Improving Powder Magnetic Core Properties via Application of Thin, Insulating Silica-Nanosheet Layers on Iron Powder Particles
Abstract
:1. Introduction
2. Results and Discussion
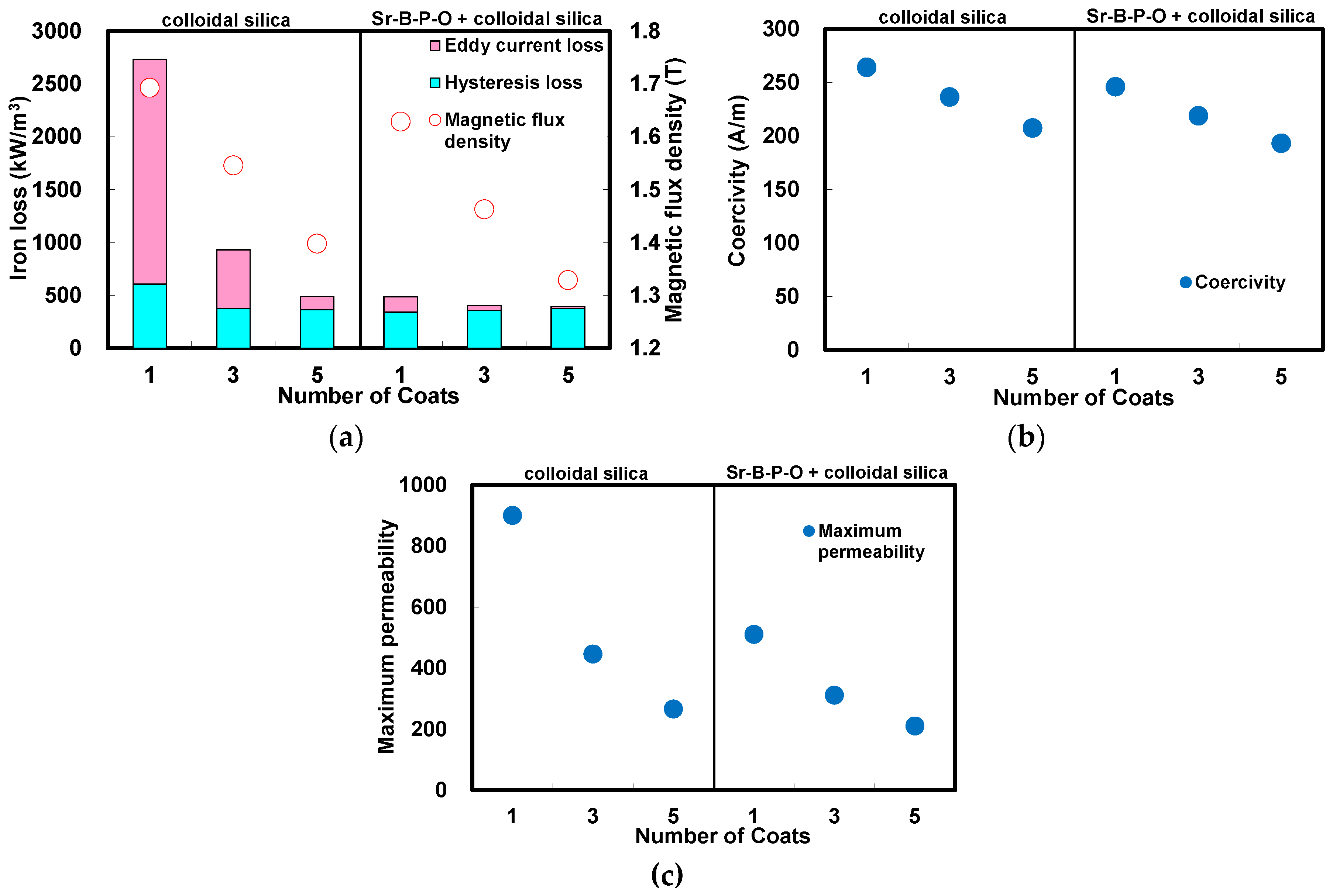
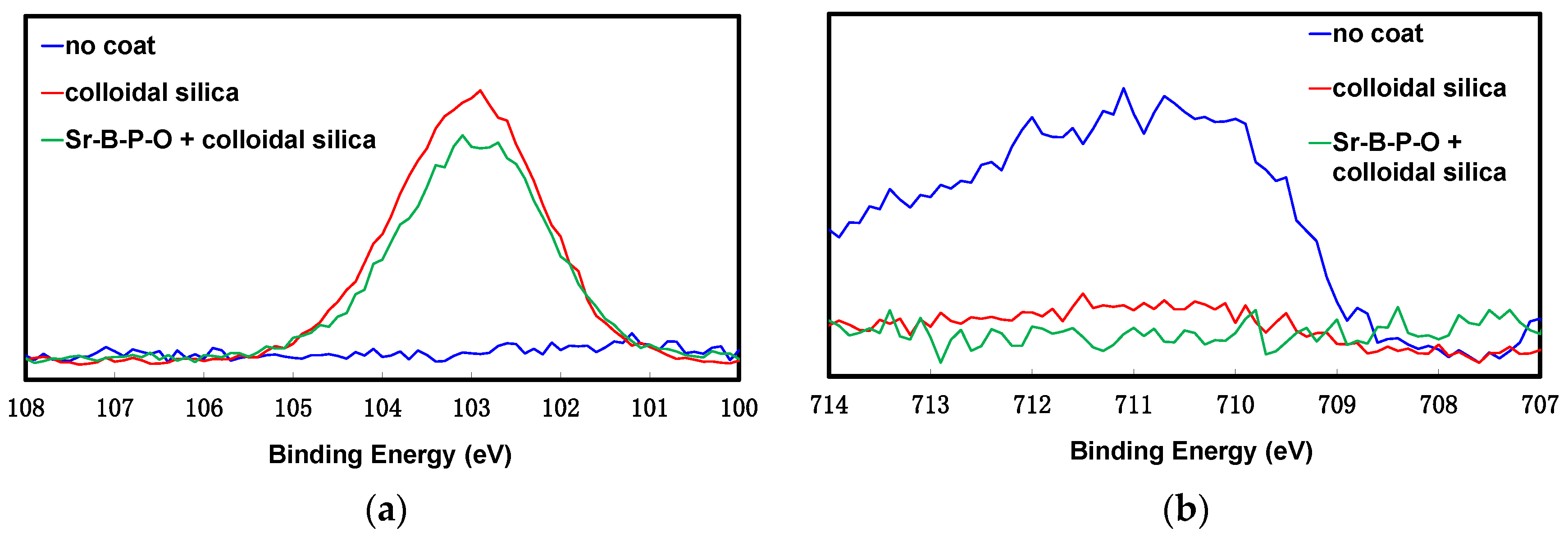
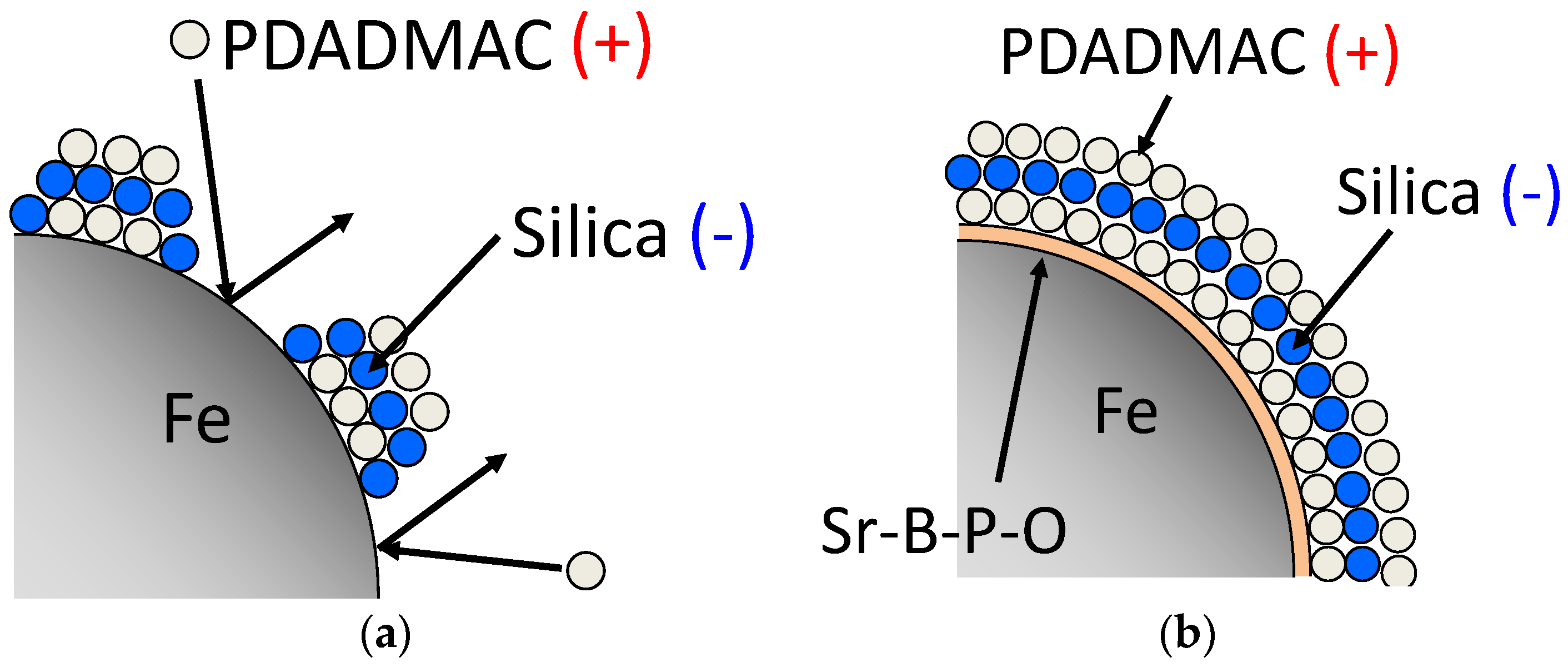
2.1. Colloidal Silica Coating of Iron Powder Particles with and without Sr-B-P-O Insulating Layers
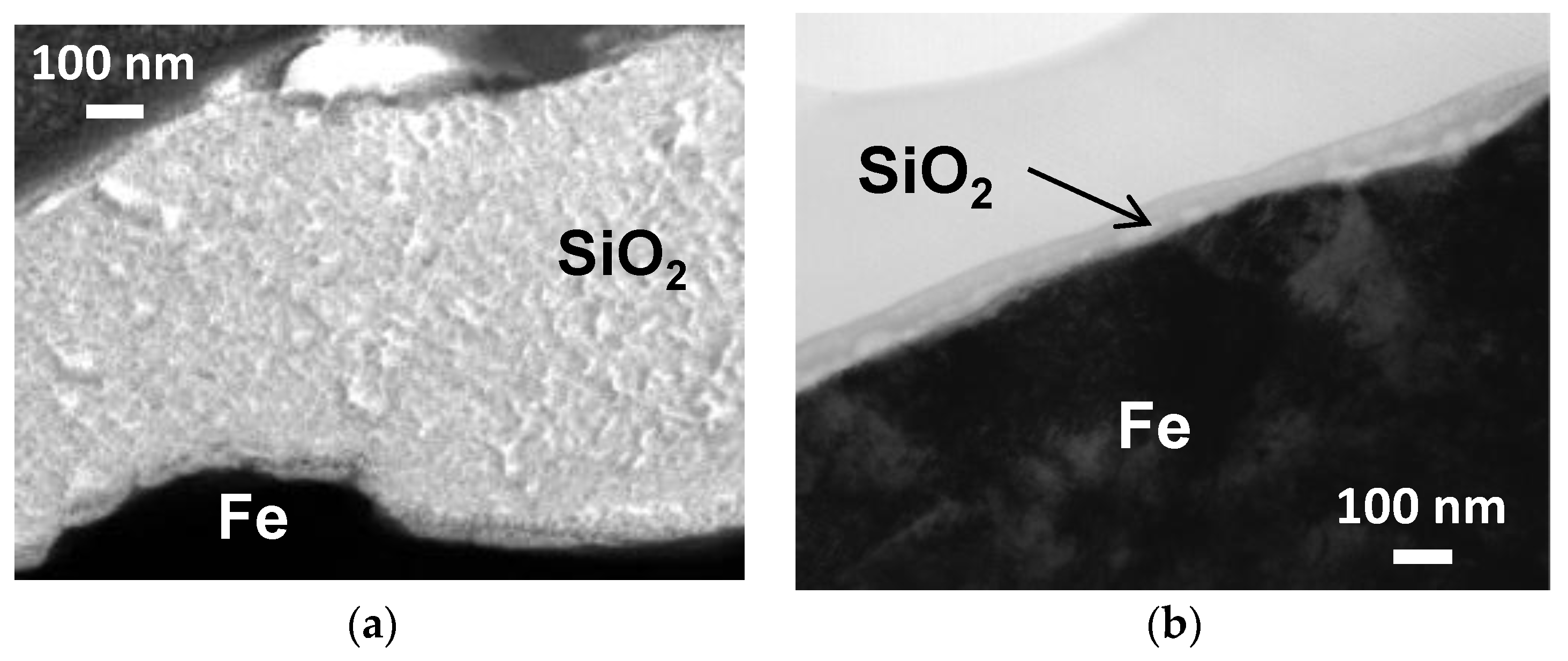
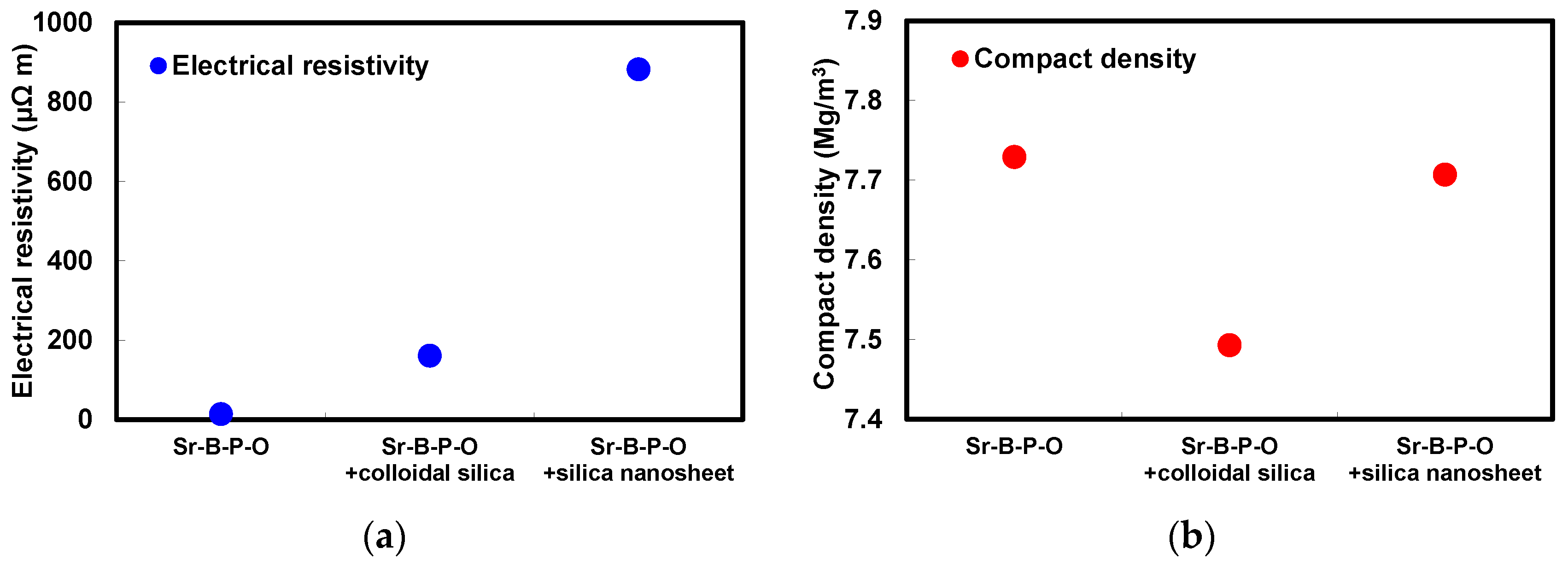
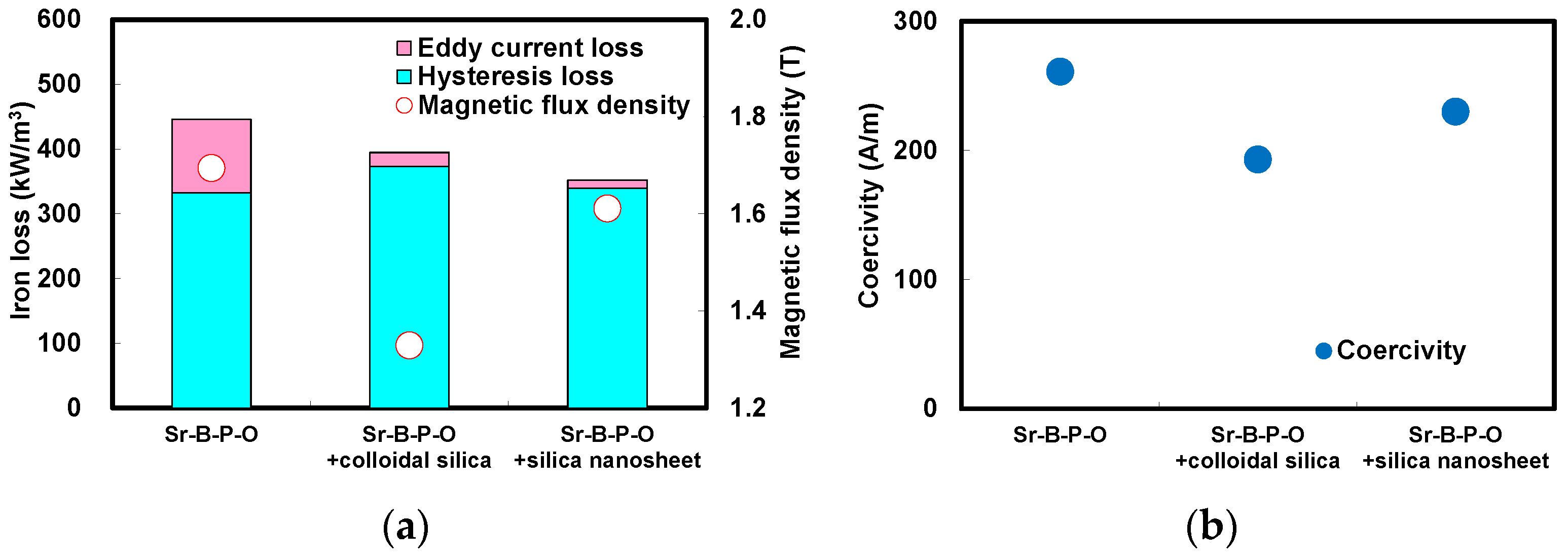
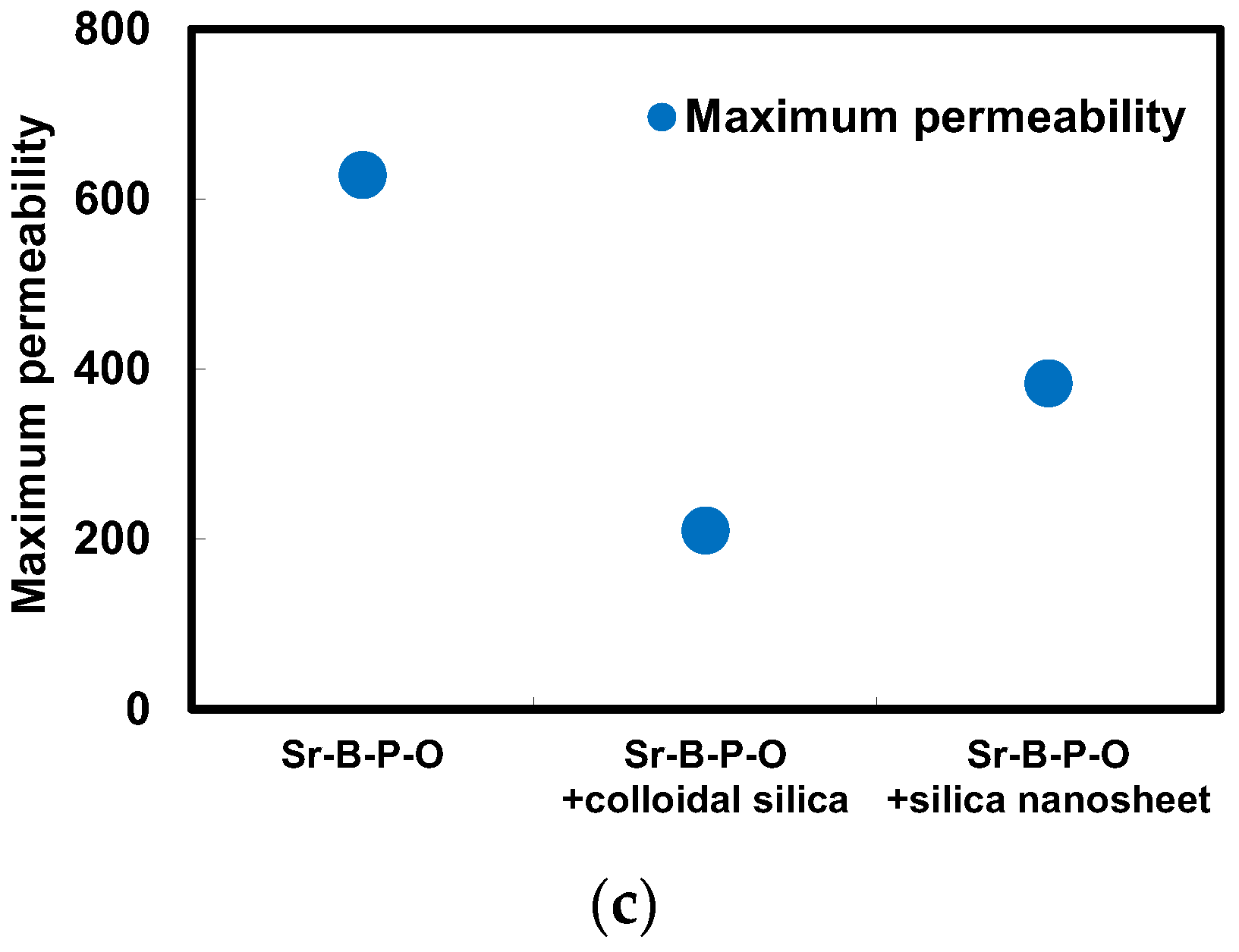
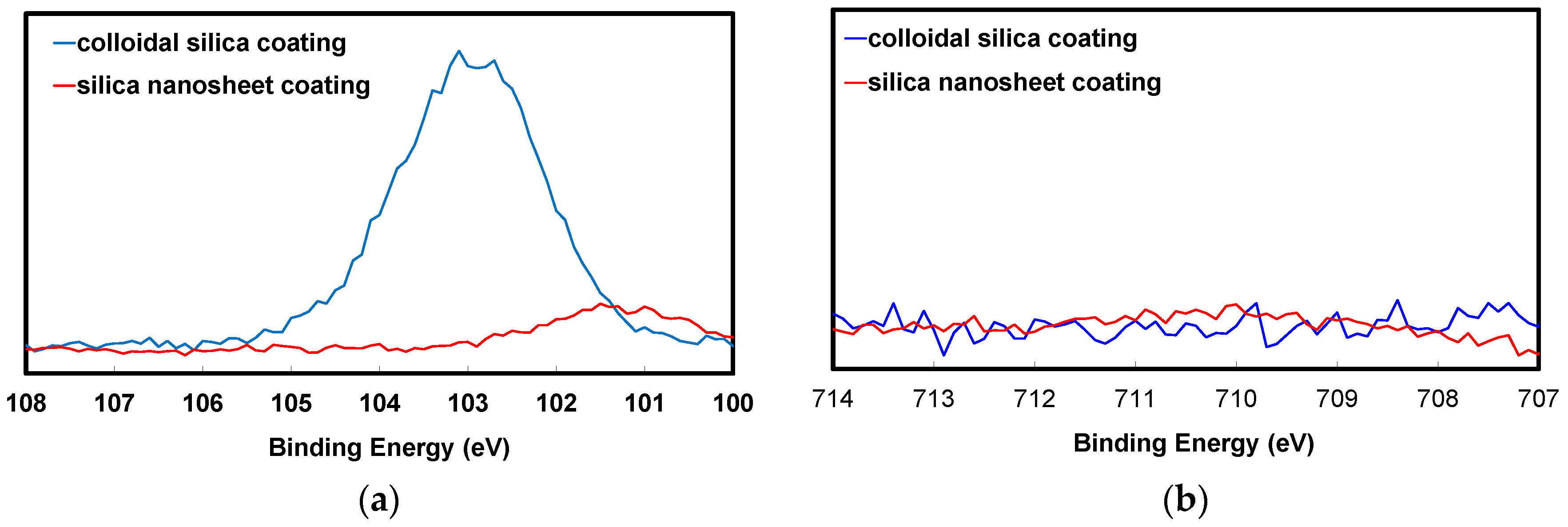
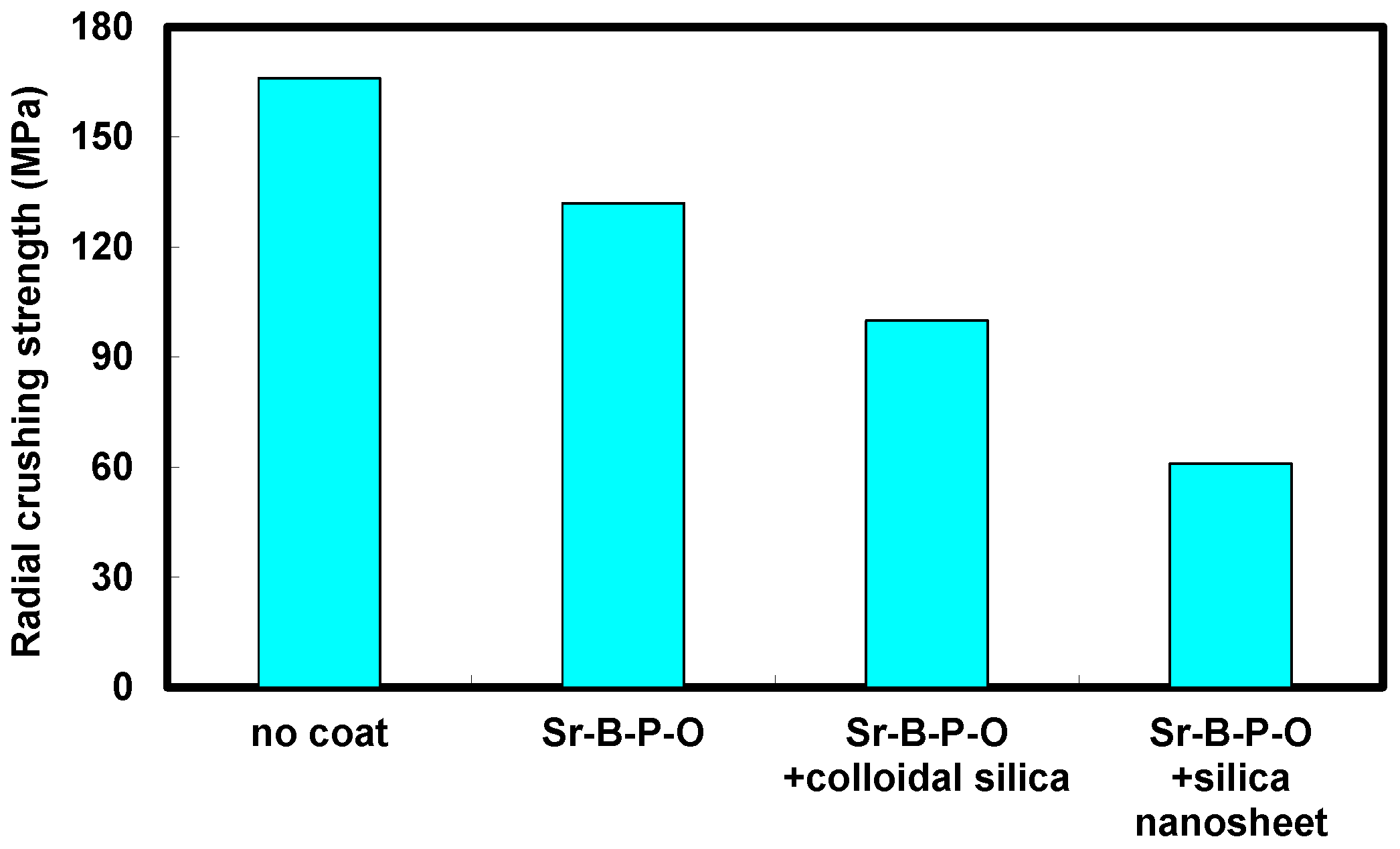
2.2. Comparison between Coloidal Silica and Silica Nanosheet Coatings
3. Materials and Methods
3.1. Preparation of Polycation and Silica Solutions
3.2. Coating Process
3.3. Preparation of Powder Magnetic Cores and Evaluation of Their Magnetic Properties and Mechanical Strength
3.4. Observation and Surface Analyses
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bas, J.A.; Calero, J.A.; Dougan, M.J. Sintered soft magnetic materials. Properties and applications. J. Magn. Magn. Mater. 2003, 254–255, 391–398. [Google Scholar] [CrossRef]
