A Sub-Microanalysis Approach in Chemical Characterisation of Gold Nanorods Formed by a Novel Polymer-Immobilised Gold Seeds Base
Abstract
:1. Introduction
2. Results and Discussion
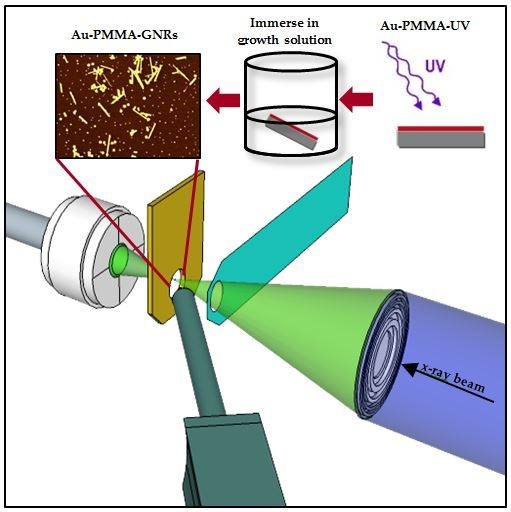
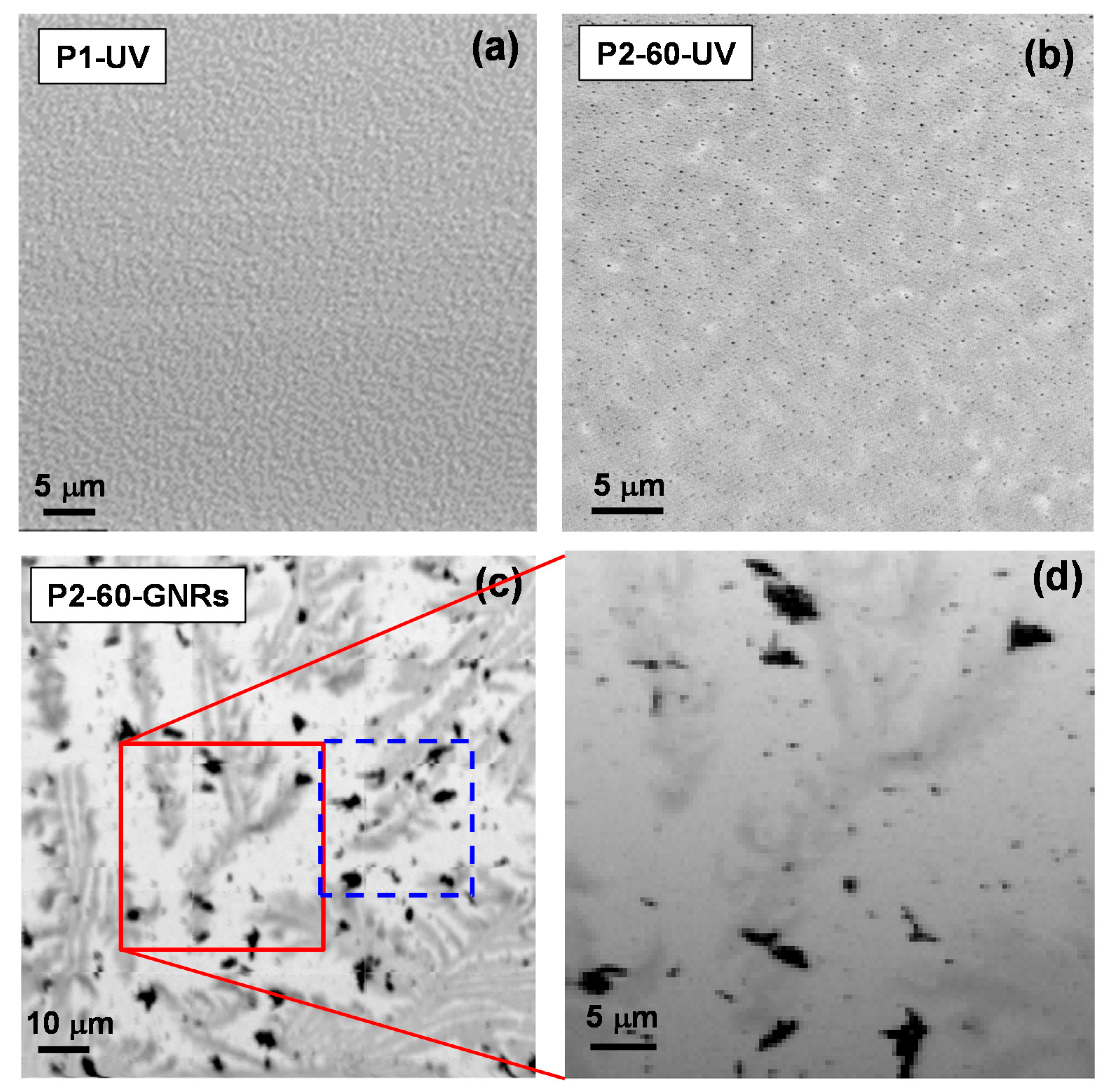
2.1. Synthesised GNRs Using Polymer-Immobilised Seed Mediated Method
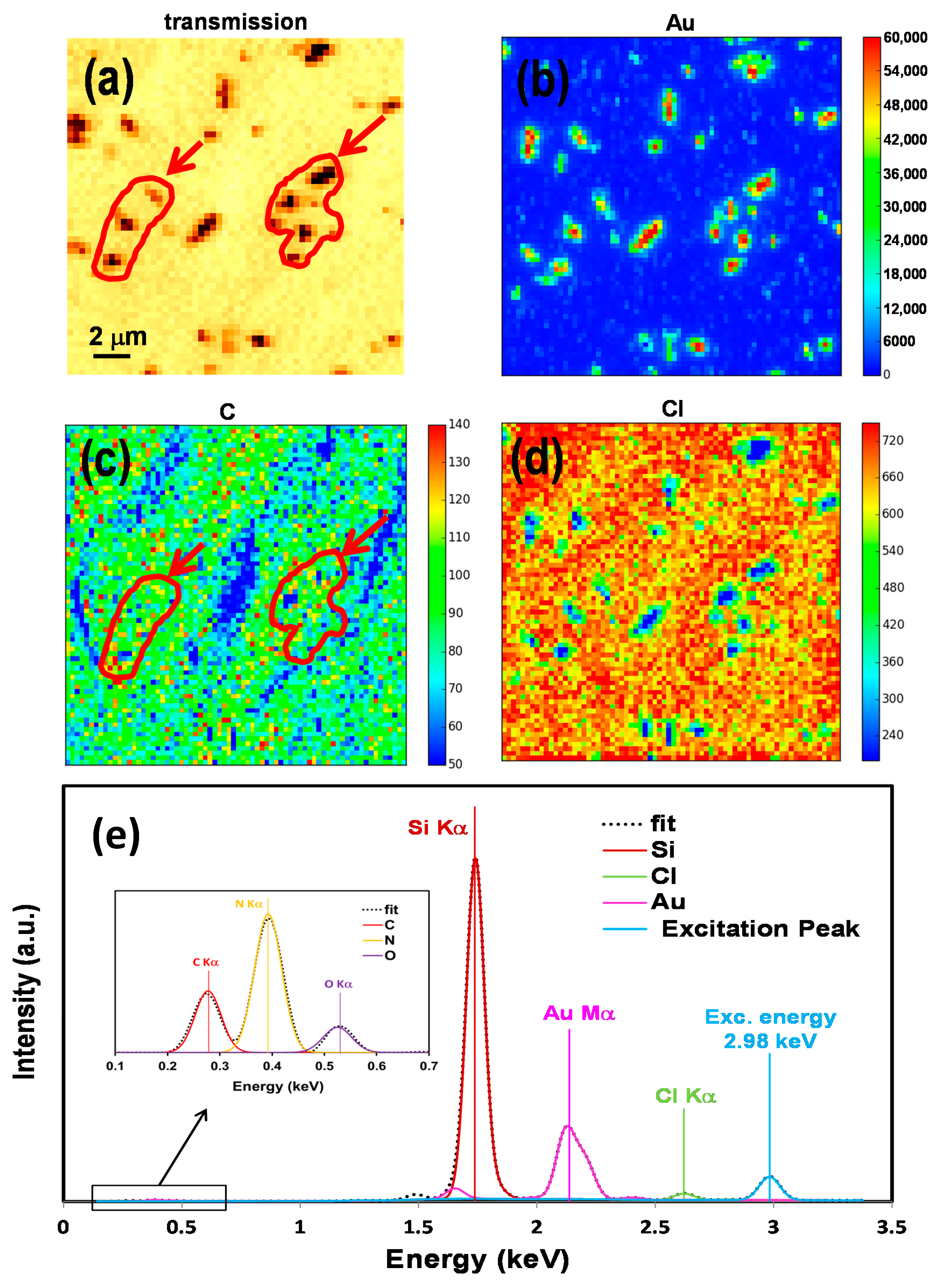
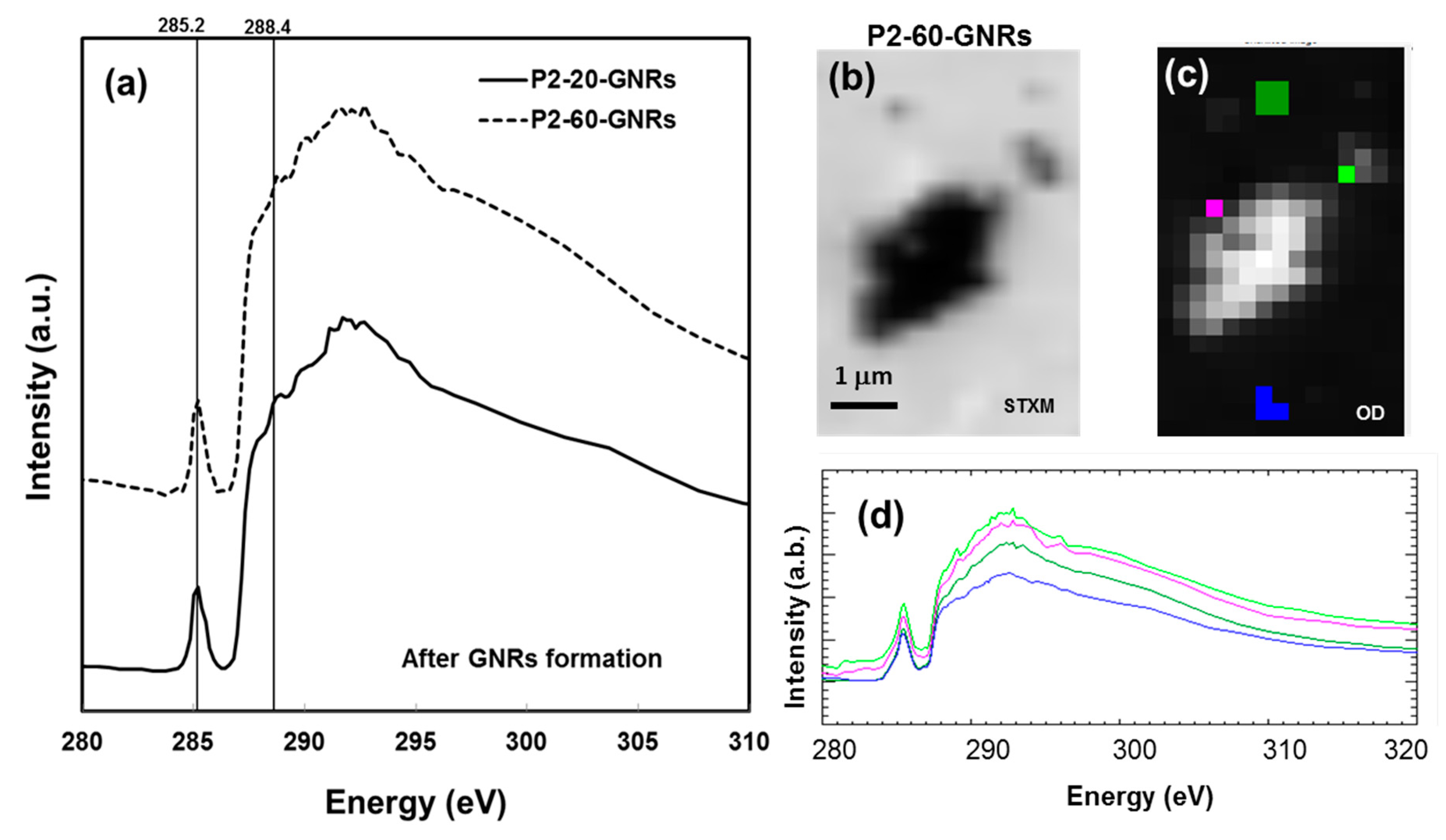
2.2. Elemental Mapping Using Soft X-Ray Fluorescence
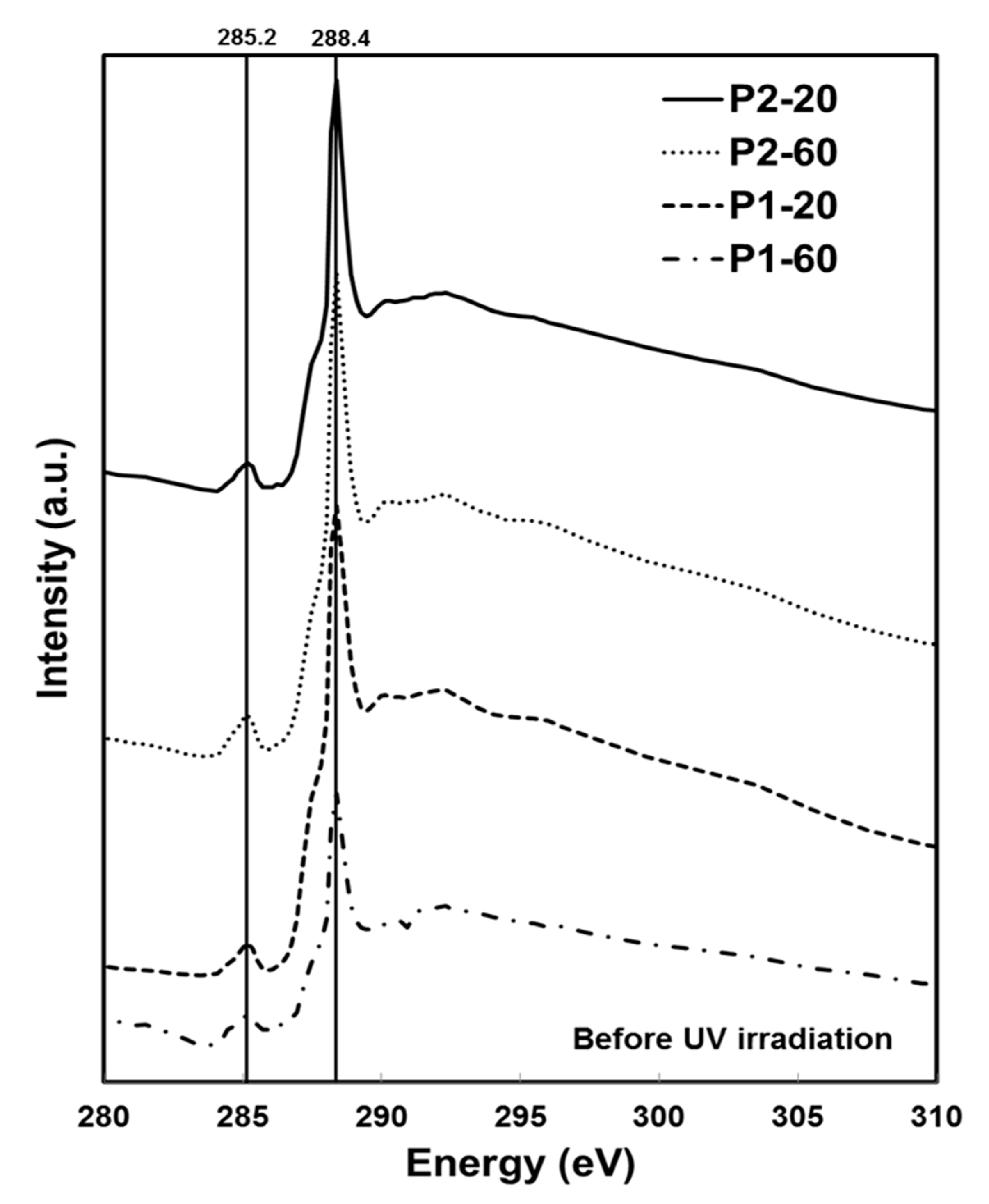
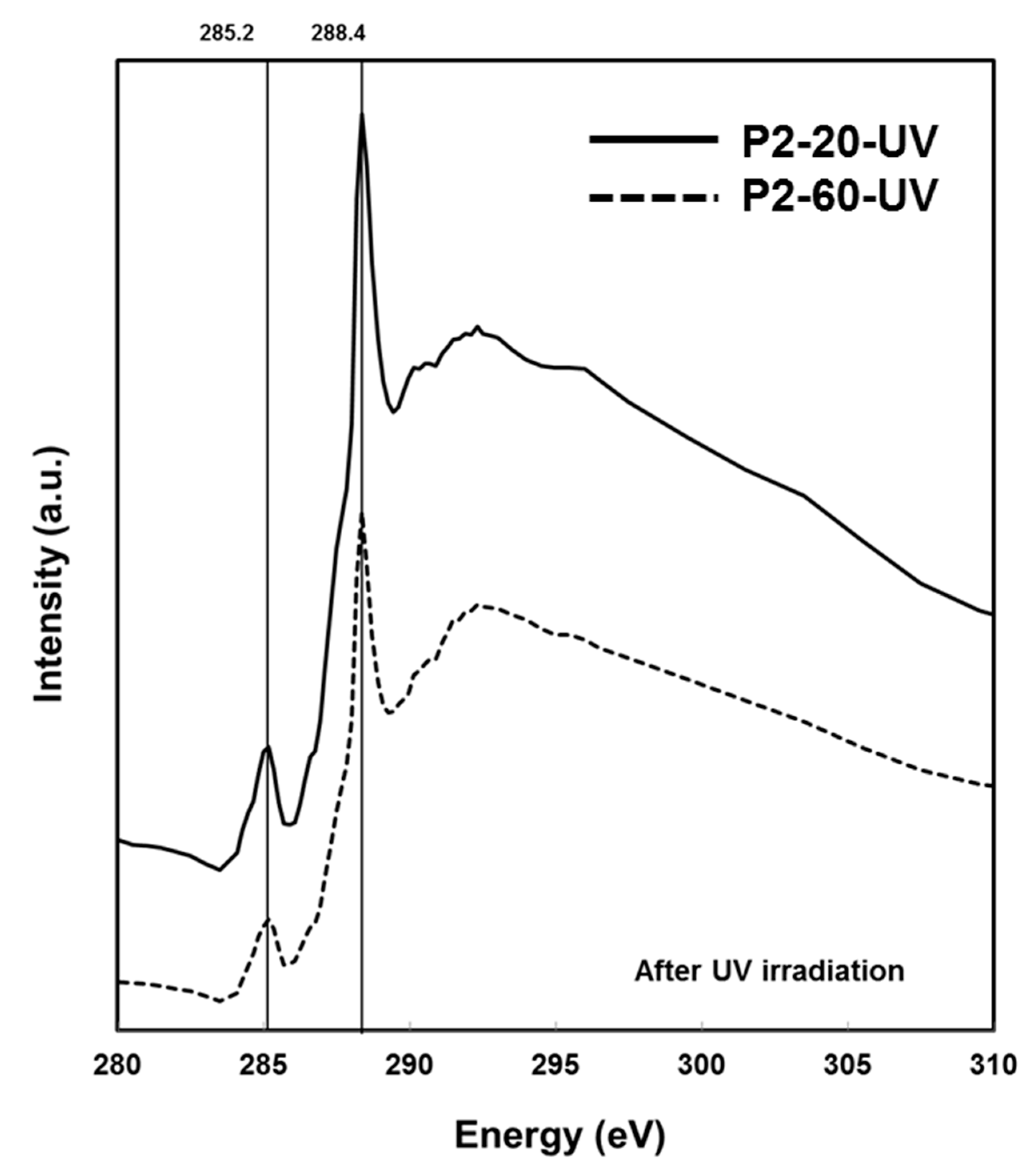
2.3. Chemical Characterisation Using Soft X-Ray Spectromicroscopy
3. Conclusions
4. Materials and Methods
4.1. Synthesis of Au–PMMA Nanocomposite
4.2. Synthesis of Polymer-Immobilised GNRs
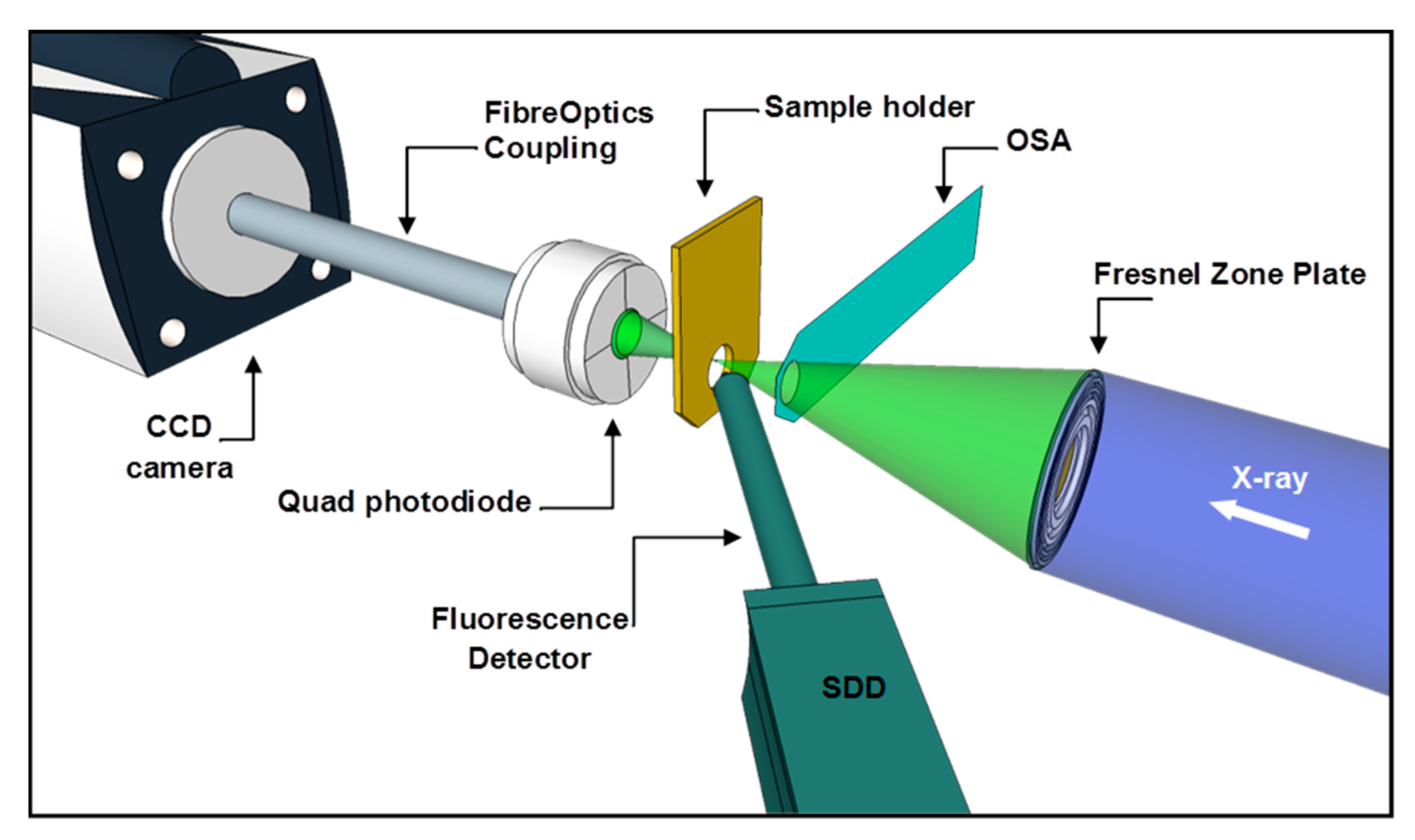
4.3. Synchrotron Based NEXAFS and XRF Measurements
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kulkarni, S.K. Nanotechnology: Principles and Practices, 3rd ed.; Springer: Berlin, Germany, 2015; p. 403. [Google Scholar]
- Zhang, H.; Jin, M.; Xia, Y. Noble-Metal Nanocrystals with Concave Surfaces: Synthesis and Applications. Angew. Chem. Int. Ed. 2012, 51, 7656–7673. [Google Scholar] [CrossRef] [PubMed]
