Lyotropic Liquid Crystal Phases from Anisotropic Nanomaterials
Abstract
:1. Introduction
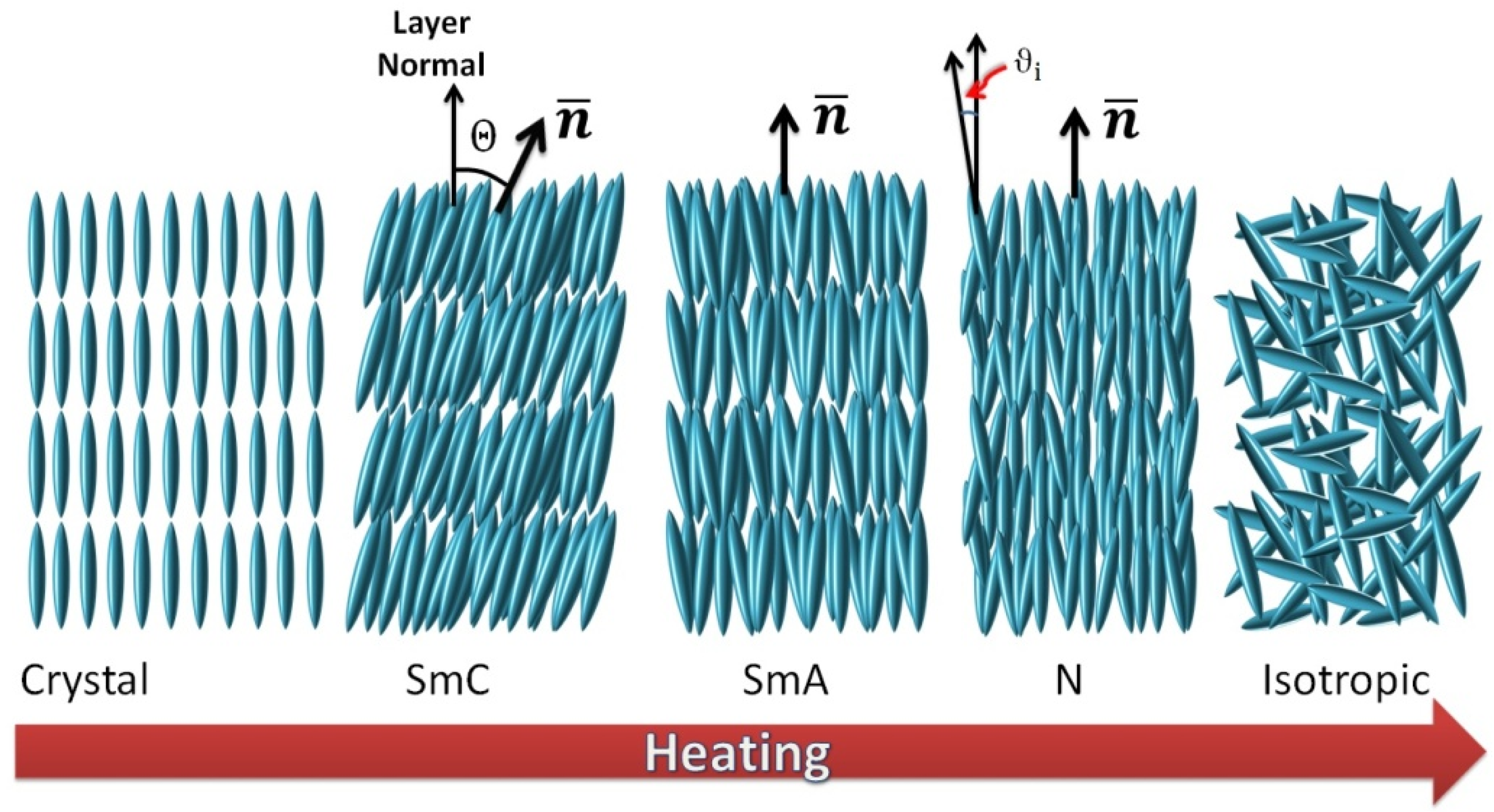
1.1. Thermotropic Liquid Crystals
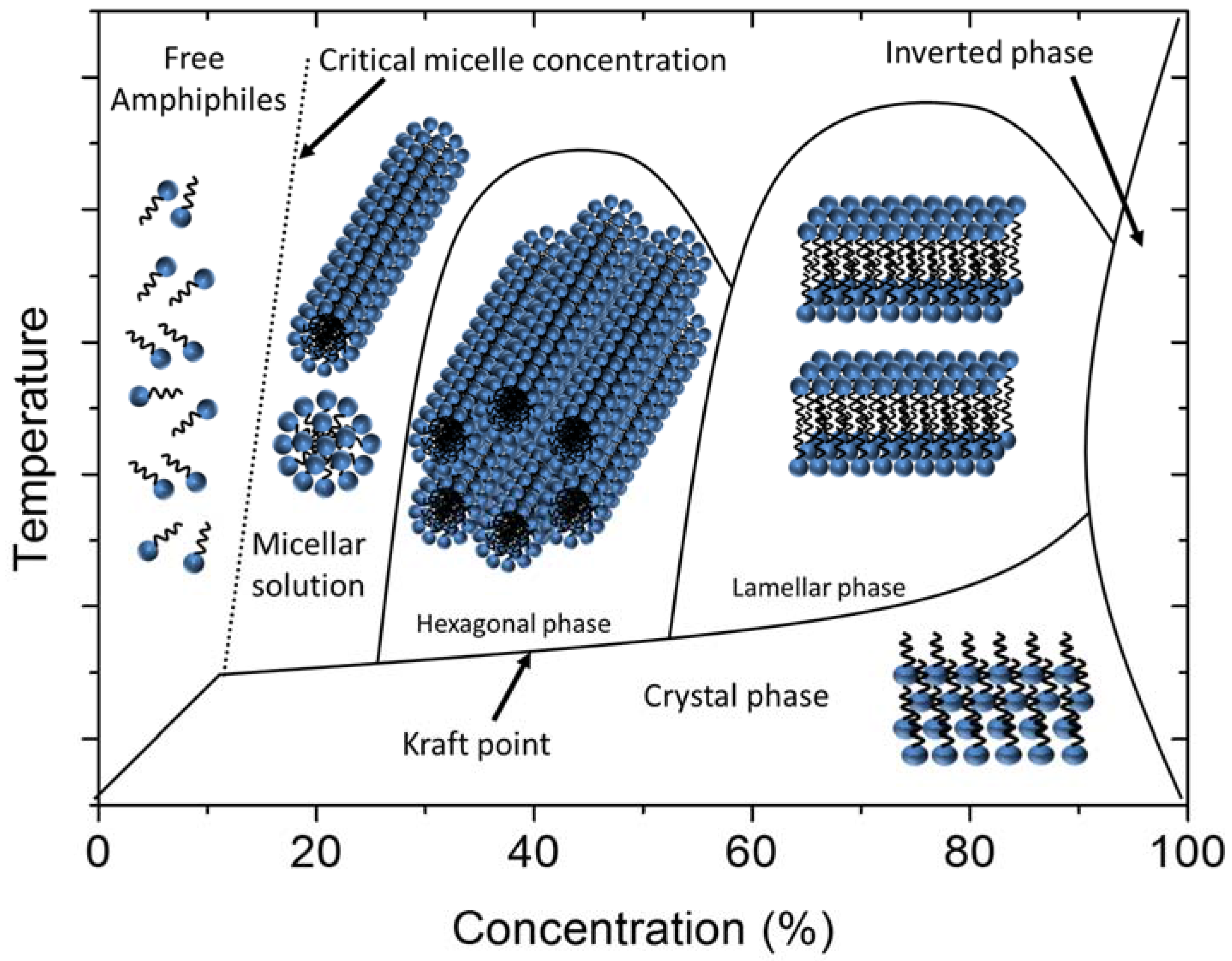
1.2. Lyotropic Liquid Crystals
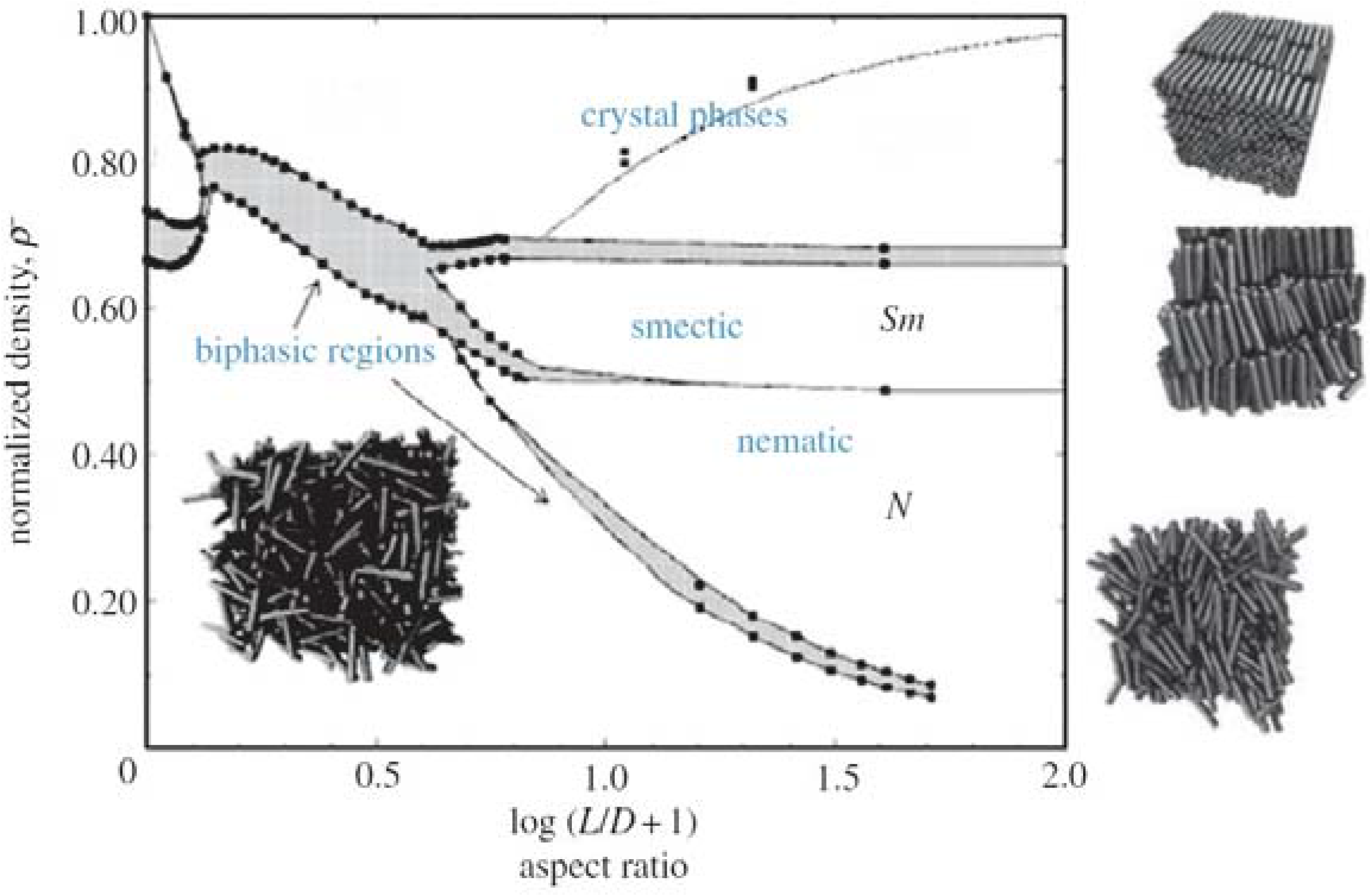
1.3. Isotropic to Nematic Transition: Maier-Saupe vs. Onsager
2. Lyotropic Phases from Nanomaterials
2.1. Inorganic and Mineral Liquid Crystals
2.2. Clay Based Liquid Crystals
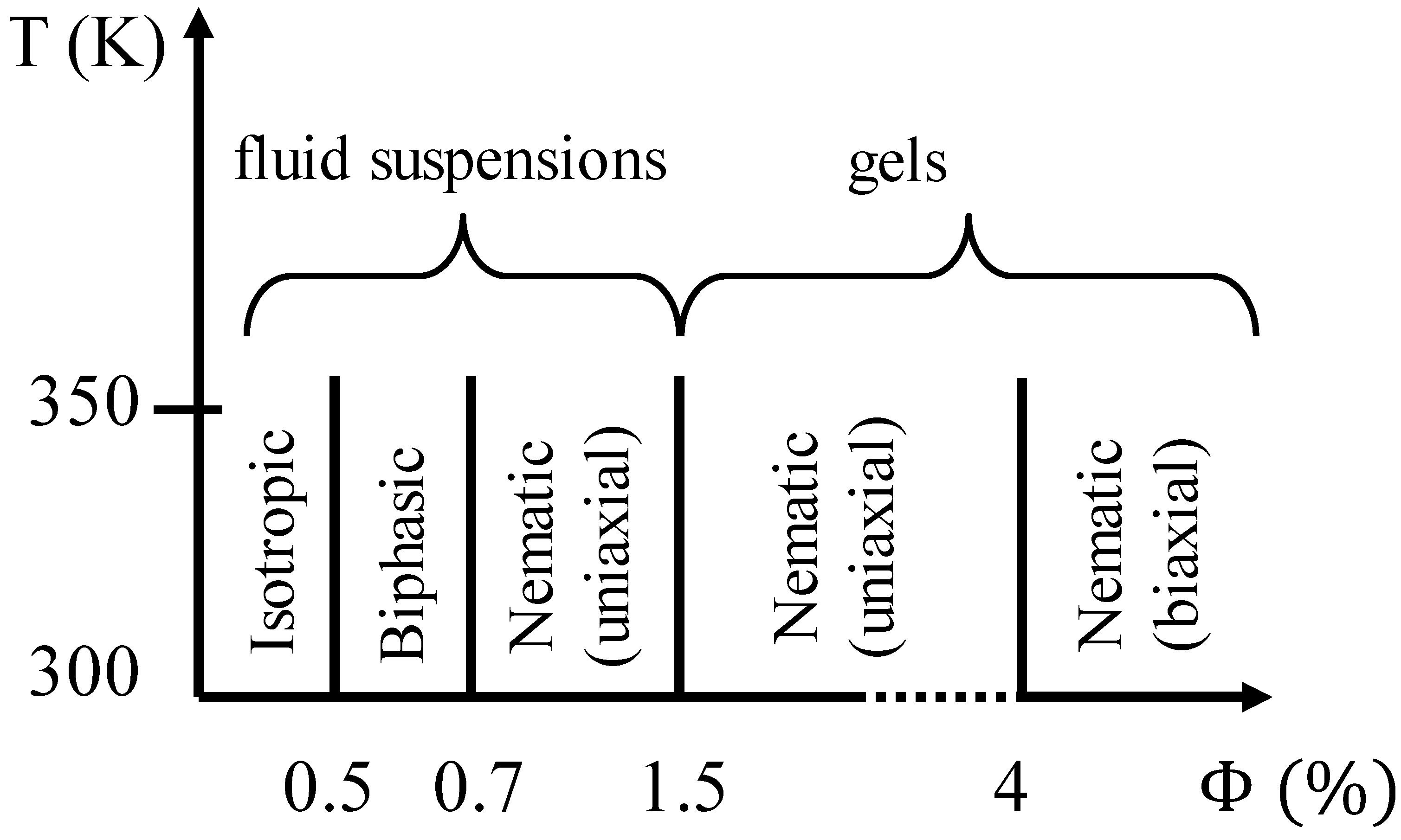
2.2.1. Bentonite
2.2.2. Laponite
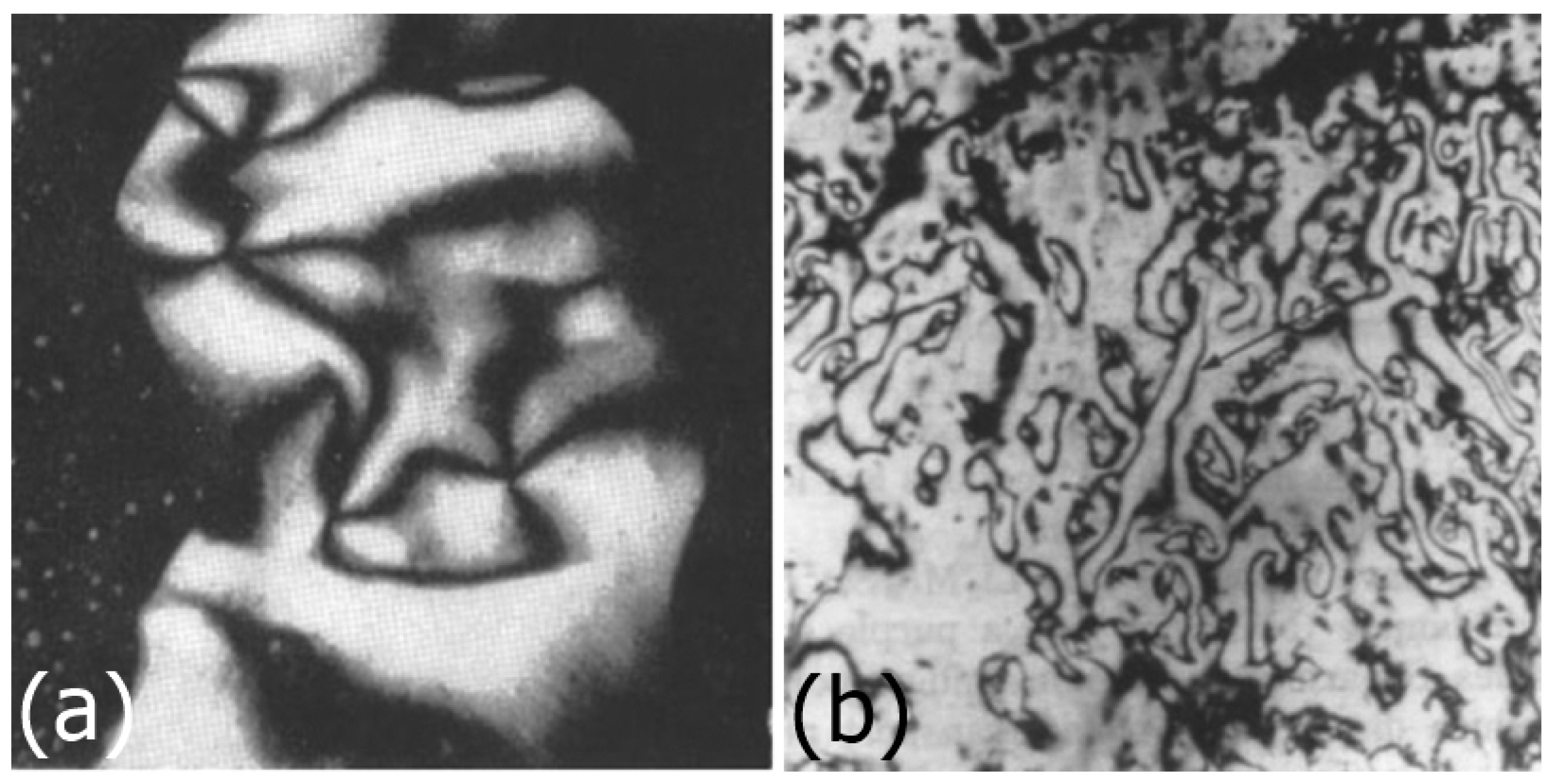
2.2.3. Imogolite
2.3. Biological Nanoparticles
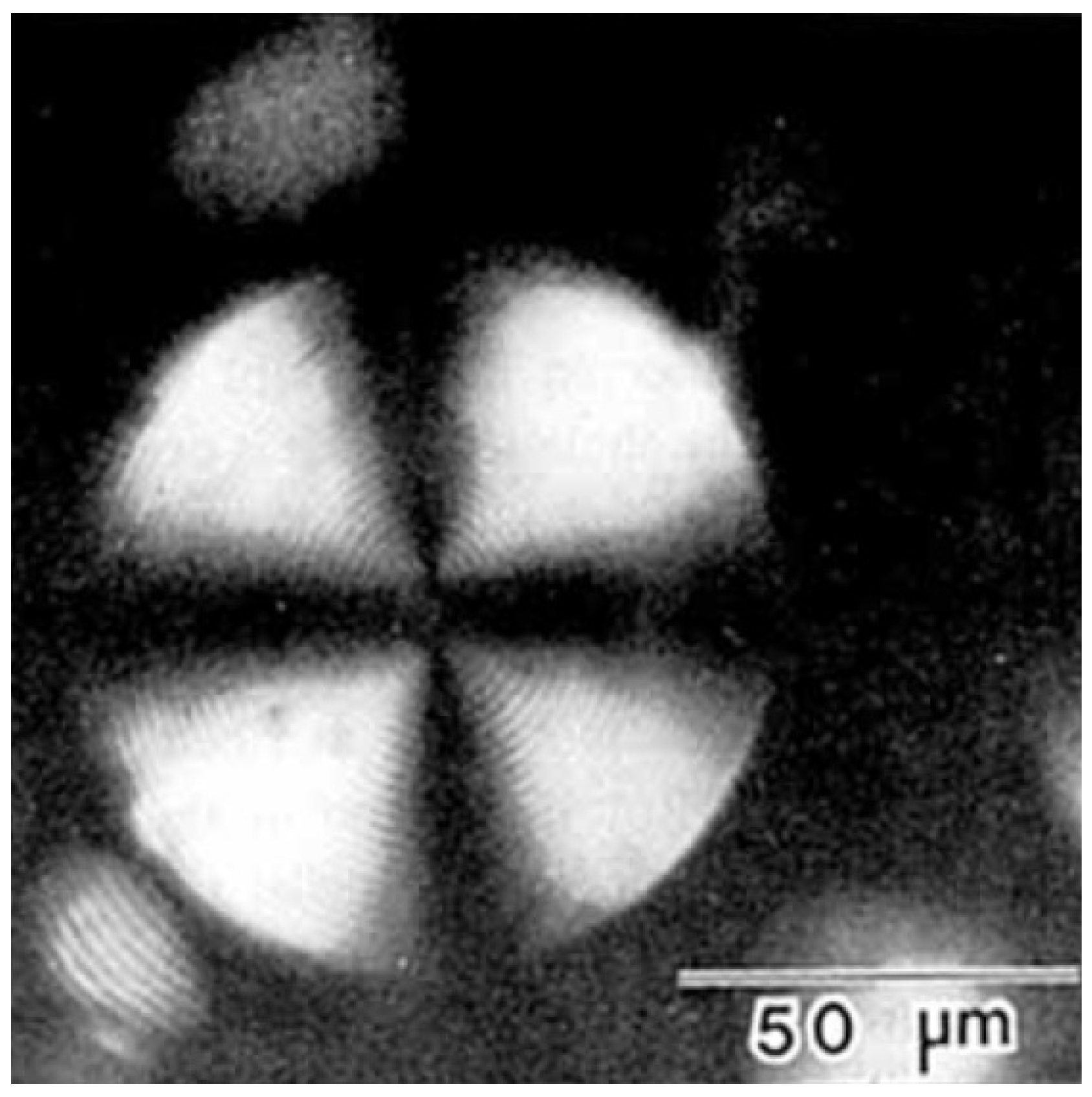
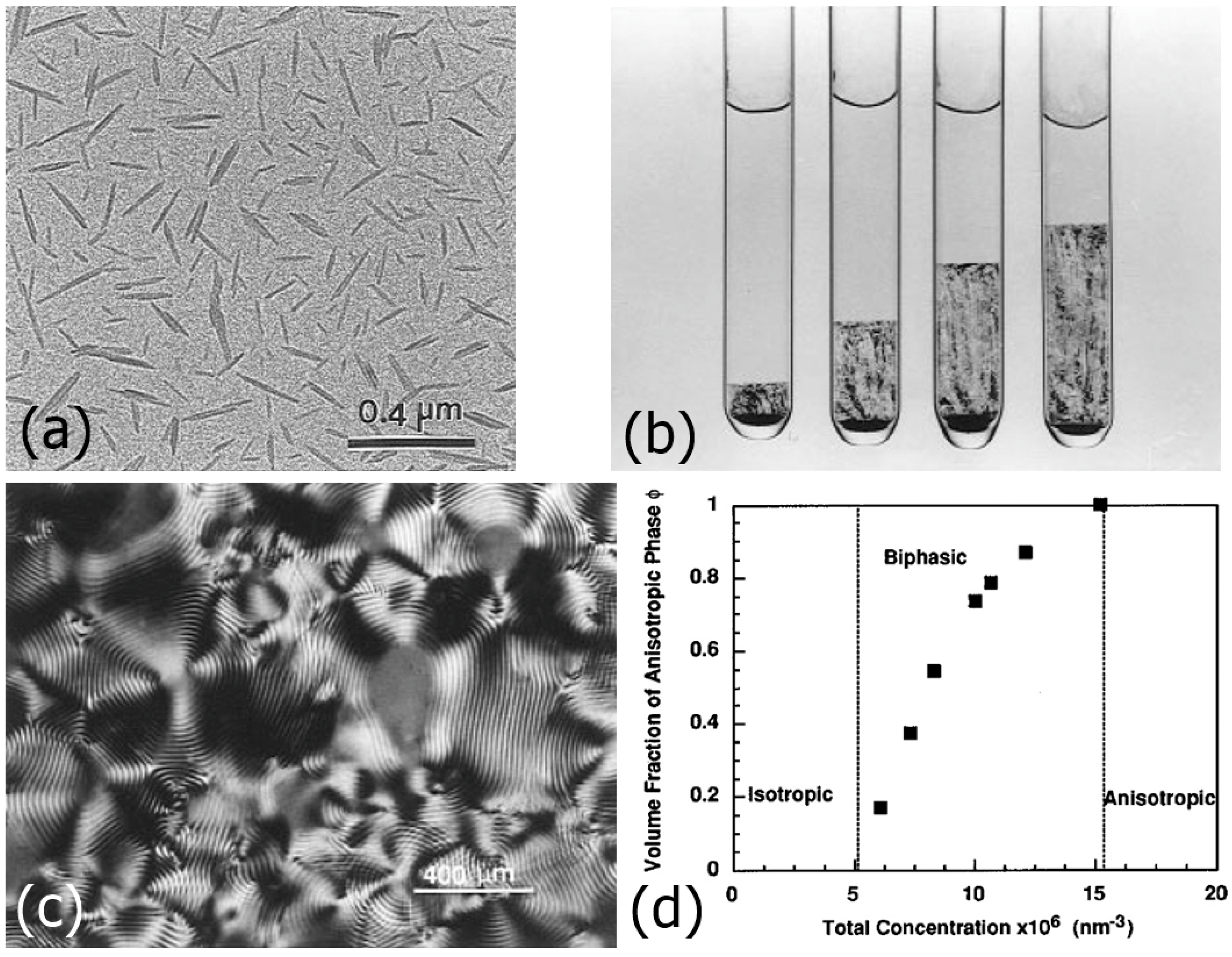
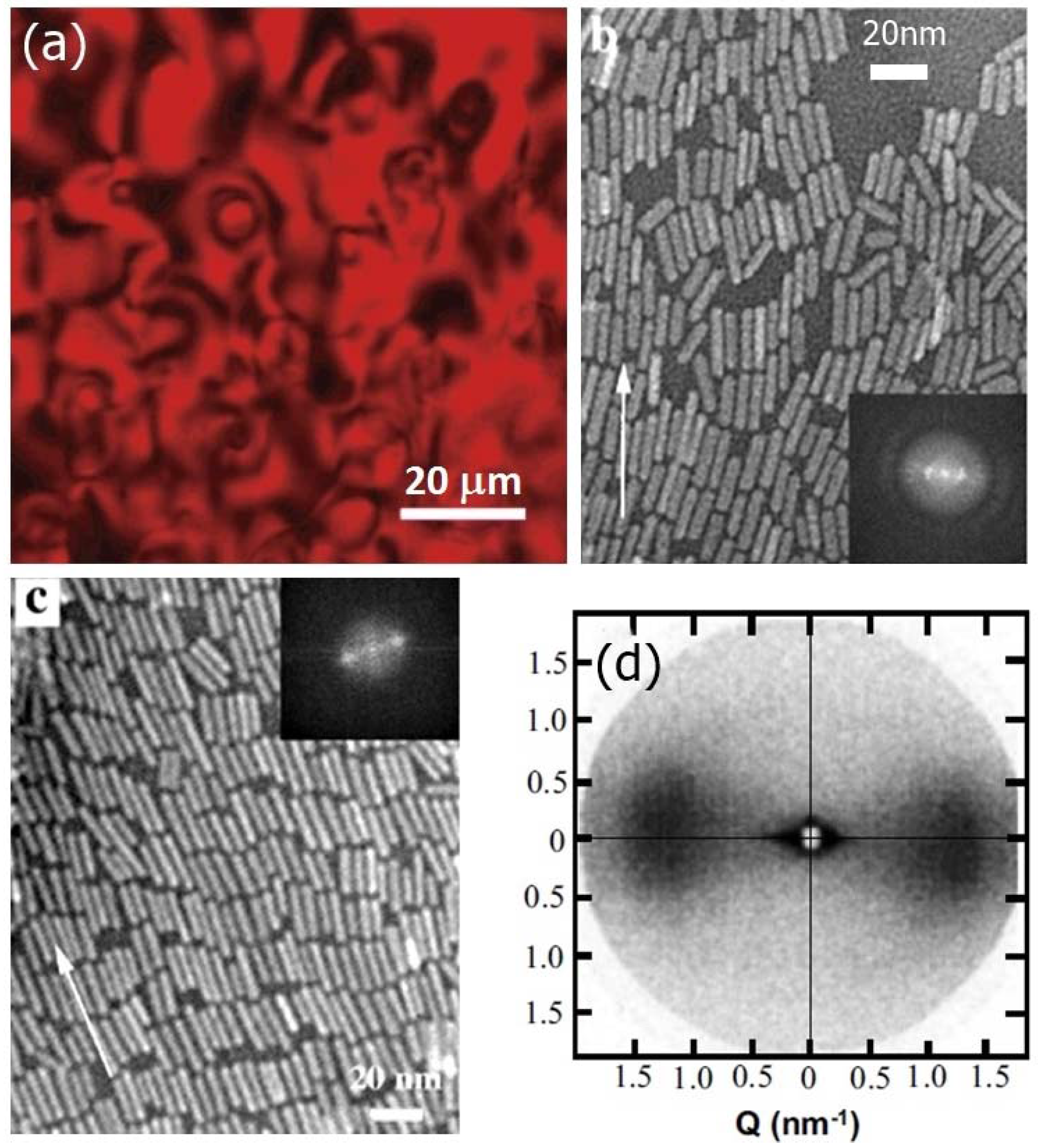
2.3.1. Tobacco Mosaic Virus (TMV)
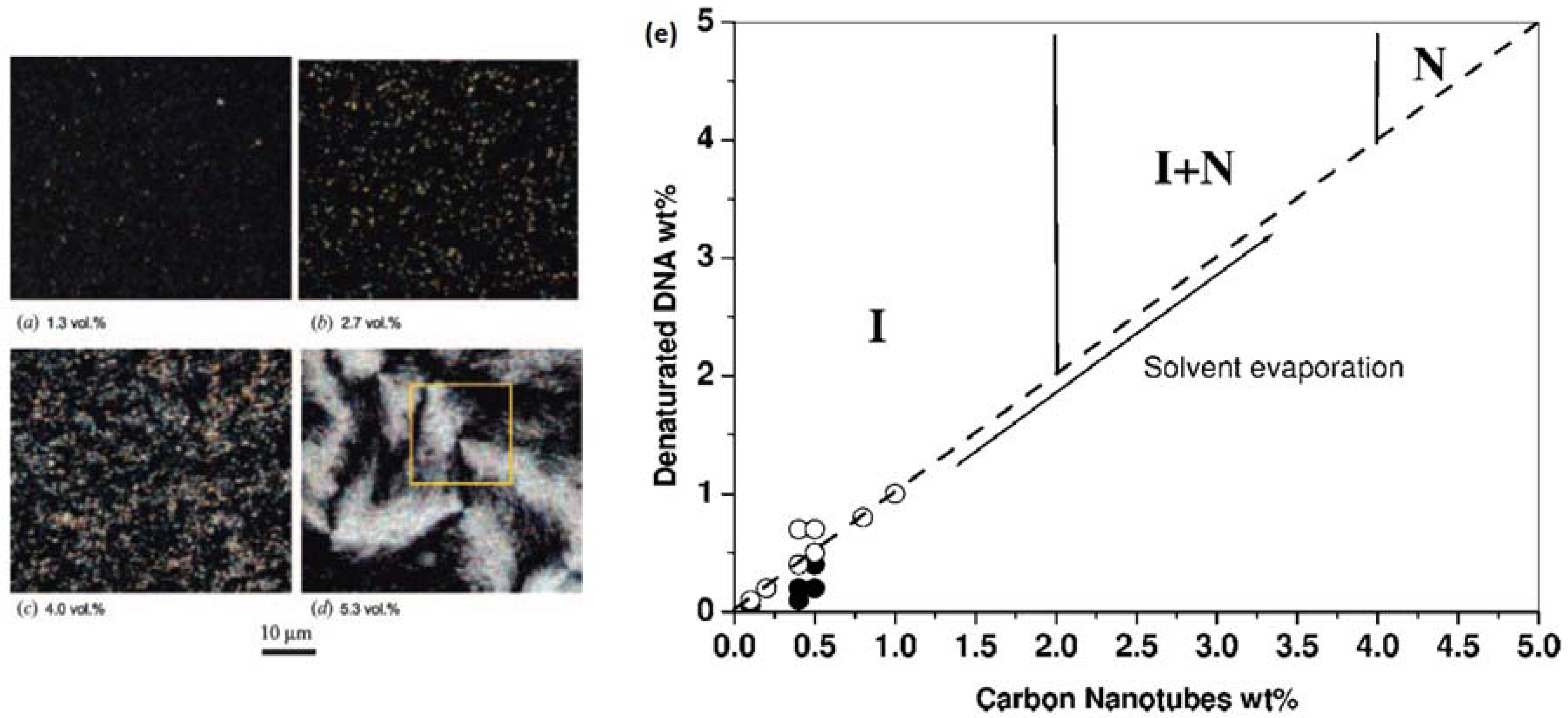
2.3.2. DNA
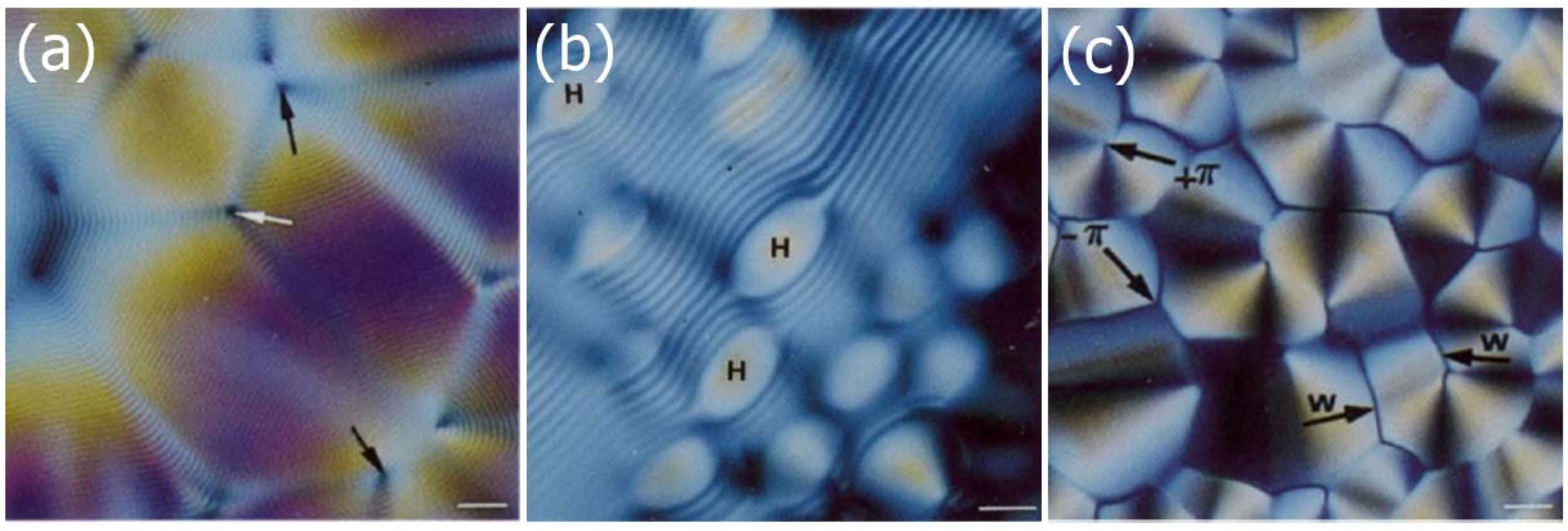
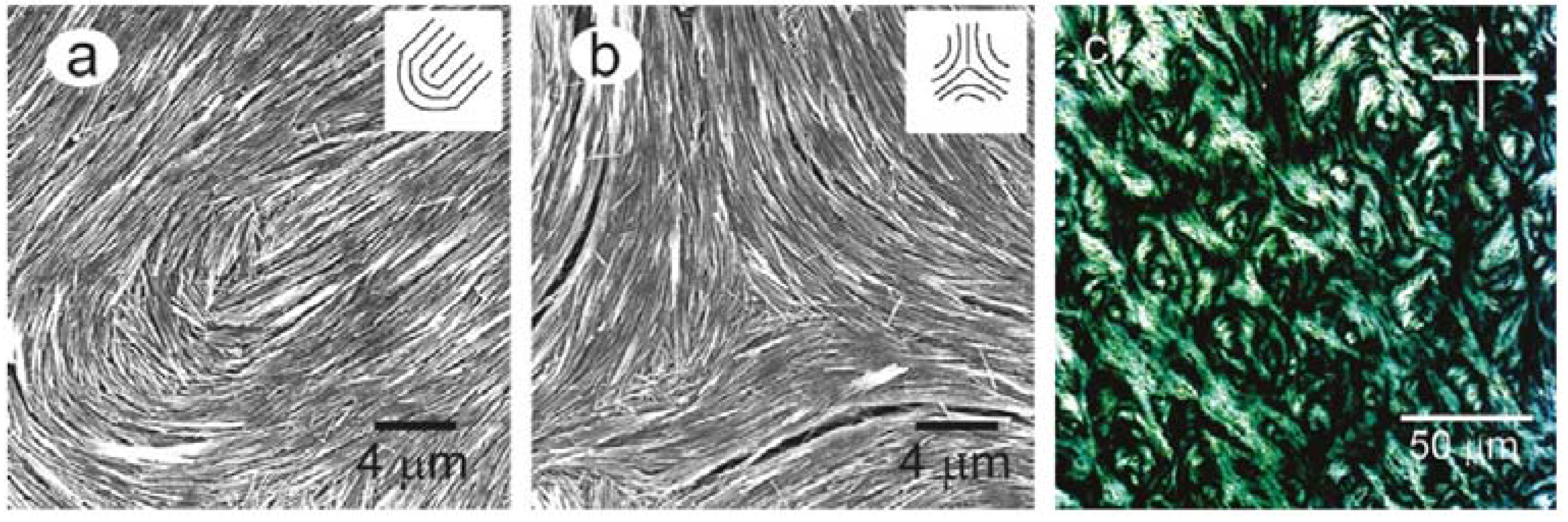
2.3.3. Cellulose Nanocrystals
2.3.4. Active Liquid Crystals
2.4. Liquid Crystals from Nanotubes and Nanorods
2.4.1. Nanotubes
2.4.2. Nanorods and Nanowires
Nanorods of ZnO
Nanorods of TiO2
CdSe Semiconductor Nanorods
2.5. Liquid Crystals from Nanoplates
2.5.1. Graphene
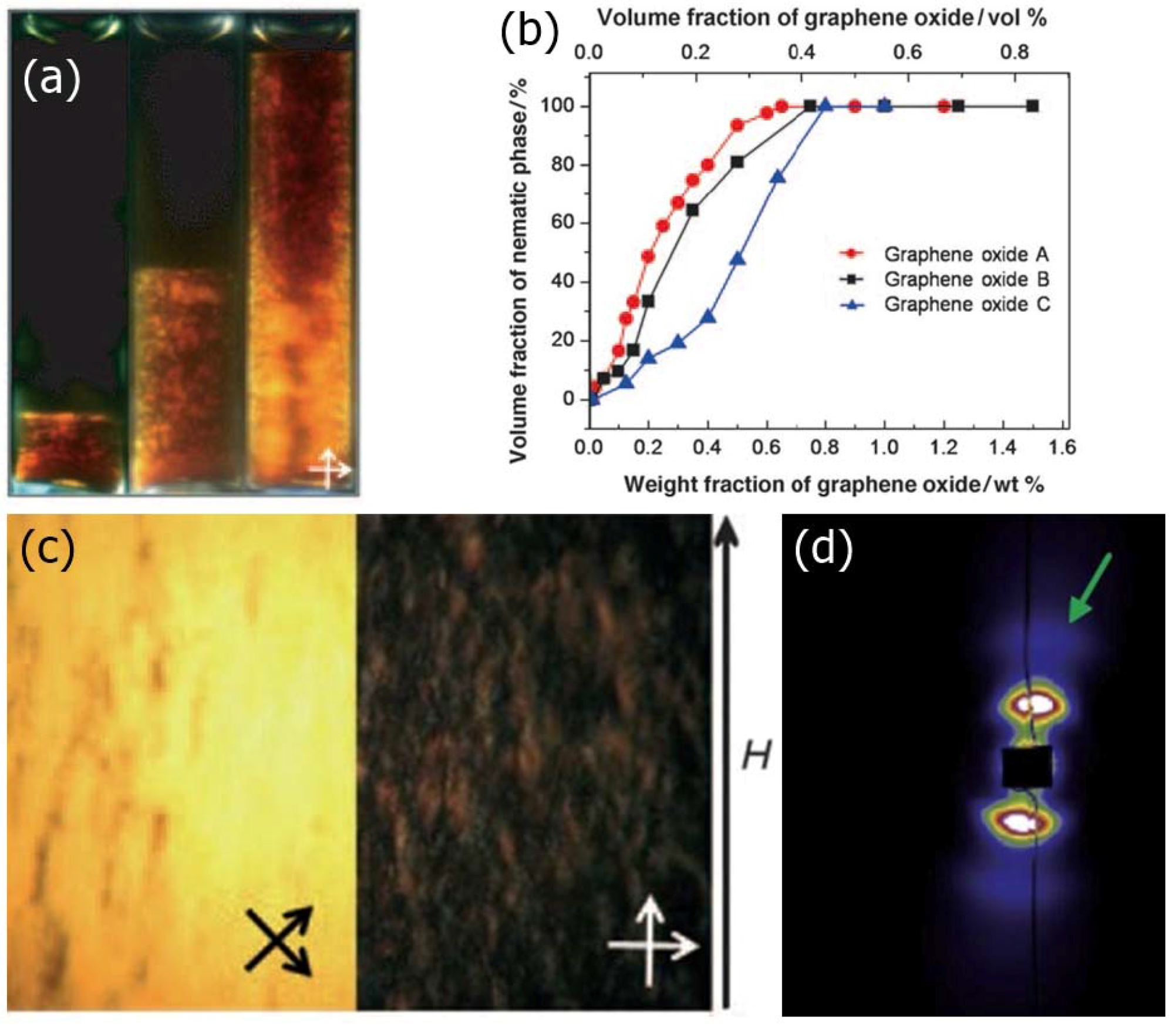
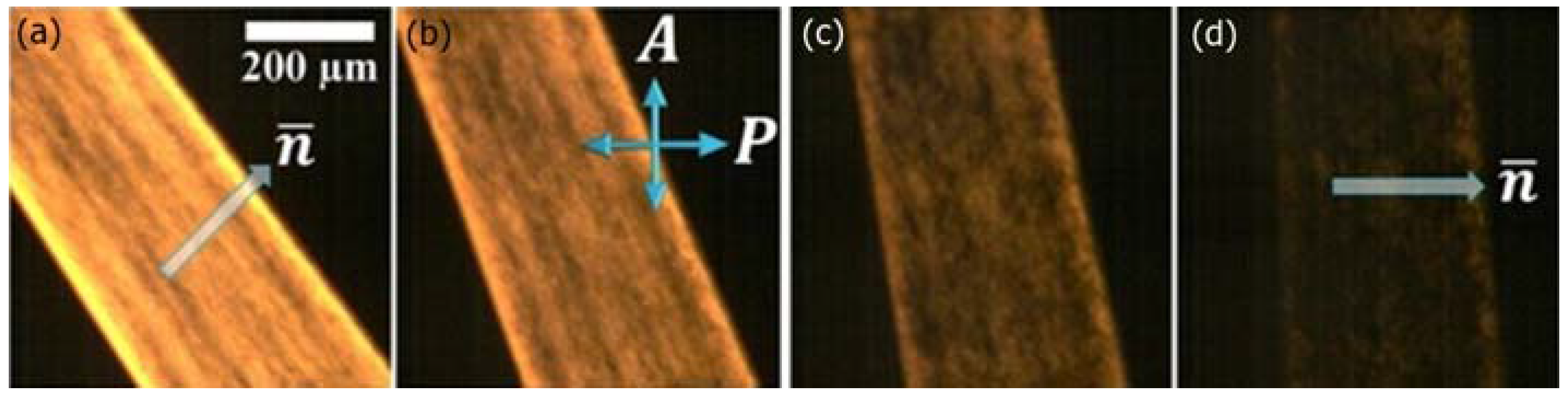
2.5.2. Graphene Oxide
2.5.3. Reduced Graphene Oxide
2.5.4. Other 2D Materials
3. Summary and Outlook
Author Contributions
Conflicts of Interest
References
- Collings, P.J. Liquid Crystals: Nature’s Delicate Phase of Matter; Princeton University Press: Princeton, NJ, USA, 1990. [Google Scholar]
- Collings, P.J.; Hird, M. Introduction to Liquid Crystals Chemistry and Physics; Taylor & Francis: London, UK; Bristol, PA, USA, 1997. [Google Scholar]