- Wu, L.Z.; Ding, J.; Jiang, H.B.; Chen, L.F.; Ong, C.K. Particle size influence to the microwave properties of iron based magnetic particulate composites. J. Magn. Magn. Mater. 2005, 285, 233–239. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, B.; Cao, Y.; Yu, R.H. Preparation and magnetic properties of novel hybrid magnetic powder cores. Mater. Res. Innov. 2014, 18, 610–614. [Google Scholar] [CrossRef]
- Liu, H.J.; Su, H.L.; Geng, W.B.; Sun, Z.G.; Song, T.T.; Tong, X.C.; Zou, Z.Q.; Wu, Y.C.; Du, Y.W. Effect of particle size distribution on the magnetic properties of Fe-Si-Al powder core. J. Supercond. Nov. Magn. 2016, 29, 463–468. [Google Scholar] [CrossRef]
- Xie, D.-Z.; Lin, K.-H.; Lin, S.-T. Effects of processed parameters on the magnetic performance of a powder magnetic core. J. Magn. Magn. Mater. 2014, 353, 34–40. [Google Scholar] [CrossRef]
- Liu, Y.; Yi, Y.; Shao, W.; Shao, Y. Microstructure and magnetic properties of soft magnetic powder cores of amorphous and nanocrystalline alloys. J. Magn. Magn. Mater. 2013, 330, 119–133. [Google Scholar] [CrossRef]
- Ding, W.; Wang, X.; Feng, Z.K.; Gong, R.Z. FeSiAl powder core coated by Ba-B-P-O system insulating layer. Appl. Mech. Mater. 2013, 303–306, 2469–2473. [Google Scholar]
- Ding, W.; Wu, R.; Xiu, Z.Y.; Chen, G.Q.; Song, J.B.; Liao, Y.Q.; Wu, G.H. Effect of iron particle size and volume fraction on the magnetic properties of Fe/silicate glass soft magnetic composites. J. Supercond. Nov. Magn. 2014, 27, 435–441. [Google Scholar] [CrossRef]