- Dauthal, P.; Mukhopadhyay, M. Noble Metal Nanoparticles: Plant-Mediated Synthesis, Mechanistic Aspects of Synthesis, and Applications. Ind. Eng. Chem. Res. 2016, 55, 9557–9577. [Google Scholar] [CrossRef]
- Lee, Y.H.; Shi, W.; Lee, H.K.; Jiang, R.; Phang, I.Y.; Cui, Y.; Isa, L.; Yang, Y.; Wang, J.; Li, S.; et al. Nanoscale surface chemistry directs the tunable assembly of silver octahedra into three two-dimensional plasmonic superlattices. Nat. Commun. 2015, 6, 6990. [Google Scholar] [CrossRef] [PubMed]
- Doria, G.; Conde, J.; Veigas, B.; Giestas, L.; Almeida, C.; Assunção, M.; Rosa, J.; Baptista, P.V. Noble Metal Nanoparticles for Biosensing Applications. Sensors 2012, 12, 1657–1687. [Google Scholar] [CrossRef] [PubMed]
- Bruce, P.G.; Scrosati, B.; Tarascon, J.-M. Nanomaterials for Rechargeable Lithium Batteries. Angew. Chem. Int. Ed. 2008, 47, 2930–2946. [Google Scholar] [CrossRef] [PubMed]