- Chandrasekhar, S. Liquid Crystals, 2nd ed.; Cambridge University Press: Cambridge, UK, 1992. [Google Scholar]
- Singh, S. Liquid Crystals: Fundamentals; World Scientific: Hackensack, NJ, USA, 2002. [Google Scholar]
- De Gennes, P.G.; Prost, J. The Physics of Liquid Crystals, 2nd ed.; Oxford University Press: Oxford, UK; New York, NY, USA, 1993. [Google Scholar]
- Lueder, E. Liquid Crystal Displays: Addressing Schemes and Electro-Optical Effects; John Wiley & Sons: Chichester, UK, 2001. [Google Scholar]
- Dierking, I. Textures of Liquid Crystals; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Petrov, A.G. The Lyotropic State of Matter: Molecular Physics and Living Matter Physics; Taylor & Francis: London, UK, 1999. [Google Scholar]
- Figueiredo Neto, A.M.; Salinas, S.R.A. The Physics of Lyotropic Liquid Crystals: Phase Transitions and Structural Properties; Oxford University Press: New York, NY, USA, 2005. [Google Scholar]
- Onsager, L. The effects of shape on the interaction of colloidal particles. Ann. N. Y. Acad. Sci. 1949, 51, 627–659. [Google Scholar] [CrossRef]
- Bawden, F.C.; Pirie, N.W.; Bernal, J.D.; Fankuchen, I. Liquid Crystalline Substances from Virus-infected Plants. Nature 1936, 138, 1051–1052. [Google Scholar] [CrossRef]
- Diesselhorst, H.; Freundlich, H. On the double refraction of vanadine pentoxydsol. Phys. Z. 1915, 16, 419–425. [Google Scholar]
- Mourchid, A.; Delville, A.; Lambard, J.; LeColier, E.; Levitz, P. Phase Diagram of Colloidal Dispersions of Anisotropic Charged Particles: Equilibrium Properties, Structure, and Rheology of Laponite Suspensions. Langmuir 1995, 11, 1942–1950. [Google Scholar] [CrossRef]