- Ding, W.; Jiang, L.T.; Ma, K.; Yang, W.S.; Wu, G.H. Processing and properties of soda lime silica glass glass insulation soft magnetic composite. Optoelectron. Adv. Mater. 2014, 8, 68–71. [Google Scholar]
- Dhokey, N.B.; Patil, S.; Dhandare, S.; Bandal, V.S. Role of ceramic coating on electrical and magnetic properties of iron powder. Electron. Mater. Lett. 2014, 10, 591–596. [Google Scholar] [CrossRef]
- Peng, Y.D.; Nie, J.W.; Zhang, W.J.; Ma, J.; Bao, C.X.; Cao, Y. Effect of the addition of Al2O3 nanoparticles on the magnetic properties of Fe soft magnetic composites. J. Magn. Magn. Mater. 2016, 399, 88–93. [Google Scholar] [CrossRef]
- Wang, J.; Fan, X.; Wu, Z.Y.; Li, G.Q. Synthesis, microstructure and magnetic properties of Fe3Si0.7Al0.3@SiO2 core-shell particles and Fe3Si/Al2O3 soft magnetic composite core. J. Solid State Chem. 2015, 231, 152–158. [Google Scholar] [CrossRef]
- Yaghtin, M.; Taghvaei, A.H.; Hashemi, B.; Janghorban, K. Effect of heat treatment on magnetic properties of iron-based soft magnetic composites with Al2O3 insulation coating produced by sol-gel method. J. Alloys Compd. 2013, 581, 293–297. [Google Scholar] [CrossRef]
- Wang, M.G.; Zan, Z.; Deng, N.; Zhao, Z.K. Preparation of pure iron/Ni-Zn ferrite high strength soft magnetic composite by spark plasma sintering. J. Magn. Magn. Mater. 2014, 361, 166–169. [Google Scholar] [CrossRef]