- Wen, F.; Zhang, W.; Wei, G.; Wang, Y.; Zhang, J.; Zhang, M.; Shi, L. Synthesis of Noble Metal Nanoparticles Embedded in the Shell Layer of Core-Shell Poly(styrene-co-4-vinylpyridine) Microspheres and Their Application in Catalysis. Chem. Mater. 2008, 20, 2144–2150. [Google Scholar] [CrossRef]
- Lee, J.; Mahendra, S.; Alvarez, P.J.J. Nanomaterials in the Construction Industry: A Review of Their Applications and Environmental Health and Safety Considerations. ACS Nano 2010, 4, 3580–3590. [Google Scholar] [CrossRef]
- Daniel, M.C.; Astruc, D. Gold Nanoparticles: Assembly, Supramolecular Chemistry, Quantum-Size-Related Properties, and Applications toward Biology, Catalysis, and Nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef]
- Li, N.; Zhao, P.; Astruc, D. Anisotropic gold nanoparticles: Synthesis, properties, applications, and toxicity. Angew. Chem. Int. Ed. 2014, 53, 1756–1789. [Google Scholar] [CrossRef]
- Aldeanueva-Potel, P.; Carbó-Argibay, E.; Pazos-Pérez, N.; Barbosa, S.; Pastoriza-Santos, I.; Alvarez-Puebla, R.A.; Liz-Marzán, L.M. Spiked Gold Beads as Substrates for Single-Particle SERS. ChemPhysChem 2012, 13, 2561–2565. [Google Scholar] [CrossRef] [PubMed]
