On the Use of the Electrospinning Coating Technique to Produce Antimicrobial Polyhydroxyalkanoate Materials Containing In Situ-Stabilized Silver Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Silver Nanoparticles in PHBV18 Matrices
2.3. Preparation of the Multilayer Systems
2.3.1. Preparation of the PHBV3 Film
2.3.2. Preparation of the Electrospun Fibers
2.3.3. Preparation of the Multilayer Films
2.4. Determination of Silver Content in the Active Multilayer Systems
2.5. Transmission Electronic Microscopy (TEM)
2.6. Scanning Electronic Microscopy (SEM)
2.7. Differential Scanning Calorimetry (DSC)
2.8. Mechanical Properties
2.9. Barrier Properties
2.9.1. Water Vapor Permeability (WVP)
2.9.2. Oxygen Permeability (PO2)
2.10. Antimicrobial Activity of Coated Systems
2.11. Statistical Analysis
3. Results and Discussion
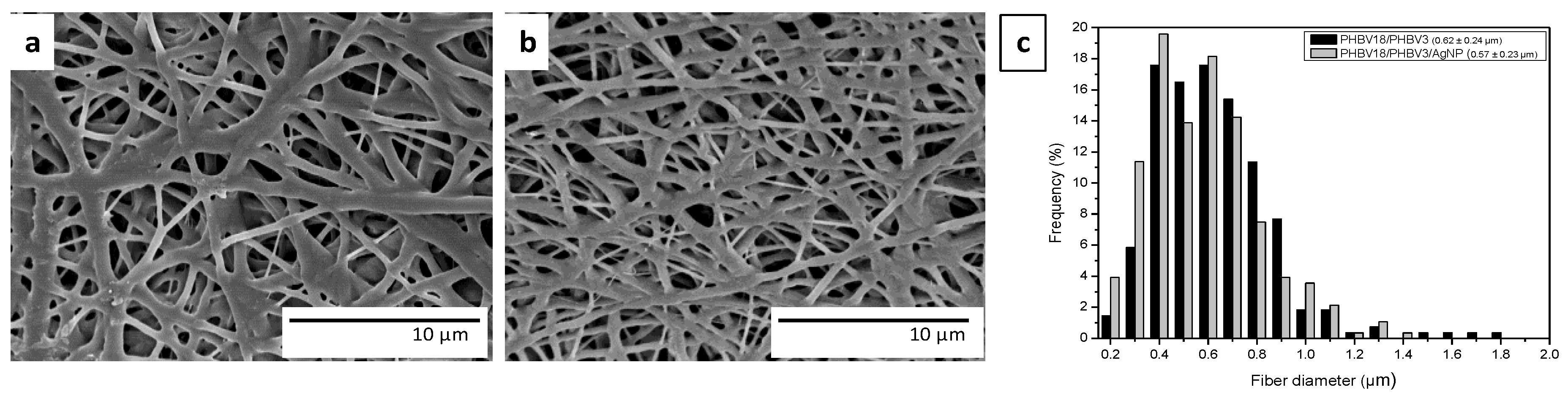
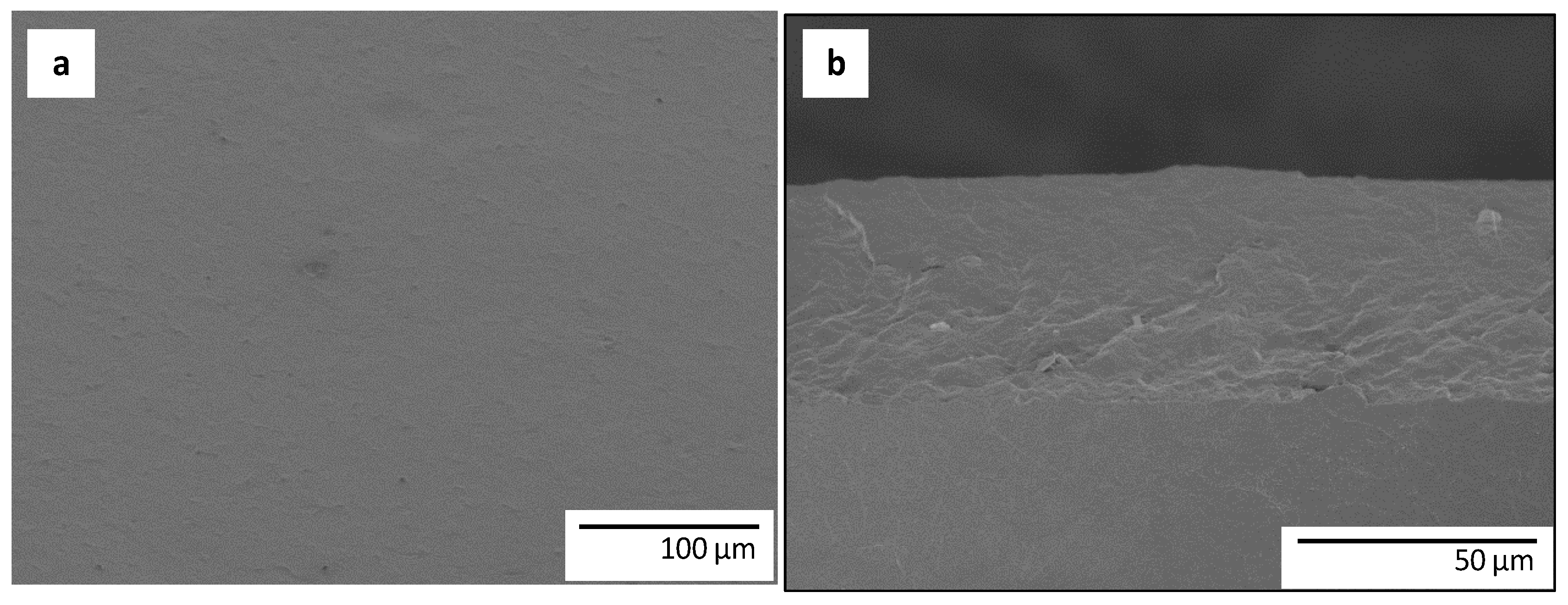
3.1. Morphology
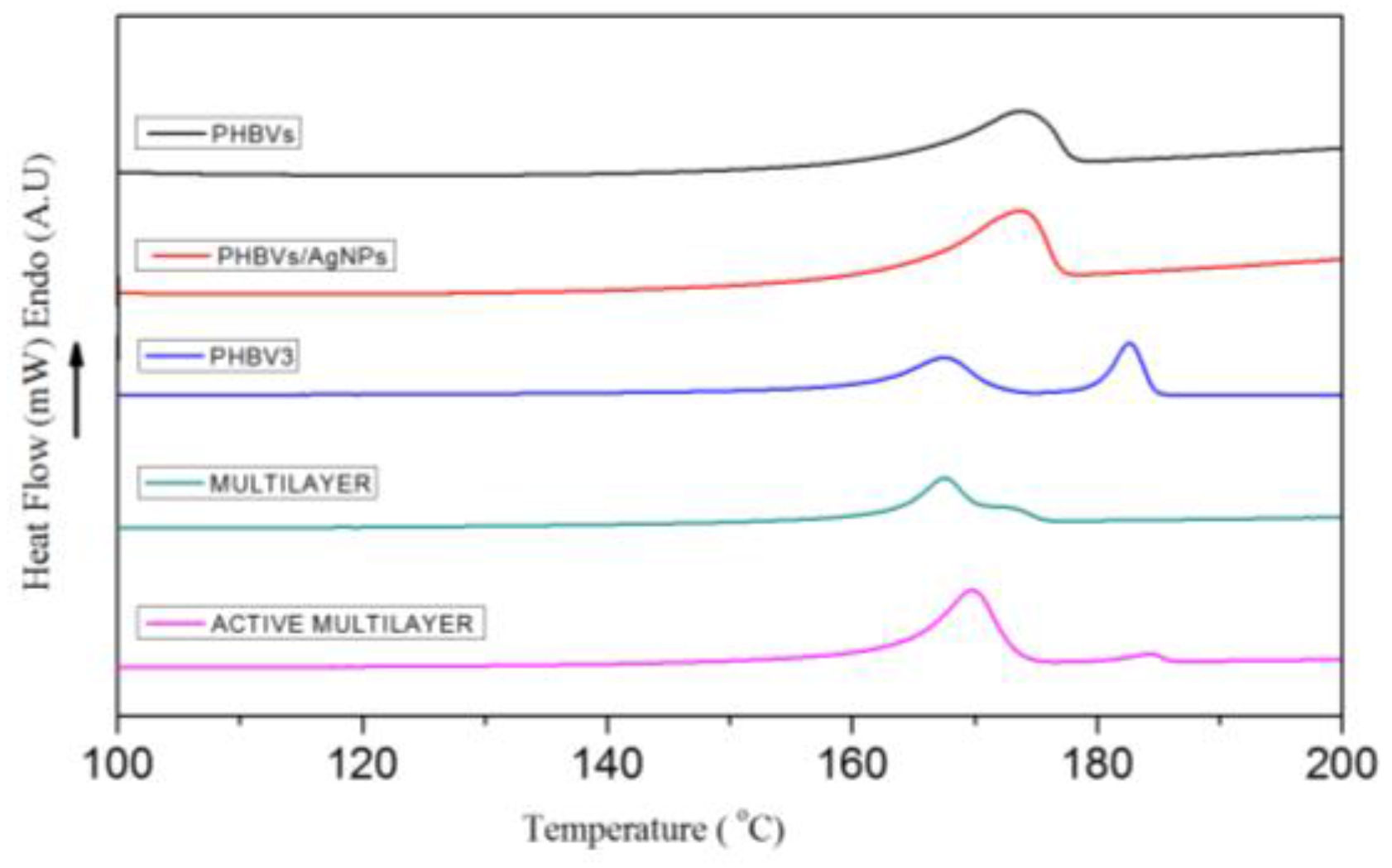
3.2. Thermal Properties
3.3. Barrier Properties
3.4. Mechanical Properties
3.5. Antimicrobial Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bhushani, J.A.; Anandharamakrishnan, C. Electrospinning and electrospraying techniques: Potential food based applications. Trends Food Sci. Technol. 2014, 38, 21–33. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Frenot, A.; Chronakis, I.S. Polymer nanofibers assembled by electrospinning. Curr. Opin. Colloid Interface Sci. 2003, 8, 64–75. [Google Scholar] [CrossRef]
- Noruzi, M. Electrospun nanofibres in agriculture and the food industry: A review. J. Sci. Food Agric. 2016, 96, 4663–4678. [Google Scholar] [CrossRef] [PubMed]
- Quirós, J.; Boltes, K.; Rosal, R. Bioactive Applications for Electrospun Fibers. Polym. Rev. 2016, 56, 631–667. [Google Scholar] [CrossRef]
- Sun, G.; Sun, L.; Xie, H.; Liu, J. Electrospinning of nanofibers for energy applications. Nanomaterials 2016, 6, 129. [Google Scholar] [CrossRef]
- Rivero, P.J.; Urrutia, A.; Goicoechea, J.; Arregui, F.J. Nanomaterials for Functional Textiles and Fibers. Nanoscale Res. Lett. 2015, 10, 1–22. [Google Scholar] [CrossRef] [PubMed]
- López-Rubio, A.; Lagaron, J.M. Whey protein capsules obtained through electrospraying for the encapsulation of bioactives. Innov. Food Sci. Emerg. Technol. 2012, 13, 200–206. [Google Scholar] [CrossRef]
