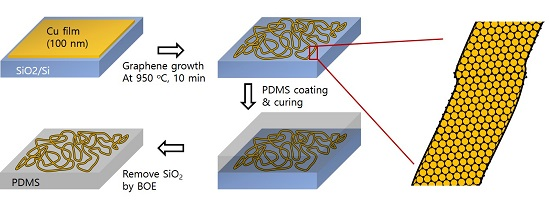
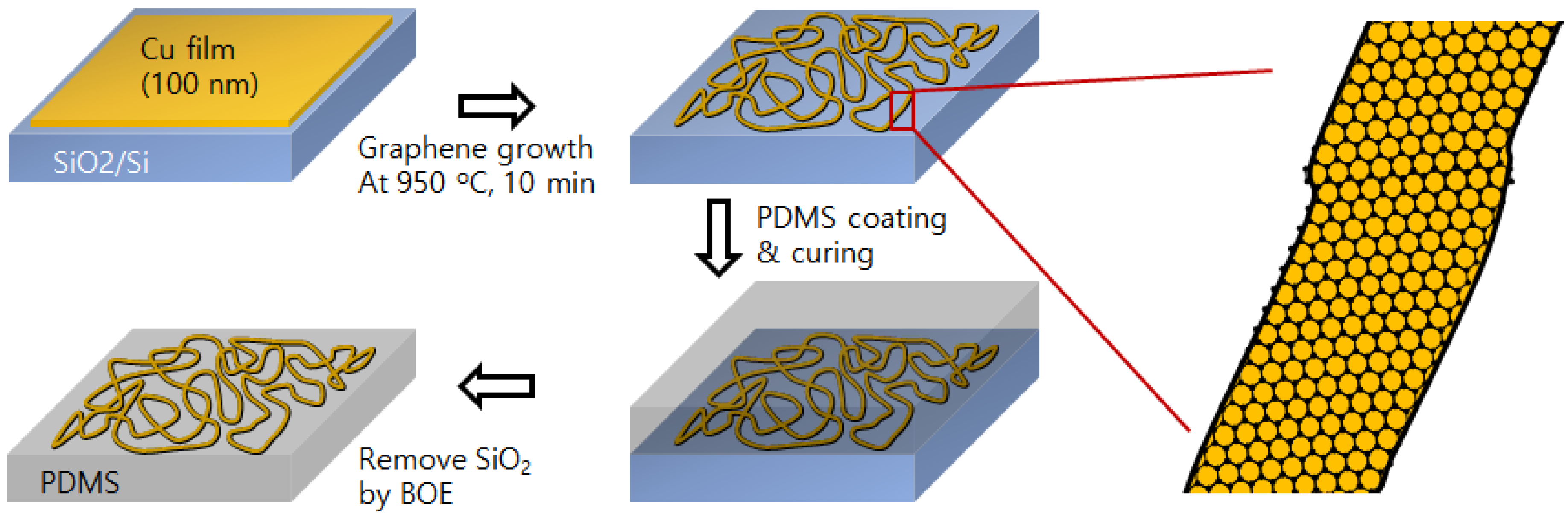
Copper Micro-Labyrinth with Graphene Skin: New Transparent Flexible Electrodes with Ultimate Low Sheet Resistivity and Superior Stability
Abstract
:1. Introduction
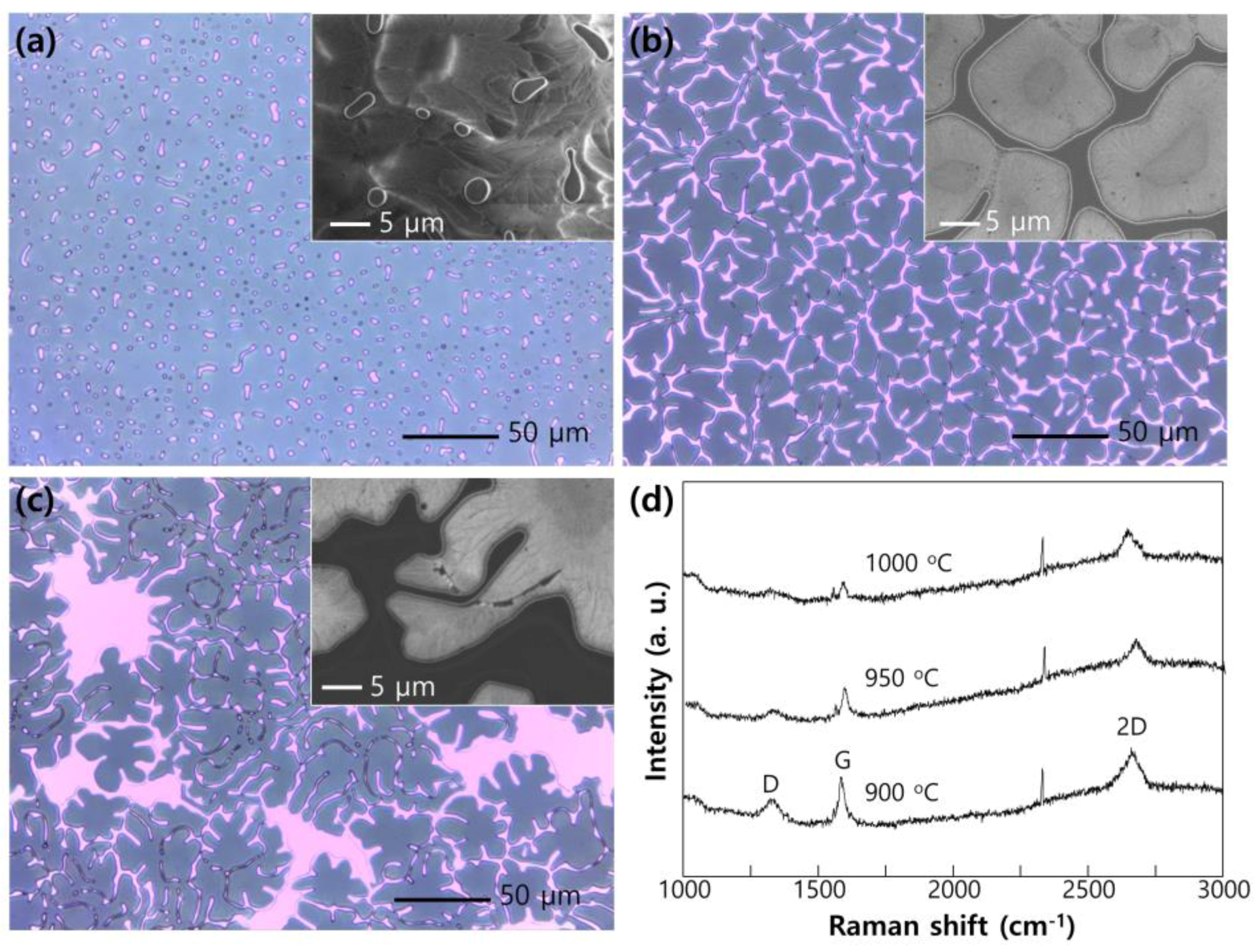
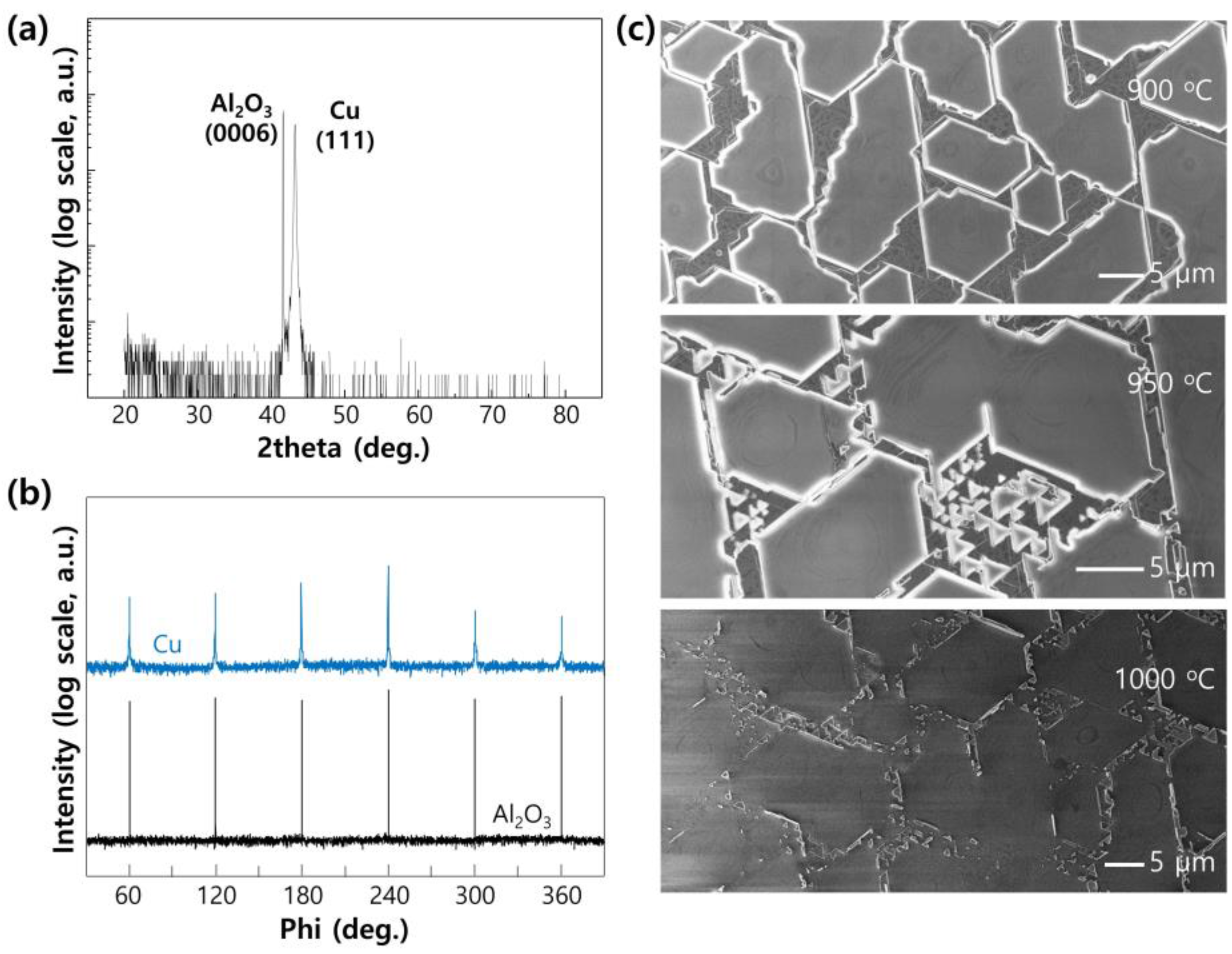
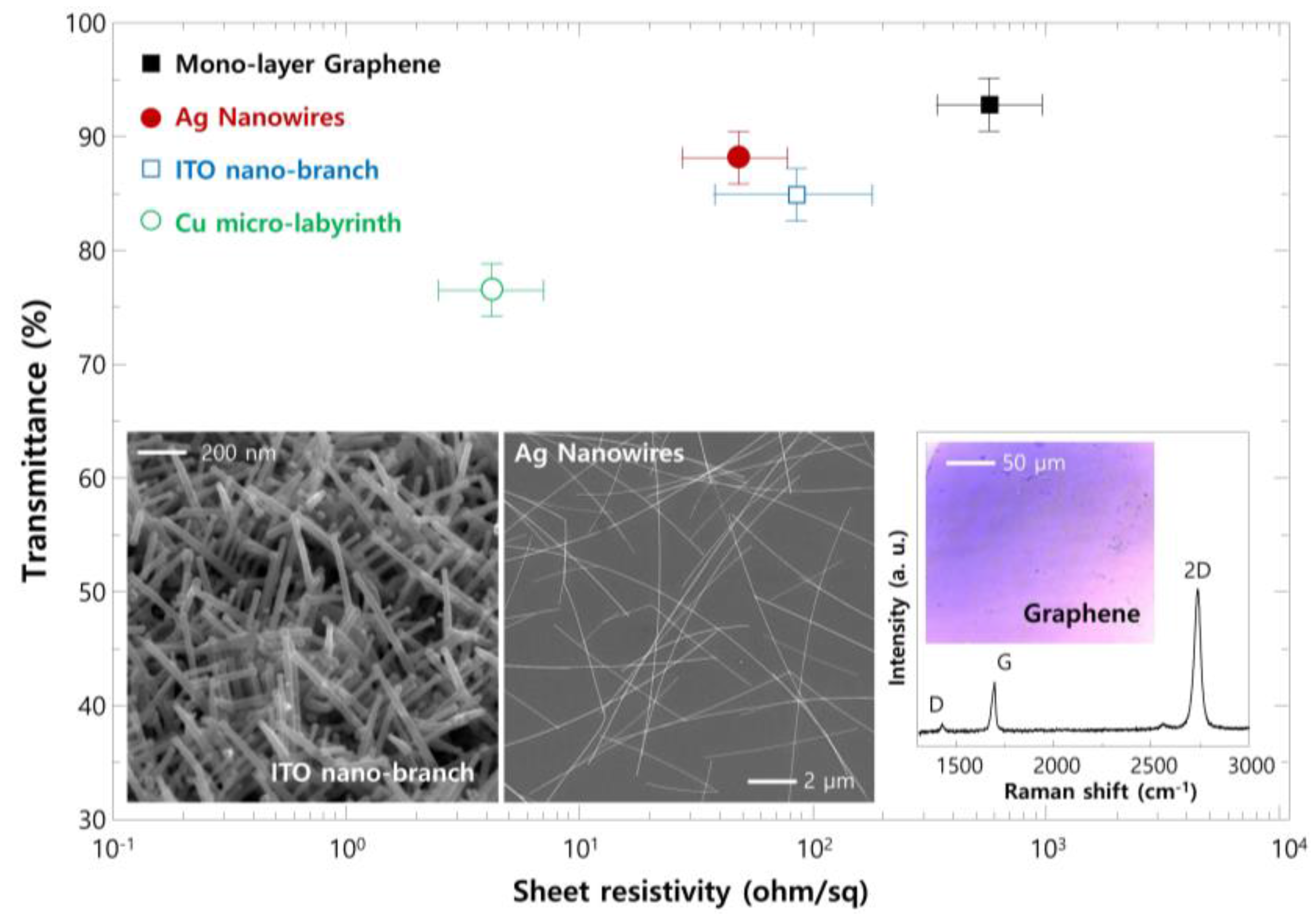
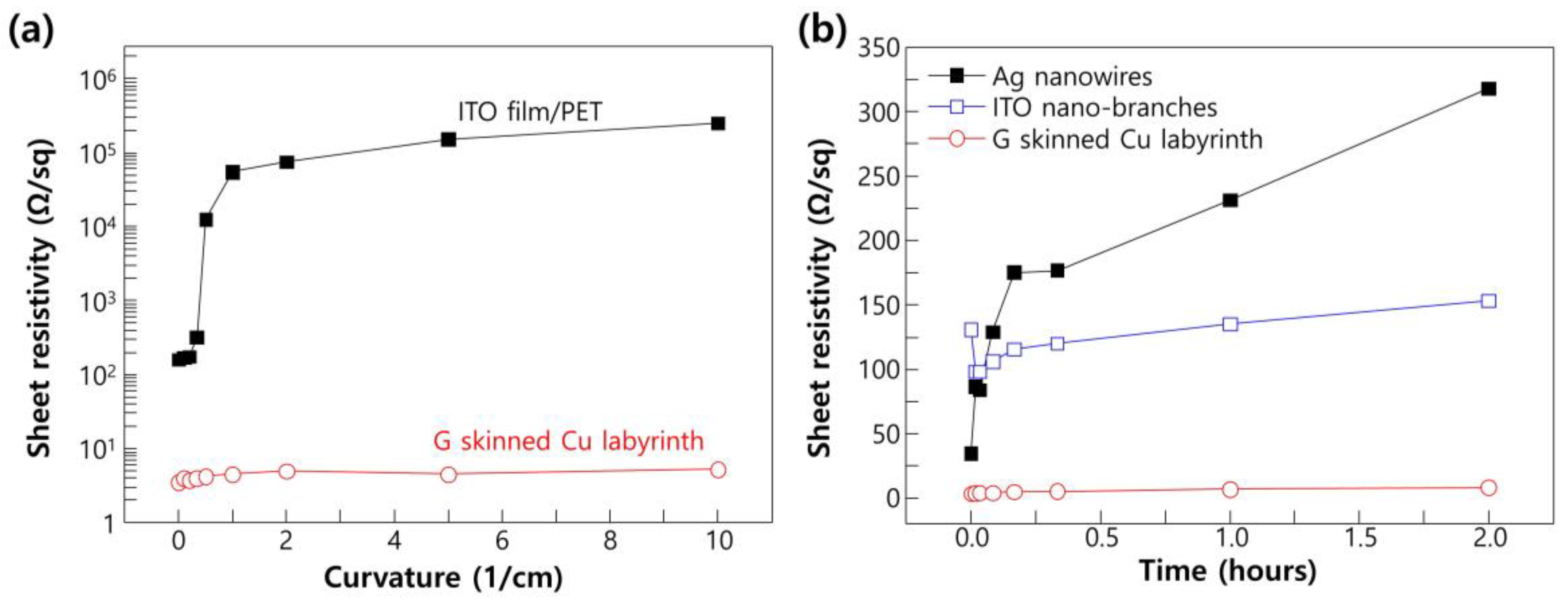
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Cu ML | Copper Micro-Labyrinth |
| Cu ML-G | Copper Micro-Labyrinth with Graphene skin |
| ITO | Indium-Tix-Oxide |