- Kannan, P.; Sampath, S.; John, S.A. Direct Growth of Gold Nanorods on Gold and Indium Tin Oxide Surfaces: Spectral, Electrochemical, and Electrocatalytic Studies. J. Phys. Chem. C 2010, 114, 21114–21122. [Google Scholar] [CrossRef]
- Lee, K.-H.; Huang, K.-M.; Tseng, W.-L.; Chiu, T.-C.; Lin, Y.-W.; Chang, H.-T. Manipulation of the Growth of Gold and Silver Nanomaterials on Glass by Seeding Approach. Langmuir 2007, 23, 1435–1442. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-K.; Na, H.-K.; Lee, Y.W.; Jang, H.; Han, S.W.; Min, D.-H. The direct growth of gold rods on graphene thin films. Chem. Commun. 2010, 46, 3185–3187. [Google Scholar] [CrossRef] [PubMed]
- Ferhan, A.R.; Guo, L.; Kim, D.-H. Influence of Ionic Strength and Surfactant Concentration on Electrostatic Surfacial Assembly of Cetyltrimethylammonium Bromide-Capped Gold Nanorods on Fully Immersed Glass. Langmuir 2010, 26, 12433–12442. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Martinez, A.; Perez-Juste, J.; Carbo-Argibay, E.; Tardajos, G.; Liz-Marzan, L.M. Gemini-Surfactant-Directed Self-Assembly of Monodisperse Gold Nanorods into Standing Superlattices. Angew. Chem. Int. Ed. 2009, 48, 9484–9488. [Google Scholar] [CrossRef] [PubMed]
