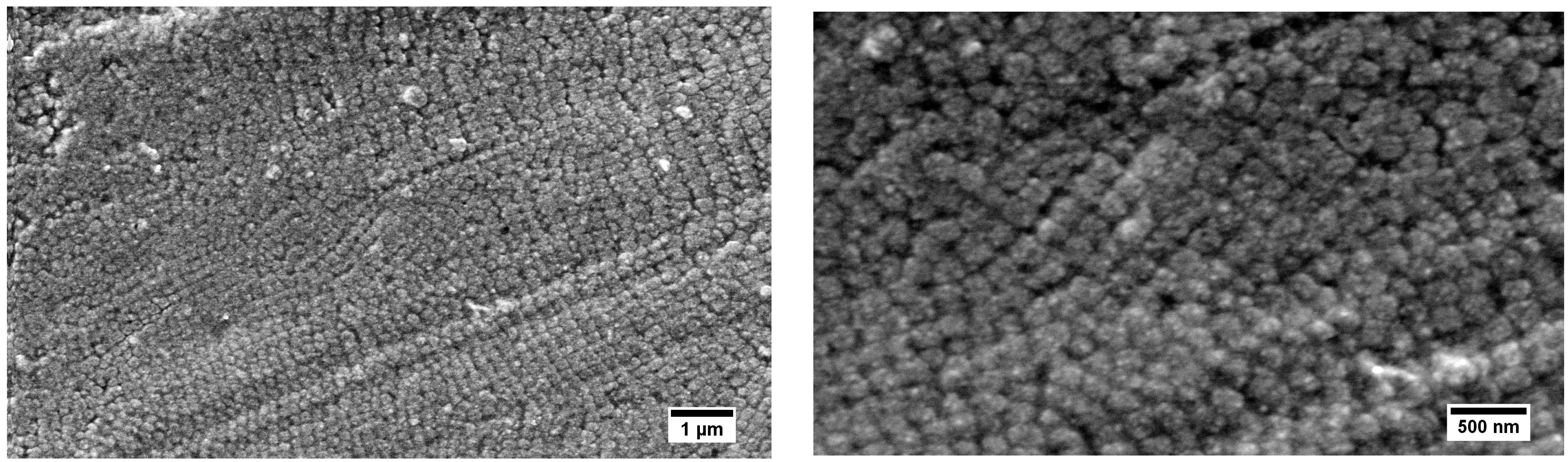
3.5. Morphological Studies on the Hybrid Particle Films
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, F.; Josephson, D.P.; Stein, A. Colloidal assembly: The road from particles to colloidal molecules and crystals. Angew. Chem. Int. Ed. 2011, 50, 360–388. [Google Scholar] [CrossRef] [PubMed]
- Vutukuri, H.R.; Stiefelhagen, J.; Vissers, T.; Imhof, A.; van Blaaderen, A. Bonding assembled colloids without loss of colloidal stability. Adv. Mater. 2012, 24, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Gasser, U. Crystallization in three- and two-dimensional colloidal suspensions. J. Phys. Condens. Matter 2009, 21. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Gradon, L.; Janeczko, S.; Iskandar, F.; Okuyama, K. Formation of highly ordered nanostructures by drying micrometer colloidal droplets. ACS Nano 2010, 4, 4717–4724. [Google Scholar] [CrossRef] [PubMed]
- Galisteo-Lopez, J.F.; Ibisate, M.; Sapienza, R.; Froufe-Perez, L.S.; Blanco, A.; Lopez, C. Self-assembled photonic structures. Adv. Mater. 2011, 23, 30–69. [Google Scholar] [CrossRef] [PubMed]
- Von Freymann, G.; Kitaev, V.; Lotsch, B.V.; Ozin, G.A. Bottom-up assembly of photonic crystals. Chem. Soc. Rev. 2013, 42, 2528–2554. [Google Scholar] [CrossRef] [PubMed]
- John, S. Strong localization of photons in certain disordered dielectric superlattices. Phys. Rev. Lett. 1987, 58, 2486–2489. [Google Scholar] [CrossRef] [PubMed]
- Yablonovitch, E. Inhibited spontaneous emission in solid-state physics and electronics. Phys. Rev. Lett. 1987, 58, 2059–2062. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Yin, Y. Responsive photonic crystals. Angew. Chem. Int. Ed. Engl. 2011, 50, 1492–1522. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Koh, C.Y.; Singer, J.P.; Jeon, S.J.; Maldovan, M.; Stein, O.; Thomas, E.L. 25th anniversary article: Ordered polymer structures for the engineering of photons and phonons. Adv. Mater. 2014, 26, 532–569. [Google Scholar] [CrossRef] [PubMed]
- Paquet, C.; Kumacheva, E. Nanostructured polymers for photonics. Mater. Today 2008, 11, 48–56. [Google Scholar] [CrossRef]
- Gallei, M. Functional polymer opals and porous materials by shear-induced assembly of tailor-made particles. Macromol. Rapid Commun. 2017. [Google Scholar] [CrossRef]
- Zhang, Y.-N.; Zhao, Y.; Lv, R.-Q. A review for optical sensors based on photonic crystal cavities. Sens. Act. A 2015, 233, 374–389. [Google Scholar] [CrossRef]
- Innocenzi, P.; Malfatti, L. Mesoporous thin films: Properties and applications. Chem. Soc. Rev. 2013, 42, 4198–4216. [Google Scholar] [CrossRef] [PubMed]
- Petkovich, N.D.; Stein, A. Controlling macro- and mesostructures with hierarchical porosity through combined hard and soft templating. Chem. Soc. Rev. 2013, 42, 3721–3739. [Google Scholar] [CrossRef] [PubMed]
- Orilall, M.C.; Wiesner, U. Block copolymer based composition and morphology control in nanostructured hybrid materials for energy conversion and storage: Solar cells, batteries, and fuel cells. Chem. Soc. Rev. 2011, 40, 520–535. [Google Scholar] [CrossRef] [PubMed]
- Whitesides, G.M. Nanoscience, nanotechnology, and chemistry. Small 2005, 1, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Piao, Y.; Burns, A.; Kim, J.; Wiesner, U.; Hyeon, T. Designed fabrication of silica-based nanostructured particle systems for nanomedicine applications. Adv. Funct. Mater. 2008, 18, 3745–3758. [Google Scholar] [CrossRef]