- Martínez-Sanz, M.; Lopez-Rubio, A.; Lagaron, J.M. Dispersing Bacterial Cellulose Nanowhiskers in Polylactides via Electrohydrodynamic Processing. J. Polym. Environ. 2014, 22, 27–40. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Ocio, M.J.; Lagaron, J.M. Development of Active Antimicrobial Fiber-Based Chitosan Polysaccharide Nanostructures using Electrospinning. Eng. Life Sci. 2008, 8, 303–314. [Google Scholar] [CrossRef]
- Martínez-Abad, A.; Sanchez, G.; Lagaron, J.M.; Ocio, M.J. Influence of speciation in the release profiles and antimicrobial performance of electrospun ethylene vinyl alcohol copolymer (EVOH) fibers containing ionic silver ions and silver nanoparticles. Colloid Polym. Sci. 2012, 291, 1381–1392. [Google Scholar] [CrossRef]
- Lagarón, J.M.; Ocio, M.J.; López-Rubio, A. Antimicrobial Packaging Polymers. A General Introduction. In Antimicrobial Polymers; John Wiley & Sons, Inc.: New York, NY, USA, 2011; pp. 1–22. [Google Scholar]
- Totaro, G.; Paltrinieri, L.; Mazzola, G.; Vannini, M.; Sisti, L.; Gualandi, C.; Ballestrazzi, A.; Valeri, S.; Pollicino, A.; Celli, A.; et al. Electrospun Fibers Containing Bio-Based Ricinoleic Acid: Effect of Amount and Distribution of Ricinoleic Acid Unit on Antibacterial Properties. Macromol. Mater. Eng. 2015, 300, 1085–1095. [Google Scholar] [CrossRef]
- Min, M.; Shi, Y.; Ma, H.; Huang, H.; Shi, J.; Chen, X.; Liu, Y.; Wang, L. Polymer-nanoparticle composites composed of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and coated silver nanoparticles. J. Macromol. Sci. Part B 2015, 54, 411–423. [Google Scholar] [CrossRef]
- Jeong, S.; Yeo, S.; Yi, S. The effect of filler particle size on the antibacterial properties of compounded polymer/silver fibers. J. Mater. Sci. 2005, 40, 5407–5411. [Google Scholar] [CrossRef]
- Busolo, M.A.; Lagaron, J.M. Antimicrobial biocomposites of melt-compounded polylactide films containing silver-based engineered clays. J. Plast. Film Sheeting 2013, 29, 290–305. [Google Scholar] [CrossRef]
- Martínez-Abad, A.; Lagarón, J.M.; Ocio, M.J. Characterization of transparent silver loaded poly(l-lactide) films produced by melt-compounding for the sustained release of antimicrobial silver ions in food applications. Food Control 2014, 43, 238–244. [Google Scholar] [CrossRef]
- Yeo, S.Y.; Tan, W.L.; Bakar, M.A.; Ismail, J. Silver sulfide/poly(3-hydroxybutyrate) nanocomposites: Thermal stability and kinetic analysis of thermal degradation. Polym. Degrad. Stab. 2010, 95, 1299–1304. [Google Scholar] [CrossRef]