- Abyaneh, M.K.; Parisse, P.; Casalis, L. Direct formation of gold nanorods on surfaces using polymer-immobilised gold seeds. Beilstein J. Nanotechnol. 2016, 7, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.; Belleville, P.; Popall, M.; Nicole, L. Applications of advanced hybrid organic–inorganic nanomaterials: From laboratory to market. Chem. Soc. Rev. 2011, 40, 696–753. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.; Soler-Illia, G.J.A.A.; Ribot, F.; Lalot, T.; Mayer, C.R.; Cabuil, V. Designed Hybrid Organic-Inorganic Nanocomposites from Functional Nanobuilding Blocks. Chem. Mater. 2001, 13, 3061–3083. [Google Scholar] [CrossRef]
- Torrisi, V.; Ruffino, F. Metal-Polymer Nanocomposites: (Co-)Evaporation/(Co)Sputtering Approaches and Electrical Properties. Coatings 2015, 5, 378–424. [Google Scholar] [CrossRef]
- Torrisi, V.; Ruffino, F.; Licciardello, A.; Grimaldi, M.G.; Marletta, G. Memory effects in annealed hybrid gold nanoparticles/block copolymer bilayers. Nanoscale Res. Lett. 2011, 6, 167. [Google Scholar] [CrossRef] [PubMed]