- Maier, W.; Saupe, A. Eine einfache molekulare Theorie des nematischen kristallinflüssigen Zustandes. Zeitschrift für Naturforsch. A 1958, 13, 564–566. [Google Scholar] [CrossRef]
- Maier, W.; Saupe, A. Eine einfache molekular-statistische Theorie der nematischen kristallinflüssigen Phase. Teil l1. Zeitschrift für Naturforsch. A 1959, 14, 882. [Google Scholar] [CrossRef]
- Maier, W.; Saupe, A. Eine einfache molekular-statistische Theorie der nematischen kristallinflüssigen Phase. Teil II. Zeitschrift für Naturforsch. A 1960, 15, 287. [Google Scholar] [CrossRef]
- Stroobants, A.; Lekkerkerker, H.N.W.; Odijk, T. Effect of electrostatic interaction on the liquid crystal phase transition in solutions of rodlike polyelectrolytes. Macromolecules 1986, 19, 2232–2238. [Google Scholar] [CrossRef]
- Vroege, G.J.; Lekkerkerker, H.N.W. Phase transitions in lyotropic colloidal and polymer liquid crystals. Rep. Fmg. Phys. 1992, 55, 1241–1309. [Google Scholar] [CrossRef]
- Bolhuis, P.; Frenkel, D. Tracing the phase boundaries of hard spherocylinders. J. Chem. Phys. 1997, 106, 666–687. [Google Scholar] [CrossRef]
- Sonin, A.S. Inorganic lyotropic liquid crystals. J. Mater. Chem. 1998, 8, 2557–2574. [Google Scholar] [CrossRef]
- Gabriel, J.-C.P.; Davidson, P. Mineral Liquid Crystals from Self-Assembly of Anisotropic Nanosystems. Top. Curr. Chem. 2003, 226, 119–172. [Google Scholar]
- Chen, W.-T.; Chen, P.-S.; Chao, C.-Y. Effect of Doped Insulating Nanoparticles on the Electro-Optical Characteristics of Nematic Liquid Crystals. Jpn. J. Appl. Phys. 2009, 48, 15006. [Google Scholar] [CrossRef]
- Reznikov, Y.; Buchnev, O.; Tereshchenko, O.; Reshetnyak, V.; Glushchenko, A.; West, J. Ferroelectric nematic suspension. Appl. Phys. Lett. 2003, 82, 1917. [Google Scholar] [CrossRef]