- Mera, G.; Gallei, M.; Bernard, S.; Ionescu, E. Ceramic nanocomposites from tailor-made preceramic polymers. Nanomaterials 2015, 5, 468–540. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Gallei, M.; Ionescu, E. Polymer–ceramic nanohybrid materials. Adv. Polym. Sci. 2015, 267, 143–185. [Google Scholar]
- Hatton, B.; Mishchenko, L.; Davis, S.; Sandhage, K.H.; Aizenberg, J. Assembly of large-area, highly ordered, crack-free inverse opal films. Proc. Natl. Acad. Sci. USA 2010, 107, 10354–10359. [Google Scholar] [CrossRef] [PubMed]
- Vasquez, Y.; Kolle, M.; Mishchenko, L.; Hatton, B.D.; Aizenberg, J. Three-phase co-assembly: In situ incorporation of nanoparticles into tunable, highly ordered, porous silica films. ACS Photon. 2014, 1, 53–60. [Google Scholar] [CrossRef]
- Schäfer, C.G.; Viel, B.; Hellmann, G.P.; Rehahn, M.; Gallei, M. Thermo-cross-linked elastomeric opal films. ACS Appl. Mater. Interfaces 2013, 5, 10623–10632. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, C.G.; Lederle, C.; Zentel, K.; Stuhn, B.; Gallei, M. Utilizing stretch-tunable thermochromic elastomeric opal films as novel reversible switchable photonic materials. Macromol. Rapid Commun. 2014, 35, 1852–1860. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, C.G.; Gallei, M.; Zahn, J.T.; Engelhardt, J.; Hellmann, G.P.; Rehahn, M. Reversible light-, thermo-, and mechano-responsive elastomeric polymer opal films. Chem. Mater. 2013, 25, 2309–2318. [Google Scholar] [CrossRef]
- Zhao, Q.; Finlayson, C.E.; Snoswell, D.R.E.; Haines, A.; Schäfer, C.; Spahn, P.; Hellmann, G.P.; Petukhov, A.V.; Herrmann, L.; Burdet, P.; et al. Large-scale ordering of nanoparticles using viscoelastic shear processing. Nat. Commun. 2016, 7, 11661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruhl, T.; Spahn, P.; Hellmann, G.P. Artificial opals prepared by melt compression. Polymer 2003, 44, 7625–7634. [Google Scholar] [CrossRef]
- Viel, B.; Ruhl, T.; Hellmann, G.P. Reversible deformation of opal elastomers. Chem. Mater. 2007, 19, 5673–5679. [Google Scholar] [CrossRef]
- Schäfer, C.G.; Winter, T.; Heidt, S.; Dietz, C.; Ding, T.; Baumberg, J.J.; Gallei, M. Smart polymer inverse-opal photonic crystal films by melt-shear organization for hybrid core–shell architectures. J. Mater. Chem. C 2015, 3, 2204–2214. [Google Scholar] [CrossRef]
- Scheid, D.; Stock, D.; Winter, T.; Gutmann, T.; Dietz, C.; Gallei, M. The pivotal step of nanoparticle functionalization for the preparation of functional and magnetic hybrid opal films. J. Mater. Chem. C 2016, 4, 2187–2196. [Google Scholar] [CrossRef]
- Vowinkel, S.; Schäfer, C.G.; Cherkashinin, G.; Fasel, C.; Roth, F.; Liu, N.; Dietz, C.; Ionescu, E.; Gallei, M. 3d-ordered carbon materials by melt-shear organization for tailor-made hybrid core–shell polymer particle architectures. J. Mater. Chem. C 2016, 4, 3976–3986. [Google Scholar] [CrossRef]
- Metz, G.; Wu, X.; Smith, S.O. Ramped-amplitude cross polarization in magic-angle-spinning NMR. J. Magn. Reson. 1994, 110, 219–227. [Google Scholar] [CrossRef]
- Bennett, A.E.; Rienstra, C.M.; Auger, M.; Lakshmi, K.V.; Griffin, R.G. Heteronuclear decoupling in rotating solids. J. Chem. Phys. 1995, 103, 6951–6958. [Google Scholar] [CrossRef]