- Yu, H.; Sun, B.; Zhang, D.; Chen, G.; Yang, X.; Yao, J. Reinforcement of biodegradable poly(3-hydroxybutyrate-co-3-hydroxyvalerate) with cellulose nanocrystal/silver nanohybrids as bifunctional nanofillers. J. Mater. Chem. B 2014, 2, 8479–8489. [Google Scholar] [CrossRef]
- Fortunati, E.; Peltzer, M.; Armentano, I.; Jiménez, A.; Kenny, J.M. Combined effects of cellulose nanocrystals and silver nanoparticles on the barrier and migration properties of PLA nano-biocomposites. J. Food Eng. 2013, 118, 117–124. [Google Scholar] [CrossRef]
- Krklješ, A.N.; Marinović-Cincović, M.T.; Kačarević-Popović, Z.M.; Nedeljković, J.M. Dynamic thermogravimetric degradation of gamma radiolytically synthesized Ag–PVA nanocomposites. Thermochim. Acta 2007, 460, 28–34. [Google Scholar] [CrossRef]
- Castro-Mayorga, J.L.; Martínez-Abad, A.; Fabra, M.F.; Lagarón, J.M.; Ocio, M.J.; Sánchez, G. Chapter 32—Silver-Based Antibacterial and Virucide Biopolymers: Usage and Potential in Antimicrobial Packaging A2—Barros-Velázquez, Jorge. In Antimicrobial Food Packaging; Academic Press: San Diego, CA, USA, 2016; pp. 407–416. [Google Scholar]
- De Lima, R.; Seabra, A.B.; Durán, N. Silver nanoparticles: A brief review of cytotoxicity and genotoxicity of chemically and biogenically synthesized nanoparticles. J. Appl. Toxicol. 2012, 32, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Liz, R.; Simard, J.-C.; Leonardi, L.B.A.; Girard, D. Silver nanoparticles rapidly induce atypical human neutrophil cell death by a process involving inflammatory caspases and reactive oxygen species and induce neutrophil extracellular traps release upon cell adhesion. Int. Immunopharmacol. 2015, 28, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Chairuangkitti, P.; Lawanprasert, S.; Roytrakul, S.; Aueviriyavit, S.; Phummiratch, D.; Kulthong, K.; Chanvorachote, P.; Maniratanachote, R. Silver nanoparticles induce toxicity in A549 cells via ROS-dependent and ROS-independent pathways. Toxicol. In Vitro 2013, 27, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Składanowski, M.; Golinska, P.; Rudnicka, K.; Dahm, H.; Rai, M. Evaluation of cytotoxicity, immune compatibility and antibacterial activity of biogenic silver nanoparticles. Med. Microbiol. Immunol. 2016, 205, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Salama, H.E.; Saad, G.R.; Sabaa, M.W. Synthesis, characterization, and biological activity of cross-linked chitosan biguanidine loaded with silver nanoparticles. J. Biomater. Sci. Polym. Ed. 2016, 27, 1880–1898. [Google Scholar] [CrossRef] [PubMed]