- Singh, U.B.; Dhar, R.; Dabrowski, R.; Pandey, M.B. Enhanced electro-optical properties of a nematic liquid crystals in presence of BaTiO3 nanoparticles. Liq. Cryst. 2014, 41, 953–959. [Google Scholar] [CrossRef]
- Al-Zangana, S.; Turner, M.; Dierking, I. A comparison between size dependent paraelectric and ferroelectric BaTiO3 nanoparticle doped nematic and ferroelectric liquid crystals. J. Appl. Phys. 2017, 121, 85105. [Google Scholar] [CrossRef]
- Hegmann, T.; Qi, H.; Marx, V.M. Nanoparticles in Liquid Crystals: Synthesis, Self-Assembly, Defect Formation and Potential Applications. J. Inorg. Organomet. Polym. Mater. 2007, 17, 483–508. [Google Scholar] [CrossRef]
- Dierking, I.; Scalia, G.; Morales, P.; LeClere, D. Aligning and Reorienting Carbon Nanotubes with Nematic Liquid Crystals. Adv. Mater. 2004, 16, 865–869. [Google Scholar] [CrossRef]
- Dierking, I.; Scalia, G.; Morales, P. Liquid crystal–carbon nanotube dispersions. J. Appl. Phys. 2005, 97, 44309. [Google Scholar] [CrossRef]
- Al-Zangana, S.; Iliut, M.; Turner, M.; Vijayaraghavan, A.; Dierking, I. Properties of a Thermotropic Nematic Liquid Crystal Doped with Graphene Oxide. Adv. Opt. Mater. 2016, 4, 1541–1548. [Google Scholar] [CrossRef]
- Saliba, S.; Mingotaud, C.; Kahn, M.L.; Marty, J.-D. Liquid crystalline thermotropic and lyotropic nanohybrids. Nanoscale 2013, 5, 6641. [Google Scholar] [CrossRef] [PubMed]
- Mukhina, M.V.; Danilov, V.V.; Orlova, A.O.; Fedorov, M.V.; Artemyev, M.V.; Baranov, A.V. Electrically controlled polarized photoluminescence of CdSe/ZnS nanorods embedded in a liquid crystal template. Nanotechnology 2012, 23, 325201. [Google Scholar] [CrossRef] [PubMed]