- Wu, S.; Sun, A.; Xu, W.; Zhang, Q.; Zhai, F.; Logan, P.; Volinsky, A.A. Iron-based soft magnetic composites with Mn–Zn ferrite nanoparticles coating obtained by sol-gel method. J. Magn. Magn. Mater. 2012, 324, 3899–3905. [Google Scholar] [CrossRef]
- Streckova, M.; Hadraba, H.; Bures, R.; Faberova, M.; Roupcova, P.; Kubena, I.; Medvecky, L.; Girman, V.; Kollar, P.; Fuzer, J.; et al. Chemical synthesis of nickel ferrite spinel designed as an insulating bilayer coating on ferromagnetic particles. Surf. Coat. Technol. 2015, 270, 66–76. [Google Scholar] [CrossRef]
- Wei, D.; Wang, X.; Nie, Y.; Feng, Z.; Gong, R.; Chen, Y.; Harris, V.G. Low loss sendust powder cores comprised of particles coated by sodium salt insulating layer. J. Appl. Phys. 2015, 117, 17A921-1-3. [Google Scholar] [CrossRef]
- Wei, D.; Wang, X.; Feng, Z.K.; Gong, R.Z. Effect of annealing temperature on the soft magnetic behavior of Sendust powder core. Appl. Mech. Mater. 2014, 556–562, 6–10. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, X.; Xiao, J.Q. Submicrometer laminated Fe/SiO2 soft magnetic composites—An effective route to materials for high-frequency applications. Adv. Mater. 2005, 17, 915–918. [Google Scholar] [CrossRef]