- Bott, J.; Störmer, A.; Franz, R. A Comprehensive Study into the Migration Potential of Nano Silver Particles from Food Contact Polyolefins. In Chemistry of Food, Food Supplements, and Food Contact Materials: From Production to Plate; American Chemical Society: Washington DC, WA, USA, 2014; pp. 51–70. [Google Scholar]
- Jeon, H.J.; Kim, J.S.; Kim, T.G.; Kim, J.H.; Yu, W.-R.; Youk, J.H. Preparation of poly(ɛ-caprolactone)-based polyurethane nanofibers containing silver nanoparticles. Appl. Surf. Sci. 2008, 254, 5886–5890. [Google Scholar] [CrossRef]
- Munteanu, B.; Aytac, Z.; Pricope, G.; Uyar, T.; Vasile, C. Polylactic acid (PLA)/Silver-NP/VitaminE bionanocomposite electrospun nanofibers with antibacterial and antioxidant activity. J. Nanopart. Res. 2014, 16, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Rujitanaroj, P.-O.; Pimpha, N.; Supaphol, P. Preparation, characterization, and antibacterial properties of electrospun polyacrylonitrile fibrous membranes containing silver nanoparticles. J. Appl. Polym. Sci. 2010, 116, 1967–1976. [Google Scholar] [CrossRef]
- Min, M.H.; Shi, Y.Y.; Chen, X.X.; Shi, J.G.; Ma, H.Y.; Huang, H.L.; Wang, L. Preparation and characteristics of electrospun silver-containing PHBV ultrafine fiber. Appl. Mech. Mater. 2014, 548–549, 34–37. [Google Scholar] [CrossRef]
- Xing, Z.C.; Chae, W.P.; Baek, J.Y.; Choi, M.J.; Jung, Y.; Kang, I.K. In vitro assessment of antibacterial activity and cytocompatibility of silver-containing phbv nanofibrous scaffolds for tissue engineering. Biomacromolecules 2010, 11, 1248–1253. [Google Scholar] [CrossRef] [PubMed]
- Castro-Mayorga, J.L.; Martínez-Abad, A.; Fabra, M.J.; Olivera, C.; Reis, M.; Lagarón, J.M. Stabilization of antimicrobial silver nanoparticles by a polyhydroxyalkanoate obtained from mixed bacterial culture. Int. J. Biol. Macromol. 2014, 71, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Abad, A.; Cabedo, L.; Oliveira, C.S.S.; Hilliou, L.; Reis, M.; Lagarón, J.M. Characterization of polyhydroxyalkanoate blends incorporating unpurified biosustainably produced poly(3-hydroxybutyrate-co-3-hydroxyvalerate. J. Appl. Polym. Sci. 2015, 133, 42633. [Google Scholar] [CrossRef]