- Majorana, Q. Sur la biréfringence magnétique. C. R. Acad. Sci. 1902, 135, 159–161. [Google Scholar]
- Freundlich, H. Die Doppelbrechung des Vanadinpentoxydsols. Berichte der Bunsengesellschaft für Phys. Chem. 1916, 22, 27–33. [Google Scholar]
- Zocher, H. Spontaneous structure formation in sols; a new kind of anisotropic liquid media. Anorg. Allg. Chem. 1925, 147, 91–110. [Google Scholar] [CrossRef]
- Yi, Y.; Clark, N.A. Orientation of chromonic liquid crystals by topographic linear channels: Multi-stable alignment and tactoid structure. Liq. Cryst. 2013, 40, 1736–1747. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Shiyanovskii, S.V.; Lavrentovich, O.D. Morphogenesis of defects and tactoids during isotropic–nematic phase transition in self-assembled lyotropic chromonic liquid crystals. J. Phys. Condens. Matter 2013, 25, 404202. [Google Scholar] [CrossRef] [PubMed]
- Bernal, J.D.; Fankuchen, I. X-ray and crystallographic studies of plant virus preparations: I. Introduction and preparation of specimens; II. Modes of aggregation of the virus particles. J. Gen. Physiol. 1941, 25, 111–146. [Google Scholar] [CrossRef] [PubMed]
- Davidson, P.; Garreau, A.; Livage, J. Nematic colloidal suspensions of V2O5 in water—Or Zocher phases revisited. Liq. Cryst. 1994, 16, 905–910. [Google Scholar] [CrossRef]
- Pelletier, O.; Sotta, P.; Davidson, P. Deuterium Nuclear Magnetic Resonance Study of the Nematic Phase of Vanadium Pentoxide Aqueous Suspensions. J. Phys. Chem. B 1999, 103, 5427–5433. [Google Scholar] [CrossRef]
- Zocher, H.; Török, C. Neuere Beiträge zur Kenntnis der Taktosole. Kolloid-Zeitschrift 1960, 173, 1–7. [Google Scholar] [CrossRef]
- Michaelev, V.A.; Tcherbakov, V.A. Zh. Obs. Khim. 1985, 55, 1223.