- Yang, B.; Wu, Z.; Zou, Z.; Yu, R. High-performance Fe/SiO2 soft magnetic composites for low-loss and high-power applications. J. Phys. D 2010, 43, 365003-1-6. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Fan, X.A.; Li, G.Q.; Wang, J.; Gan, Z.H. Intergranular insulated Fe-6.5 wt % Si/SiO2 composite compacts with tunable insulating layer thickness for low core loss applications. RSC Adv. 2015, 5, 67031–67040. [Google Scholar] [CrossRef]
- Liu, Y.D.; Fang, F.F.; Choi, H.J. Core-shell-structured silica-coated magnetic carbonyl iron microbead and its magnetorheology with anti-acidic characteristics. Colloid Polym. Sci. 2011, 289, 1295–1298. [Google Scholar] [CrossRef]
- Liu, Y.D.; Choi, H.J.; Choi, S.-B. Controllable fabrication of silica encapsulated soft magnetic microspheres with enhanced oxidation-resistance and their rheology under magnetic field. Colloid. Surf. A Physicochem. Eng. Asp. 2012, 403, 133–138. [Google Scholar] [CrossRef]
- Desautels, R.D.; Freeland, J.W.; Rowe, M.P.; van Lierop, J. The role of interfacial metal silicates on the magnetism in FeCo/SiO2 and Fe49%Co49%V2%/SiO2 core/shell nanoparticles. J. Appl. Phys. 2015, 117, 17C728-1-4. [Google Scholar] [CrossRef]
- Desautels, R.D.; Rowe, M.P.; Jones, M.; Whallen, A.; van Lierop, J. Spontaneously formed interfacial metal silicates and their effect on the magnetism of superparamagnetic FeCo/SiO2 core/shell nanoparticles. Langmuir 2015, 31, 2879–2884. [Google Scholar] [CrossRef] [PubMed]