- Villa, A.; Dimitratos, N.; Chan-Thaw, C.E.; Hammond, C.; Veith, G.M.; Wang, D.; Manzoli, M.; Pratia, L.; Hutchings, G.J. Characterisation of gold catalysts. Chem. Soc. Rev. 2016, 45, 4953–4994. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, E.; Suzer, S. Au nanoparticles in PMMA matrix: In situ synthesis and the effect of Au nanoparticles on PMMA conductivity. Appl. Surf. Sci. 2010, 256, 6630–6633. [Google Scholar] [CrossRef]
- Alsawafta, M.; Badilescu, S.; Paneri, A.; Truong, V.-V.; Packirisamy, M. Gold-Poly(methyl methacrylate) Nanocomposite Films for Plasmonic Biosensing Applications. Polymers 2011, 3, 1833–1848. [Google Scholar] [CrossRef]
- Abyaneh, M.K.; Paramanik, D.; Varma, S.; Gosavi, S.W.; Kulkarni, S.K. Formation of gold nanoparticles in polymethylmethacrylate by UV irradiation. J. Phys. D Appl. Phys. 2007, 40, 3771–3779. [Google Scholar] [CrossRef]
- Eve, S.; Mohr, J. Study of the surface modification of the PMMA by UV-radiation. Procedia Eng. 2009, 1, 237–240. [Google Scholar] [CrossRef]