- Castro-Mayorga, J.L.; Fabra, M.J.; Lagaron, J.M. Stabilized nanosilver based antimicrobial poly(3-hydroxybutyrate-co-3-hydroxyvalerate) nanocomposites of interest in active food packaging. Innov. Food Sci. Emerg. Technol. 2016, 33, 524–533. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, H. Facile synthesis of anisotropic silver nanoparticles and their surface-enhanced Raman scattering properties. J. Mol. Struct. 2014, 1060, 1–5. [Google Scholar] [CrossRef]
- Saquing, C.D.; Manasco, J.L.; Khan, S.A. Electrospun nanoparticle-nanofiber composites via a one-step synthesis. Small 2009, 5, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Radheshkumar, C.; Münstedt, H. Morphology and mechanical properties of antimicrobial polyamide/silver composites. Mater. Lett. 2005, 59, 1949–1953. [Google Scholar] [CrossRef]
- Martínez-Sanz, M.; Villano, M.; Oliveira, C.; Albuquerque, M.G.E.; Majone, M.; Reis, M.; Lopez-Rubio, A.; Lagaron, J.M. Characterization of polyhydroxyalkanoates synthesized from microbial mixed cultures and of their nanobiocomposites with bacterial cellulose nanowhiskers. New Biotechnol. 2014, 31, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Ambrosio-Martin, J.; Fabra, M.J.; López-Rubio, A.; Gorrasi, G.; Sorrentino, A.; Lagaron, J.M. Assessment of Ball Milling as a Compounding Technique to Develop Nanocomposites of Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate) and Bacterial Cellulose Nanowhiskers. J. Polym. Environ. 2016, 24, 241–254. [Google Scholar] [CrossRef]
- Kanmani, P.; Lim, S.T. Synthesis and structural characterization of silver nanoparticles using bacterial exopolysaccharide and its antimicrobial activity against food and multidrug resistant pathogens. Process Biochem. 2013, 48, 1099–1106. [Google Scholar] [CrossRef]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.-H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.-Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Grigor’Eva, A.; Saranina, I.; Tikunova, N.; Safonov, A.; Timoshenko, N.; Rebrov, A.; Ryabchikova, E. Fine mechanisms of the interaction of silver nanoparticles with the cells of Salmonella typhimurium and Staphylococcus aureus. BioMetals 2013, 26, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Echegoyen, Y.; Nerín, C. Nanoparticle release from nano-silver antimicrobial food containers. Food Chem. Toxicol. 2013, 62, 16–22. [Google Scholar] [CrossRef] [PubMed]