- Vowinkel, S.; Malz, F.; Rode, K.; Gallei, M. Single-source macroporous hybrid materials by melt-shear organization of core–shell particles. J. Mater. Sci. 2017, 52, 11179–11190. [Google Scholar] [CrossRef]
- Grünberg, A.; Breitzke, H.; Buntkowsky, G. Spectroscopic properties of inorganic and organometallic compounds: Techniques, materials and applications. In Solid State Nmr of Immobilized Catalysts and Nanocatalysts; The Royal Chemical Society: Cambridge, UK, 2012; Volume 43, pp. 289–323. [Google Scholar]
- Gutmann, T.; Gruenberg, A.; Rothermel, N.; Werner, M.; Srour, M.; Abdulhussain, S.; Tan, S.; Xu, Y.; Breitzke, H.; Buntkowsky, G. Solid-state NMR concepts for the investigation of supported transition metal catalysts and nanoparticles. Solid State Nucl. Magn. Reson. 2013, 55–56, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gutmann, T.; Buntkowsky, G. Solid-state nmr studies of supported transition metal catalysts and nanoparticles. In Modern Magnetic Resonance; Webb, G.A., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–21. [Google Scholar]
- Lesage, A. Recent advances in solid-state NMR spectroscopy of spin I = 1/2 nuclei. Phys. Chem. Chem. Phys. 2009, 11, 6876–6891. [Google Scholar] [CrossRef] [PubMed]
- Bonhomme, C.; Gervais, C.; Laurencin, D. Recent NMR developments applied to organic-inorganic materials. Prog. Nucl. Magn. Reson. Spectrosc. 2014, 77, 1–48. [Google Scholar] [CrossRef] [PubMed]
- Moran, R.F.; Dawson, D.M.; Ashbrook, S.E. Exploiting NMR spectroscopy for the study of disorder in solids. Int. Rev. Phys. Chem. 2017, 36, 39–115. [Google Scholar] [CrossRef]
- Marchetti, A.; Chen, J.E.; Pang, Z.F.; Li, S.H.; Ling, D.S.; Deng, F.; Kong, X.Q. Understanding surface and interfacial chemistry in functional nanomaterials via solid-state NMR. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Geppi, M.; Borsacchi, S.; Mollica, G.; Veracini, C.A. Applications of solid-state NMR to the study of organic/inorganic multicomponent materials. Appl. Spectrosc. Rev. 2009, 44, 1–89. [Google Scholar] [CrossRef]
- Roehrich, A.; Drobny, G. Solid-state NMR studies of biomineralization peptides and proteins. Acc. Chem. Res. 2013, 46, 2136–2144. [Google Scholar] [CrossRef] [PubMed]
- Albert, K.; Bayer, E. Characterization of bonded phases by solid-state NMR-spectroscopy. J. Chromat. 1991, 544, 345–370. [Google Scholar] [CrossRef]
- Bluemel, J. Linkers and catalysts immobilized on oxide supports: New insights by solid-state NMR spectroscopy. Coord. Chem. Rev. 2008, 252, 2410–2423. [Google Scholar] [CrossRef]
- Zhou, Z.; Franz, A.W.; Hartmann, M.; Seifert, A.; Mueller, T.J.J.; Thiel, W.R. Novel organic/inorganic hybrid materials by covalent anchoring of phenothiazines on MCM-41. Chem. Mater. 2008, 20, 4986–4992. [Google Scholar] [CrossRef]
- Simon, P.F.W.; Ulrich, R.; Spiess, H.W.; Wiesner, U. Block copolymer-ceramic hybrid materials from organically modified ceramic precursors. Chem. Mat. 2001, 13, 3464–3486. [Google Scholar] [CrossRef]
- Davis, S.R.; Brough, A.R.; Atkinson, A. Formation of silica/epoxy hybrid network polymers. J. Non-Cryst. Solids 2003, 315, 197–205. [Google Scholar] [CrossRef]
- Bourgeat-Lami, E.; Tissot, I.; Lefebvre, F. Synthesis and characterization of SiOH-functionalized polymer latexes using methacryloxy propyl trimethoxysilane in emulsion polymerization. Macromolecules 2002, 35, 6185–6191. [Google Scholar] [CrossRef]