- Nakaya, M.; Nishida, R.; Muramatsu, A. Preparation of air-stable iron-oxide-coated metallic iron nanoparticles. J. Chem. Chem. Eng. 2013, 7, 1050–1053. [Google Scholar]
- Tajima, S.; Hattori, T.; Kondoh, M.; Kishimoto, H.; Sugiyama, T.; Kikko, T. Properties of high-density magnetic composite fabricated from iron powder coated with a new type phosphate insulator. IEEE Trans. Mag. 2005, 41, 3280–3282. [Google Scholar] [CrossRef]
- Decher, G. Fuzzy nanoassemblies: Toward layered polymeric multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Schmitt, J.; Decher, G.; Dressick, W.J.; Brandow, S.L.; Geer, R.E.; Shashidhar, R.; Calvert, J.M. Metal nanoparticle/polymer superlattice films: Fabrication and control of layer structure. Adv. Mater. 1997, 9, 61–65. [Google Scholar] [CrossRef]
- Caruso, F.; Shi, X.; Caruso, R.A.; Susha, A. Hollow titania spheres from layered precursor deposition on sacrificial colloidal core particles. Adv. Mater. 2001, 13, 740–744. [Google Scholar] [CrossRef]
- Caruso, R.A.; Susha, A.; Caruso, F. Multilayered titania, silica, and laponite nanoparticle coatings on polystyrene colloidal templates and resulting inorganic hollow spheres. Chem. Mater. 2001, 13, 400–409. [Google Scholar] [CrossRef]
- Sasaki, T.; Ebina, Y.; Tanaka, T.; Harada, M.; Watanabe, M.; Decher, G. Layer-by-layer assembly of titania nanosheet/polycation composite films. Chem. Mater. 2001, 13, 4661–4667. [Google Scholar] [CrossRef]