- Choi, J.O.; Moore, J.A.; Corelli, J.C.; Silverman, J.P.; Bakhru, H. Degradation of poly(methylmethacrylate) by deep ultraviolet, X-ray, electron beam, and proton beam irradiations. J. Vac. Sci. Technol. 1988, 6, 2286–2289. [Google Scholar] [CrossRef]
- PyMca X-Ray Fluorescence Toolkit. Available online: http://pymca.sourceforge.net/ (accessed on 12 October 2017).
- Lerotic, M.; Mak, R.; Wirick, S.; Meirer, F.; Jacobsen, C. MANTiS: A program for the analysis of X-ray spectromicroscopy data. J. Synchrotron Rad. 2014, 21, 1206–1212. [Google Scholar] [CrossRef] [PubMed]
- Araki, T.; Ade, H.; Stubbs, J.M.; Sundberg, D.C.; Mitchell, G.E.; Kortright, J.B.; Kilcoyne, A.L.D. Resonant soft X-ray scattering from structured polymer nanoparticles. Appl. Phys. Lett. 2006, 89, 124106. [Google Scholar] [CrossRef]
- Wang, J.; Morin, C.; Li, L.; Hitchcock, A.P.; Scholl, A.; Doran, A. Radiation damage in soft X-ray microscopy. J. Electron Spectrosc. Relat. Phenom. 2009, 170, 25–36. [Google Scholar] [CrossRef]
- Gregoratti, L.; Amati, M.; Abyaneh, M.K. Damage formation and characterization with scanning photoemission spectromicroscopy. Proc. SPIE—Int. Soc. Opt. Eng. 2011, 8077, 80770K. [Google Scholar]
- Zhang, X.; Jacobsen, C.; Lindaas, S.; Williams, S. Exposure strategies for polymethyl methacrylate from in situ X-ray absorption near edge structure spectroscopy. J. Vac. Sci. Technol. B 1995, 13, 1477. [Google Scholar] [CrossRef]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abyaneh, M.K.; Araki, T.; Kaulich, B. A Sub-Microanalysis Approach in Chemical Characterisation of Gold Nanorods Formed by a Novel Polymer-Immobilised Gold Seeds Base. Nanomaterials 2017, 7, 331. https://doi.org/10.3390/nano7100331
Abyaneh MK, Araki T, Kaulich B. A Sub-Microanalysis Approach in Chemical Characterisation of Gold Nanorods Formed by a Novel Polymer-Immobilised Gold Seeds Base. Nanomaterials. 2017; 7(10):331. https://doi.org/10.3390/nano7100331
Chicago/Turabian StyleAbyaneh, Majid Kazemian, Tohru Araki, and Burkhard Kaulich. 2017. "A Sub-Microanalysis Approach in Chemical Characterisation of Gold Nanorods Formed by a Novel Polymer-Immobilised Gold Seeds Base" Nanomaterials 7, no. 10: 331. https://doi.org/10.3390/nano7100331