- Renker, S.; Mahajan, S.; Babski, D.T.; Schnell, I.; Jain, A.; Gutmann, J.; Zhang, Y.M.; Gruner, S.M.; Spiess, H.W.; Wiesner, U. Nanostructure and shape control in polymer-ceramic hybrids from poly(ethylene oxide)-block-poly(hexyl methacrylate) and aluminosilicates derived from them. Macromol. Chem. Phys. 2004, 205, 1021–1030. [Google Scholar] [CrossRef]
- Ravarian, R.; Wei, H.; Rawal, A.; Hook, J.; Chrzanowski, W.; Dehghani, F. Molecular interactions in coupled PMMA-bioglass hybrid networks. J. Mat. Chem. B 2013, 1, 1835–1845. [Google Scholar] [CrossRef]
- Dubois, C.; Herzog, N.; Ruettiger, C.; Geissler, A.; Grange, E.; Kunz, U.; Kleebe, H.J.; Biesalski, M.; Meckel, T.; Gutmann, T.; et al. Fluid flow programming in paper-derived silica-polymer hybrids. Langmuir 2017, 33, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Ni, K.F.; Shan, G.R.; Weng, Z.X.; Sheibat-Othman, N.; Fevotte, G.; Lefebvre, F.; Bourgeat-Lami, E. Synthesis of hybrid core−shell nanoparticles by emulsion (co)polymerization of styrene and γ-methacryloxypropyltrimethoxysilane. Macromolecules 2005, 38, 7321–7329. [Google Scholar] [CrossRef]
- Cao, Z.H.; Shan, G.R.; Fevotte, G.; Sheibat-Othman, N.; Bourgeat-Lami, E. Miniemulsion copolymerization of styrene and γ-methacryloxypropyltrimethoxysilane: Kinetics and mechanism. Macromolecules 2008, 41, 5166–5173. [Google Scholar] [CrossRef]
- Bounor-Legaré, V.; Angelloz, C.; Blanc, P.; Cassagnau, P.; Michel, A. A new route for organic-inorganic hybrid material synthesis through reactive processing without solvent. Polymer 2004, 45, 1485–1493. [Google Scholar] [CrossRef]
- Ding, T.; Cao, G.; Schäfer, C.G.; Zhao, Q.; Gallei, M.; Smoukov, S.K.; Baumberg, J.J. Revealing invisible photonic inscriptions: Images from strain. ACS Appl. Mater. Interfaces 2015, 7, 13497–13502. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, C.G.; Vowinkel, S.; Hellmann, G.P.; Herdt, T.; Contiu, C.; Schneider, J.J.; Gallei, M. A polymer based and template-directed approach towards functional multidimensional microstructured organic/inorganic hybrid materials. J. Mater. Chem. C 2014, 2, 7960–7975. [Google Scholar] [CrossRef]
- Schäfer, C.G.; Biesalski, M.; Hellmann, G.P.; Rehahn, M.; Gallei, M. Paper-supported elastomeric opal films for enhanced and reversible solvatochromic response. J. Nanophot. 2013, 7. [Google Scholar] [CrossRef]
- Schäfer, C.G.; Smolin, D.A.; Hellmann, G.P.; Gallei, M. Fully reversible shape transition of soft spheres in elastomeric polymer opal films. Langmuir 2013, 29, 11275–11283. [Google Scholar] [CrossRef] [PubMed]





© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vowinkel, S.; Paul, S.; Gutmann, T.; Gallei, M. Free-Standing and Self-Crosslinkable Hybrid Films by Core–Shell Particle Design and Processing. Nanomaterials 2017, 7, 390. https://doi.org/10.3390/nano7110390
Vowinkel S, Paul S, Gutmann T, Gallei M. Free-Standing and Self-Crosslinkable Hybrid Films by Core–Shell Particle Design and Processing. Nanomaterials. 2017; 7(11):390. https://doi.org/10.3390/nano7110390
Chicago/Turabian StyleVowinkel, Steffen, Stephen Paul, Torsten Gutmann, and Markus Gallei. 2017. "Free-Standing and Self-Crosslinkable Hybrid Films by Core–Shell Particle Design and Processing" Nanomaterials 7, no. 11: 390. https://doi.org/10.3390/nano7110390