- Sasaki, T.; Ebina, Y.; Fukuda, K.; Tanaka, M.; Harada, M.; Watanabe, M. Titania nanostructured films derived from a titania nanosheet/polycation multilayer assembly via heat treatment and UV irradiation. Chem. Mater. 2002, 14, 3524–3530. [Google Scholar] [CrossRef]
- Nakano, H.; Ishii, M.; Nakamura, H. Preparation and structure of novel siloxene nanosheets. Chem. Commun. 2005, 2945–2947. [Google Scholar] [CrossRef] [PubMed]
- Nakano, H.; Ohtani, O.; Mitsuoka, T.; Akimoto, Y.; Nakamura, H. Synthesis of amorphous silica nanosheets and their photoluminescence. J. Am. Ceram. Soc. 2005, 88, 3522–3524. [Google Scholar] [CrossRef]
- Nakano, H.; Mitsuoka, T.; Harada, M.; Horibuchi, K.; Nozaki, H.; Takahashi, N.; Nonaka, T.; Seno, Y.; Nakamura, H. Soft synthesis of single-crystal silicon monolayer sheets. Angew. Chem. Int. Ed. 2006, 45, 6303–6306. [Google Scholar] [CrossRef] [PubMed]
- Tajima, S.; Hattori, T.; Kondoh, M.; Sugiyama, M.; Higashiyama, K.; Kishimoto, H.; Kikko, T. Properties of high density magnetic composite (HDMC) by warm compaction using die wall lubrication. Mater. Trans. 2004, 45, 1891–1894. [Google Scholar] [CrossRef]
- Tajima, S.; Hattori, T.; Kondoh, M.; Okajima, H.; Sugiyama, M.; Kikko, T. Magnetic properties and microstructure of high-density sintered iron formed by warm compaction using die wall lubrication. Mater. Trans. 2005, 46, 1402–1406. [Google Scholar] [CrossRef]