- Davidson, P.; Gabriel, J.C.; Levelut, A.M.; Batail, P. A New Nematic Suspension Based on All-Inorganic Polymer Rods. Europhys. Lett. 1993, 21, 317–322. [Google Scholar] [CrossRef]
- Zocher, H.; Török, C. Crystals of higher order and their relation to other superphases. Acta Crystallogr. 1967, 22, 751–755. [Google Scholar] [CrossRef]
- Gabriel, J.-C.P.; Davidson, P. New Trends in Colloidal Liquid Crystals Based on Mineral Moieties. Adv. Mater. 2000, 12, 9–20. [Google Scholar] [CrossRef]
- Veerman, J.A.C.; Frenkel, D. Phase behavior of disklike hard-core mesogens. Phys. Rev. A 1992, 45, 5632–5648. [Google Scholar] [CrossRef] [PubMed]
- Langmuir, I. The Role of Attractive and Repulsive Forces in the Formation of Tactoids, Thixotropic Gels, Protein Crystals and Coacervates. J. Chem. Phys. 1938, 6, 873–896. [Google Scholar] [CrossRef]
- Gabriel, J.-C.P.; Sanchez, C.; Davidson, P. Observation of Nematic Liquid-Crystal Textures in Aqueous Gels of Smectite Clays. J. Phys. Chem. 1996, 100, 11139–11143. [Google Scholar] [CrossRef]
- Kajiwara, K.; Donkai, N.; Hiragi, Y.; Inagaki, H. Lyotropic mesophase of imogolite, 1. Effect of polydispersity on phase diagram. Makromol. Chem. 1986, 187, 2883–2893. [Google Scholar] [CrossRef]
- Kajiwara, K.; Donkai, N.; Fujiyoshi, Y.; Inagaki, H. Lyotropic mesophase of imogolite, 2. Microscopic observation of imogolite mesophase. Makromol. Chemie 1986, 187, 2895–2907. [Google Scholar] [CrossRef]
- Zugenmaier, P. Crystalline Cellulose and Cellulose Derivatives; Springer: Berlin, Germany, 2010. [Google Scholar]
- Lin, N.; Huang, J.; Dufresne, A. Preparation, properties and applications of polysaccharide nanocrystals in advanced functional nanomaterials: A review. Nanoscale 2012, 4, 3274. [Google Scholar] [CrossRef] [PubMed]
- Oldenbourg, R.; Wen, X.; Meyer, R.B.; Caspar, D.L.D. Orientational Distribution Function in Nematic Tobacco-Mosaic-Virus Liquid Crystals Measured by X-Ray Diffraction. Phys. Rev. Lett. 1988, 61, 1851–1854. [Google Scholar] [CrossRef] [PubMed]
- Rischkov, V.L.; Smirnova, V.A. Liquid Crystals of the Virus of the Tobacco Mosaic (Nicotiana virus 1). Comptes Rendus l’Academie Sci. l’URSS 1941, 31, 930. [Google Scholar]
- Fraden, S.; Maret, G.; Caspar, D.L.D. Angular correlations and the isotropic-nematic phase transition in suspensions of tobacco mosaic virus. Phys. Rev. E 1993, 48, 2816–2837. [Google Scholar] [CrossRef]
- Graf, H.; Löwen, H. Phase diagram of tobacco mosaic virus solutions. Phys. Rev. E 1999, 59, 1932–1942. [Google Scholar] [CrossRef]
- Fowler, C.E.; Shenton, W.; Stubbs, G.; Mann, S. Tobacco Mosaic Virus Liquid Crystals as Templates for the Interior Design of Silica Mesophases and Nanoparticles. Adv. Mater. 2001, 13, 1266–1269. [Google Scholar] [CrossRef]
- Flynn, C.E.; Lee, S.-W.; Peelle, B.R.; Belcher, A.M. Viruses as vehicles for growth, organization and assembly of materials. Acta Mater. 2003, 51, 5867–5880. [Google Scholar] [CrossRef]
- Dogic, Z.; Fraden, S. Ordered phases of filamentous viruses. Curr. Opin. Colloid Interface Sci. 2006, 11, 47–55. [Google Scholar] [CrossRef]
- Dogic, Z.; Fraden, S. Cholesteric Phase in Virus Suspensions. Langmuir 2000, 16, 7820–7824. [Google Scholar] [CrossRef]
- Dogic, Z.; Fraden, S. Smectic Phase in a Colloidal Suspension of Semiflexible Virus Particles. Phys. Rev. Lett. 1997, 78, 2417–2420. [Google Scholar] [CrossRef]
- Livolant, F.; Leforestier, A. Condensed phases of DNA: Structures and phase transitions. Prog. Polym. Sci. 1996, 21, 1115–1164. [Google Scholar] [CrossRef]
- Strzelecka, T.E.; Davidson, M.W.; Rill, R.L. Multiple liquid crystal phases of DNA at high concentrations. Nature 1988, 331, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Livolant, F.; Levelut, A.M.; Doucet, J.; Benoit, J.P. The highly concentrated liquid-crystalline phase of DNA is columnar hexagonal. Nature 1989, 339, 724–726. [Google Scholar] [CrossRef] [PubMed]
- Leforstier, A.; Livolant, F. DNA liquid crystalline blue phases. Electron microscopy evidence and biological implications. Liq. Cryst. 1994, 17, 651–658. [Google Scholar] [CrossRef]
- Nakata, M.; Zanchetta, G.; Chapman, B.D.; Jones, C.D.; Cross, J.O.; Pindak, R.; Bellini, T.; Clark, N.A. End-to-End Stacking and Liquid Crystal Condensation of 6- to 20–Base Pair DNA Duplexes. Science 2007, 318, 1276–1279. [Google Scholar] [CrossRef] [PubMed]
- Zanchetta, G.; Nakata, M.; Buscaglia, M.; Clark, N.A.; Bellini, T. Liquid crystal ordering of DNA and RNA oligomers with partially overlapping sequences. J. Phys. Condens. Matter 2008, 20, 494214. [Google Scholar] [CrossRef]
- Zanchetta, G.; Nakata, M.; Buscaglia, M.; Bellini, T.; Clark, N.A. Phase separation and liquid crystallization of complementary sequences in mixtures of nanoDNA oligomers. Proc. Natl. Acad. Sci. USA 2008, 105, 1111–1117. [Google Scholar] [CrossRef] [PubMed]
- Bellini, T.; Zanchetta, G.; Fraccia, T.P.; Cerbino, R.; Tsai, E.; Smith, G.P.; Moran, M.J.; Walba, D.M.; Clark, N.A. Liquid crystal self-assembly of random-sequence DNA oligomers. Proc. Natl. Acad. Sci. USA 2012, 109, 1110–1115. [Google Scholar] [CrossRef] [PubMed]
- Fraccia, T.P.; Smith, G.P.; Bethge, L.; Zanchetta, G.; Nava, G.; Klussmann, S.; Clark, N.A.; Bellini, T. Liquid Crystal Ordering and Isotropic Gelation in Solutions of Four-Base-Long DNA Oligomers. ACS Nano 2016, 10, 8508–8516. [Google Scholar] [CrossRef] [PubMed]
- Zanchetta, G.; Giavazzi, F.; Nakata, M.; Buscaglia, M.; Cerbino, R.; Clark, N.A.; Bellini, T. Right-handed double-helix ultrashort DNA yields chiral nematic phases with both right- and left-handed director twist. Proc. Natl. Acad. Sci. USA 2010, 107, 17497–17502. [Google Scholar] [CrossRef] [PubMed]
- Stegemeyer, H.; Siemensmeyer, K.; Sucrow, W.; Appel, L. Liquid Crystalline Norcholesterylesters: Influence of the Axial Methylgroups on the Phase Transitions and the Cholesteric Helix. Zeitschrift für Naturforsch. A 1989, 44, 1127–1130. [Google Scholar] [CrossRef]
- Slaney, A.J.; Nishiyama, I.; Styring, P.; Goodby, J.W. Twist inversion in a cholesteric material containing a single chiral centre. J. Mater. Chem. 1992, 2, 805–810. [Google Scholar] [CrossRef]
- Dierking, I.; Gieβelmann, F.; Zugenmaier, P.; Kuczynskit, W.; Lagerwall, S.T.; Stebler, B. Investigations of the structure of a cholesteric phase with a temperature induced helix inversion and of the succeeding SmC* phase in thin liquid crystal cells. Liq. Cryst. 1993, 13, 45–55. [Google Scholar] [CrossRef]
- Styring, P.; Vuijk, J.D.; Nishiyama, I.; Slaney, A.J.; Goodby, J.W. Inversion of chirality-dependent properties in optically active liquid crystals. J. Mater. Chem. 1993, 3, 399–405. [Google Scholar] [CrossRef]
- Dierking, I.; Gießelmann, F.; Zugenmaier, P.; Mohr, K.; Zaschke, H.; Kuczynski, W. The Origin of the Helical Twist Inversion in Single Component Cholesteric Liquid Crystals. Zeitschrift für Naturforsch. A 1994, 49, 1081–1086. [Google Scholar] [CrossRef]
- Dierking, I.; Gieβelmann, F.; Zugenmaier, P.; Mohr, K.; Zaschke, H.; Kuczynski, W. New diastereomeric compound with cholesteric twist inversion. Liq. Cryst. 1995, 18, 443–449. [Google Scholar] [CrossRef]
- De Michele, C.; Zanchetta, G.; Bellini, T.; Frezza, E.; Ferrarini, A. Hierarchical Propagation of Chirality through Reversible Polymerization: The Cholesteric Phase of DNA Oligomers. ACS Macro Lett. 2016, 5, 208–212. [Google Scholar] [CrossRef]
- Tan, H.; Yang, S.; Shen, G.; Yu, R.; Wu, Z. Signal-Enhanced Liquid-Crystal DNA Biosensors Based on Enzymatic Metal Deposition. Angew. Chem. Int. Ed. 2010, 49, 8608–8611. [Google Scholar] [CrossRef] [PubMed]
- Nakata, M.; Zanchetta, G.; Buscaglia, M.; Bellini, T.; Clark, N.A. Liquid Crystal Alignment on a Chiral Surface: Interfacial Interaction with Sheared DNA Films. Langmuir 2008, 24, 10390–10394. [Google Scholar] [CrossRef] [PubMed]
- Rojas, O.J. Cellulose Chemistry and Properties: Fibers, Nanocelluloses and Advanced Materials; Springer: Cham, Switzerland, 2016. [Google Scholar]