| Sample code | Composition |
|---|---|
| poly(3-hydroxybutyrate-co-3 mol %-3-hydroxyvalerate) (PHBV3) | 100% commercial PHBV3 |
| PHBVs (a mixture of PHBV3 and PHBV18) | 92% commercial PHBV3 + 8% Mixed microbial culture derived poly(3-hydroxybutyrate-co-18 mol %-3-hydroxyvalerate) (PHBV18) |
| PHBVs/silver nanoparticles (AgNPs) | PHBVs + Silver nanoparticles |
| Multilayer | Substrate PHBV3 + PHBVs coating |
| Active Multilayer | Substrate PHBV3 + PHBVs/AgNPs coating |
| Sample | Tm1 (°C) | Tm2 (°C) | Tc (°C) | ΔHm (J/g) |
|---|---|---|---|---|
| PHBVs | 173.7 ± 0.2 a | - | 113.4 ± 0.5 a | 61 ± 1 a |
| PHBVs/AgNPs | 173.8 ± 0.1 a | - | 114.9 ± 0.3 a | 65 ± 1 a |
| PHBV3 | 168.7 ± 1.0 b | 181.8 ± 0.2 a | 114.7 ± 0.0 a | 72 ± 1 b |
| Multilayer | 168.5 ± 1.4 b | - | 117.3 ± 0.1 b | 73 ± 1 b |
| Active Multilayer | 170.4 ± 0.8 a,b | 184.8 ± 0.7 b | 117.4 ± 0.6 b | 74 ± 1 b |
| Sample | WVP (Kg·m/Pa·s·m2) | PO2 (m3·m/m2·s·Pa) 80% RH |
|---|---|---|
| PHBV3 | (1.10 ± 0.02) × 10−15 a | (2.06 ± 0.09) × 10−19 a |
| Multilayer | (1.25 ± 0.25) × 10−15 a | (2.13 ± 0.12) × 10−19 a |
| Active Multilayer | (1.59 ± 0.38) × 10−15 a | (2.17 ± 0.19) × 10−19 a |
| Sample | E (GPa) | EAB (%) | TS (MPa) |
|---|---|---|---|
| PHBV3 | 2.6 ± 0.1 a | 1.5 ± 0.2 a | 33.9 ± 6.9 a |
| Multilayer | 2.6 ± 0.2 a | 1.8 ± 0.2 a | 37.1 ± 2.8 a |
| Active Multilayer | 2.6 ± 0.1 a | 2.4 ± 0.6 a | 29.9 ± 3.5 a |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro-Mayorga, J.L.; Fabra, M.J.; Cabedo, L.; Lagaron, J.M. On the Use of the Electrospinning Coating Technique to Produce Antimicrobial Polyhydroxyalkanoate Materials Containing In Situ-Stabilized Silver Nanoparticles. Nanomaterials 2017, 7, 4. https://doi.org/10.3390/nano7010004
Castro-Mayorga JL, Fabra MJ, Cabedo L, Lagaron JM. On the Use of the Electrospinning Coating Technique to Produce Antimicrobial Polyhydroxyalkanoate Materials Containing In Situ-Stabilized Silver Nanoparticles. Nanomaterials. 2017; 7(1):4. https://doi.org/10.3390/nano7010004
Chicago/Turabian StyleCastro-Mayorga, Jinneth Lorena, Maria Jose Fabra, Luis Cabedo, and Jose Maria Lagaron. 2017. "On the Use of the Electrospinning Coating Technique to Produce Antimicrobial Polyhydroxyalkanoate Materials Containing In Situ-Stabilized Silver Nanoparticles" Nanomaterials 7, no. 1: 4. https://doi.org/10.3390/nano7010004