- Ristić, D.; Ivanda, M.; Speranza, G.; Siketić, Z.; Bogdanović-Radović, I.; Marciuš, M.; Ristić, M.; Gamulin, O.; Musić, S.; Furić, K.; et al. Local size distribution of oxygen in silicon-rich oxide thin films: a tool to investigate phase separation. J. Phys. Chem. C 2012, 116, 10039–10047. [Google Scholar] [CrossRef]
- Noma, Y.; Saga, Y.; Une, N. Amorphous silica-coated graphite particles for thermally conductive and electrically insulating resins. Carbon 2014, 78, 204–211. [Google Scholar] [CrossRef]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishizaki, T.; Nakano, H.; Tajima, S.; Takahashi, N. Improving Powder Magnetic Core Properties via Application of Thin, Insulating Silica-Nanosheet Layers on Iron Powder Particles. Nanomaterials 2017, 7, 1. https://doi.org/10.3390/nano7010001
Ishizaki T, Nakano H, Tajima S, Takahashi N. Improving Powder Magnetic Core Properties via Application of Thin, Insulating Silica-Nanosheet Layers on Iron Powder Particles. Nanomaterials. 2017; 7(1):1. https://doi.org/10.3390/nano7010001
Chicago/Turabian StyleIshizaki, Toshitaka, Hideyuki Nakano, Shin Tajima, and Naoko Takahashi. 2017. "Improving Powder Magnetic Core Properties via Application of Thin, Insulating Silica-Nanosheet Layers on Iron Powder Particles" Nanomaterials 7, no. 1: 1. https://doi.org/10.3390/nano7010001