- George, J.; Sabapathi, S.N. Cellulose nanocrystals: Synthesis, functional properties, and applications. Nanotechnol. Sci. Appl. 2015, 8, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Gray, D.; Mu, X. Chiral Nematic Structure of Cellulose Nanocrystal Suspensions and Films; Polarized Light and Atomic Force Microscopy. Materials (Basel). 2015, 8, 7873–7888. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.M.; Kimura, T.; Revol, J.-F.; Gray, D.G. Effects of Ionic Strength on the Isotropic–Chiral Nematic Phase Transition of Suspensions of Cellulose Crystallites. Langmuir 1996, 12, 2076–2082. [Google Scholar] [CrossRef]
- Beck-Candanedo, S.; Roman, M.; Gray, D.G. Effect of Reaction Conditions on the Properties and Behavior of Wood Cellulose Nanocrystal Suspensions. Biomacromolecules 2005, 6, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Ureña-Benavides, E.E.; Ao, G.; Davis, V.A.; Kitchens, C.L. Rheology and Phase Behavior of Lyotropic Cellulose Nanocrystal Suspensions. Macromolecules 2011, 44, 8990–8998. [Google Scholar] [CrossRef]
- Lagerwall, J.P.F.; Schütz, C.; Salajkova, M.; Noh, J.; Hyun Park, J.; Scalia, G.; Bergström, L. Cellulose nanocrystal-based materials: From liquid crystal self-assembly and glass formation to multifunctional thin films. NPG Asia Mater. 2014, 6, e80. [Google Scholar] [CrossRef]
- Honorato-Rios, C.; Kuhnhold, A.; Bruckner, J.R.; Dannert, R.; Schilling, T.; Lagerwall, J.P.F. Equilibrium Liquid Crystal Phase Diagrams and Detection of Kinetic Arrest in Cellulose Nanocrystal Suspensions. Front. Mater. 2016, 3, 21. [Google Scholar] [CrossRef]
- Gray, D. Recent Advances in Chiral Nematic Structure and Iridescent Color of Cellulose Nanocrystal Films. Nanomaterials 2016, 6, 213. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Meng, Y.; Wang, S.; Li, Y.; Fu, S.; Ma, L.; Harper, D. Rheological behavior of cellulose nanocrystal suspension: Influence of concentration and aspect ratio. J. Appl. Polym. Sci. 2014, 131, 40525. [Google Scholar] [CrossRef]
- Shafiei-Sabet, S.; Hamad, W.Y.; Hatzikiriakos, S.G. Ionic strength effects on the microstructure and shear rheology of cellulose nanocrystal suspensions. Cellulose 2014, 21, 3347–3359. [Google Scholar] [CrossRef]
- Wu, Q.; Meng, Y.; Concha, K.; Wang, S.; Li, Y.; Ma, L.; Fu, S. Influence of temperature and humidity on nano-mechanical properties of cellulose nanocrystal films made from switchgrass and cotton. Ind. Crops Prod. 2013, 48, 28–35. [Google Scholar] [CrossRef]
- Park, J.H.; Noh, J.; Schütz, C.; Salazar-Alvarez, G.; Scalia, G.; Bergström, L.; Lagerwall, J.P.F. Macroscopic Control of Helix Orientation in Films Dried from Cholesteric Liquid-Crystalline Cellulose Nanocrystal Suspensions. ChemPhysChem 2014, 15, 1477–1484. [Google Scholar] [CrossRef] [PubMed]
- Querejeta-Fernández, A.; Chauve, G.; Methot, M.; Bouchard, J.; Kumacheva, E. Chiral Plasmonic Films Formed by Gold Nanorods and Cellulose Nanocrystals. J. Am. Chem. Soc. 2014, 136, 4788–4793. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Campbell, M.G.; Evans, J.S.; Smalyukh, I.I. Orientationally Ordered Colloidal Co-Dispersions of Gold Nanorods and Cellulose Nanocrystals. Adv. Mater. 2014, 26, 7178–7184. [Google Scholar] [CrossRef] [PubMed]
- Chu, G.; Wang, X.; Chen, T.; Gao, J.; Gai, F.; Wang, Y.; Xu, Y. Optically Tunable Chiral Plasmonic Guest–Host Cellulose Films Weaved with Long-range Ordered Silver Nanowires. ACS Appl. Mater. Interfaces 2015, 7, 11863–11870. [Google Scholar] [CrossRef] [PubMed]
- Menzel, A.M. Tuned, driven, and active soft matter. Phys. Rep. 2015, 554, 1–45. [Google Scholar] [CrossRef]
- Ramaswamy, S. Active matter. J. Stat. Mech. Theory Exp. 2017, 2017, 54002. [Google Scholar] [CrossRef]
- Bukusoglu, E.; Bedolla Pantoja, M.; Mushenheim, P.C.; Wang, X.; Abbott, N.L. Design of Responsive and Active (Soft) Materials Using Liquid Crystals. Annu. Rev. Chem. Biomol. Eng. 2016, 7, 163–196. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, T.; Chen, D.T.N.; DeCamp, S.J.; Heymann, M.; Dogic, Z. Spontaneous motion in hierarchically assembled active matter. Nature 2012, 491, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Fowler, N.; Dierking, D.I. Kibble-Zurek Scaling during Defect Formation in a Nematic Liquid Crystal. ChemPhysChem 2017, 18, 812–816. [Google Scholar] [CrossRef] [PubMed]
- Dierking, I.; Marshall, O.; Wright, J.; Bulleid, N. Annihilation dynamics of umbilical defects in nematic liquid crystals under applied electric fields. Phys. Rev. E 2005, 71, 61709. [Google Scholar] [CrossRef] [PubMed]
- Dierking, I.; Ravnik, M.; Lark, E.; Healey, J.; Alexander, G.P.; Yeomans, J.M. Anisotropy in the annihilation dynamics of umbilic defects in nematic liquid crystals. Phys. Rev. E 2012, 85, 21703. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Turiv, T.; Guo, Y.; Wei, Q.-H.; Lavrentovich, O.D. Command of active matter by topological defects and patterns. Science 2016, 354, 882–885. [Google Scholar] [CrossRef] [PubMed]
- Zakri, C. Carbon nanotubes and liquid crystalline phases. Liq. Cryst. Today 2007, 16, 1–11. [Google Scholar] [CrossRef]
- Lagerwall, J.P.F.; Scalia, G. Carbon nanotubes in liquid crystals. J. Mater. Chem. 2008, 18, 2890–2898. [Google Scholar] [CrossRef]
- Yadav, S.P.; Singh, S. Carbon nanotube dispersion in nematic liquid crystals: An overview. Prog. Mater. Sci. 2016, 80, 38–76. [Google Scholar] [CrossRef]
- Lynch, M.D.; Patrick, D.L. Organizing Carbon Nanotubes with Liquid Crystals. Nano Lett. 2002, 2, 1197–1201. [Google Scholar] [CrossRef]
- Yakemseva, M.; Dierking, I.; Kapernaum, N.; Usoltseva, N.; Giesselmann, F. Dispersions of multi-wall carbon nanotubes in ferroelectric liquid crystals. Eur. Phys. J. E 2014, 37, 7. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Bisoyi, H.K. Aligned Carbon Nanotubes in the Supramolecular Order of Discotic Liquid Crystals. Angew. Chem. Int. Ed. 2007, 46, 1501–1503. [Google Scholar] [CrossRef] [PubMed]
- Bisoyi, H.K.; Kumar, S. Carbon nanotubes in triphenylene and rufigallol-based room temperature monomeric and polymeric discotic liquid crystals. J. Mater. Chem. 2008, 18, 3032. [Google Scholar] [CrossRef]
- Lagerwall, J.P.F.; Scalia, G.; Haluska, M.; Dettlaff-Weglikowska, U.; Giesselmann, F.; Roth, S. Simultaneous alignment and dispersion of carbon nanotubes with lyotropic liquid crystals. Phys. Status Solidi 2006, 243, 3046–3049. [Google Scholar] [CrossRef]
- Lagerwall, J.; Scalia, G.; Haluska, M.; Dettlaff-Weglikowska, U.; Roth, S.; Giesselmann, F. Nanotube Alignment Using Lyotropic Liquid Crystals. Adv. Mater. 2007, 19, 359–364. [Google Scholar] [CrossRef]
- Jiang, W.; Yu, B.; Liu, W.; Hao, J. Carbon Nanotubes Incorporated within Lyotropic Hexagonal Liquid Crystal Formed in Room-Temperature Ionic Liquids. Langmuir 2007, 23, 8549–8553. [Google Scholar] [CrossRef] [PubMed]
- Scalia, G.; von Bühler, C.; Hägele, C.; Roth, S.; Giesselmann, F.; Lagerwall, J.P.F. Spontaneous macroscopic carbon nanotube alignment via colloidal suspension in hexagonal columnar lyotropic liquid crystals. Soft Matter 2008, 4, 570–576. [Google Scholar] [CrossRef]
- Badaire, S.; Zakri, C.; Maugey, M.; Derré, A.; Barisci, J.N.; Wallace, G.; Poulin, P. Liquid Crystals of DNA-Stabilized Carbon Nanotubes. Adv. Mater. 2005, 17, 1673–1676. [Google Scholar] [CrossRef]
- Song, W. Nematic Liquid Crystallinity of Multiwall Carbon Nanotubes. Science 2003, 302, 1363. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Windle, A.H. Isotropic–Nematic Phase Transition of Dispersions of Multiwall Carbon Nanotubes. Macromolecules 2005, 38, 6181–6188. [Google Scholar] [CrossRef]
- Somoza, A.M.; Sagui, C.; Roland, C. Liquid-crystal phases of capped carbon nanotubes. Phys. Rev. B 2001, 63, 81403. [Google Scholar] [CrossRef]
- Puech, N.; Blanc, C.; Grelet, E.; Zamora-Ledezma, C.; Maugey, M.; Zakri, C.; Anglaret, E.; Poulin, P. Highly Ordered Carbon Nanotube Nematic Liquid Crystals. J. Phys. Chem. C 2011, 115, 3272–3278. [Google Scholar] [CrossRef]
- Zakri, C.; Blanc, C.; Grelet, E.; Zamora-Ledezma, C.; Puech, N.; Anglaret, E.; Poulin, P. Liquid crystals of carbon nanotubes and graphene. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2013, 371, 20120499. [Google Scholar] [CrossRef] [PubMed]
- Rai, P.K.; Pinnick, R.A.; Parra-Vasquez, A.N.G.; Davis, V.A.; Schmidt, H.K.; Hauge, R.H.; Smalley, R.E.; Pasquali, M. Isotropic–Nematic Phase Transition of Single-Walled Carbon Nanotubes in Strong Acids. J. Am. Chem. Soc. 2006, 128, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Windle, A.H. Size-Dependence and Elasticity of Liquid-Crystalline Multiwalled Carbon Nanotubes. Adv. Mater. 2008, 20, 3149–3154. [Google Scholar] [CrossRef]
- Sharma, V.; Park, K.; Srinivasarao, M. Colloidal dispersion of gold nanorods: Historical background, optical properties, seed-mediated synthesis, shape separation and self-assembly. Mater. Sci. Eng. R Rep. 2009, 65, 1–38. [Google Scholar] [CrossRef]
- Stamatoiu, O.; Mirzaei, J.; Feng, X.; Hegmann, T. Nanoparticles in Liquid Crystals and Liquid Crystalline Nanoparticles. Top. Curr. Chem. 2012, 318, 331–393. [Google Scholar] [PubMed]
- Liu, Q.; Cui, Y.; Gardner, D.; Li, X.; He, S.; Smalyukh, I.I. Self-Alignment of Plasmonic Gold Nanorods in Reconfigurable Anisotropic Fluids for Tunable Bulk Metamaterial Applications. Nano Lett. 2010, 10, 1347–1353. [Google Scholar] [CrossRef] [PubMed]
- Umadevi, S.; Feng, X.; Hegmann, T. Large Area Self-Assembly of Nematic Liquid-Crystal-Functionalized Gold Nanorods. Adv. Funct. Mater. 2013, 23, 1393–1403. [Google Scholar] [CrossRef]
- Saliba, S.; Davidson, P.; Impéror-Clerc, M.; Mingotaud, C.; Kahn, M.L.; Marty, J.-D. Facile direct synthesis of ZnO nanoparticles within lyotropic liquid crystals: towards organized hybrid materials. J. Mater. Chem. 2011, 21, 18191. [Google Scholar] [CrossRef]
- Zhang, S.; Majewski, P.W.; Keskar, G.; Pfefferle, L.D.; Osuji, C.O. Lyotropic Self-Assembly of High-Aspect-Ratio Semiconductor Nanowires of Single-Crystal ZnO. Langmuir 2011, 27, 11616–11621. [Google Scholar] [CrossRef] [PubMed]
- Dierking, I.; Archer, P. Imaging liquid crystal defects. RSC Adv. 2013, 3, 26433–26437. [Google Scholar] [CrossRef]
- Zhang, S.; Pelligra, C.I.; Keskar, G.; Majewski, P.W.; Ren, F.; Pfefferle, L.D.; Osuji, C.O. Liquid Crystalline Order and Magnetocrystalline Anisotropy in Magnetically Doped Semiconducting ZnO Nanowires. ACS Nano 2011, 5, 8357–8364. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.H.; Bai, Y.; Wang, F.M.; Liu, N. Fabrication and Characterizes of TiO2 Nanomaterials Templated by Lyotropic Liquid Crystal. Adv. Mater. Res. 2012, 399–401, 532–537. [Google Scholar] [CrossRef]
- Ren, Z.; Chen, C.; Hu, R.; Mai, K.; Qian, G.; Wang, Z. Two-Step Self-Assembly and Lyotropic Liquid Crystal Behavior of TiO2 Nanorods. J. Nanomater. 2012, 2012, 180989. [Google Scholar] [CrossRef]
- Li, L.; Walda, J.; Manna, L.; Alivisatos, A.P. Semiconductor Nanorod Liquid Crystals. Nano Lett. 2002, 2, 557–560. [Google Scholar] [CrossRef]
- Li, L.-S.; Alivisatos, A.P. Semiconductor Nanorod Liquid Crystals and Their Assembly on a Substrate. Adv. Mater. 2003, 15, 408–411. [Google Scholar] [CrossRef]
- Kim, F.; Kwan, S.; Akana, J.; Yang, P. Langmuir–Blodgett Nanorod Assembly. J. Am. Chem. Soc. 2001, 123, 4360–4361. [Google Scholar] [CrossRef] [PubMed]
- Thorkelsson, K.; Bai, P.; Xu, T. Self-assembly and applications of anisotropic nanomaterials: A review. Nano Today 2015, 10, 48–66. [Google Scholar] [CrossRef]
- Georgakilas, V.; Otyepka, M.; Bourlinos, A.B.; Chandra, V.; Kim, N.; Kemp, K.C.; Hobza, P.; Zboril, R.; Kim, K.S. Functionalization of Graphene: Covalent and Non-Covalent Approaches, Derivatives and Applications. Chem. Rev. 2012, 112, 6156–6214. [Google Scholar] [CrossRef] [PubMed]
- Rezapour, M.R.; Myung, C.W.; Yun, J.; Ghassami, A.; Li, N.; Yu, S.U.; Hajibabaei, A.; Park, Y.; Kim, K.S. Graphene and Graphene Analogs toward Optical, Electronic, Spintronic, Green-Chemical, Energy-Material, Sensing, and Medical Applications. ACS Appl. Mater. Interfaces 2017, 9, 24393–24406. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Yin, Z.; Wu, S.; Qi, X.; He, Q.; Zhang, Q.; Yan, Q.; Boey, F.; Zhang, H. Graphene-Based Materials: Synthesis, Characterization, Properties, and Applications. Small 2011, 7, 1876–1902. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Joung, D.; Zhai, L.; Das, S.; Khondaker, S.I.; Seal, S. Graphene based materials: Past, present and future. Prog. Mater. Sci. 2011, 56, 1178–1271. [Google Scholar] [CrossRef]
- Huang, X.; Qi, X.; Boey, F.; Zhang, H. Graphene-based composites. Chem. Soc. Rev. 2012, 41, 666–686. [Google Scholar] [CrossRef] [PubMed]
- Brimicombe, P.D.; Gleeson, H.F. Private communication, 2008.
- Behabtu, N.; Lomeda, J.R.; Green, M.J.; Higginbotham, A.L.; Sinitskii, A.; Kosynkin, D.V.; Tsentalovich, D.; Parra-Vasquez, A.N.G.; Schmidt, J.; Kesselman, E.; et al. Spontaneous high-concentration dispersions and liquid crystals of graphene. Nat. Nanotechnol. 2010, 5, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Han, T.H.; Lee, S.H.; Kim, J.Y.; Ahn, C.W.; Yun, J.M.; Kim, S.O. Graphene Oxide Liquid Crystals. Angew. Chem. Int. Ed. 2011, 50, 3043–3047. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Gao, C. Graphene chiral liquid crystals and macroscopic assembled fibres. Nat. Commun. 2011, 2, 571. [Google Scholar] [CrossRef] [PubMed]
- Al-Zangana, S.; Iliut, M.; Turner, M.; Vijayaraghavan, A.; Dierking, I. Confinement effects on lyotropic nematic liquid crystal phases of graphene oxide dispersions. 2D Mater. 2017, 4, 41004. [Google Scholar] [CrossRef]
- Dan, B.; Behabtu, N.; Martinez, A.; Evans, J.S.; Kosynkin, D.V.; Tour, J.M.; Pasquali, M.; Smalyukh, I.I. Liquid crystals of aqueous, giant graphene oxide flakes. Soft Matter 2011, 7, 11154–11159. [Google Scholar] [CrossRef]
- Jalili, R.; Aboutalebi, S.H.; Esrafilzadeh, D.; Shepherd, R.L.; Chen, J.; Aminorroaya-Yamini, S.; Konstantinov, K.; Minett, A.I.; Razal, J.M.; Wallace, G.G. Scalable One-Step Wet-Spinning of Graphene Fibers and Yarns from Liquid Crystalline Dispersions of Graphene Oxide: Towards Multifunctional Textiles. Adv. Funct. Mater. 2013, 23, 5345–5354. [Google Scholar] [CrossRef]
- Al-Zangana, S.; Iliut, M.; Boran, G.; Turner, M.; Vijayaraghavan, A.; Dierking, I. Dielectric spectroscopy of isotropic liquids and liquid crystal phases with dispersed graphene oxide. Sci. Rep. 2016, 6, 31885. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.-Z.; Hong, S.-H.; Song, J.-K. Electro-optical switching of graphene oxide liquid crystals with an extremely large Kerr coefficient. Nat. Mater. 2014, 13, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kim, S.O. Liquid crystals: Electric fields line up graphene oxide. Nat. Mater. 2014, 13, 325–326. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ledezma, C.; Puech, N.; Zakri, C.; Grelet, E.; Moulton, S.E.; Wallace, G.G.; Gambhir, S.; Blanc, C.; Anglaret, E.; Poulin, P. Liquid Crystallinity and Dimensions of Surfactant-Stabilized Sheets of Reduced Graphene Oxide. J. Phys. Chem. Lett. 2012, 3, 2425–2430. [Google Scholar] [CrossRef] [PubMed]
- Lagerwall, J.P.F.; Scalia, G. Liquid Crystals with Nano and Microparticles; World Scientific: Singapore, 2017. [Google Scholar]
- Narayan, R.; Kim, J.E.; Kim, J.Y.; Lee, K.E.; Kim, S.O. Graphene Oxide Liquid Crystals: Discovery, Evolution and Applications. Adv. Mater. 2016, 28, 3045–3068. [Google Scholar] [CrossRef] [PubMed]
- Lekkerkerker, H.N.W.; Vroege, G.J. Liquid crystal phase transitions in suspensions of mineral colloids: New life from old roots. Philos. Trans. A Math. Phys. Eng. Sci. 2013, 371, 20120263. [Google Scholar] [CrossRef] [PubMed]




© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dierking, I.; Al-Zangana, S. Lyotropic Liquid Crystal Phases from Anisotropic Nanomaterials. Nanomaterials 2017, 7, 305. https://doi.org/10.3390/nano7100305
Dierking I, Al-Zangana S. Lyotropic Liquid Crystal Phases from Anisotropic Nanomaterials. Nanomaterials. 2017; 7(10):305. https://doi.org/10.3390/nano7100305
Chicago/Turabian StyleDierking, Ingo, and Shakhawan Al-Zangana. 2017. "Lyotropic Liquid Crystal Phases from Anisotropic Nanomaterials" Nanomaterials 7, no. 10: 305. https://doi.org/10.3390/nano7100305