| TFE | Transparent Flexible Electrodes |
References
- Liu, D.; Kelly, T.L. Perovskite solar cells with a planar heterojunction structure prepared using room-temperature solution processing techniques. Nat. Photonics 2014, 8, 133–138. [Google Scholar] [CrossRef]
- Han, T.-H.; Lee, Y.; Choi, M.-R.; Woo, S.-H.; Bae, S.-H.; Hong, B.H.; Ahn, J.-H.; Lee, T.-W. Extremely efficient flexible organic light-emitting diodes with modified graphene anode. Nat. Photonics 2012, 6, 105–110. [Google Scholar] [CrossRef]
- Mates, J.E.; Bayer, I.S.; Palumbo, J.M.; Carroll, P.J.; Megaridis, C.M. Extremely stretchable and conductive water-repellent coatings for low-cost ultra-flexible electronics. Nat. Commun. 2015, 6, 8874. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Li, L.; Chen, D.; Hajagos, T.; Ren, Z.; Chou, S.-Y.; Hu, W.; Pei, Q. Intrinsically stretchable and transparent thin-film transistors based on printable silver nanowires, carbon nanotubes and an elastomeric dielectric. Nat. Commun. 2015, 6, 7647. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Lin, N.; Chen, Y.; Wang, Z.; Xie, Q.; Zheng, T.; Gao, N.; Li, S.; Kang, J.; Cai, D.; et al. Copper Nanowires as Fully Transparent Conductive Electrodes. Sci. Rep. 2013, 3, 670–692. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Chang, S.; Zhou, X.; Kong, J.; Palacios, T.; Gradecak, S. Flexible Graphene Electrode-Based Organic Photovoltaics with Record-High Efficiency. Nano Lett. 2014, 14, 5148–5154. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Zhao, C.; Pan, W.; Cui, Y.; Wu, H. Direct Writing of Half-Meter Long CNT Based Fiber for Flexible Electronics. Nano Lett. 2015, 15, 1609–1614. [Google Scholar] [CrossRef] [PubMed]
- Vosgueritchian, M.; Lipomi, D.J.; Bao, Z. Highly Conductive and Transparent PEDOT: PSS Films with a Fluorosurfactant for Stretchable and Flexible Transparent Electrodes. Adv. Funct. Mater. 2012, 22, 421–428. [Google Scholar] [CrossRef]
- Yu, H.K.; Kim, S.; Koo, B.; Jung, G.H.; Lee, B.; Ham, J.; Lee, J.-L. Nano-branched transparent conducting oxides: Beyond the brittleness limit for flexible electrode applications. Nanoscale 2012, 4, 6831–6834. [Google Scholar] [CrossRef] [PubMed]
- Susrutha, B.; Giribabu, L.; Singh, S.P. Recent advances in flexible perovskite solar cells. Chem. Commun. 2015, 51, 14696–14707. [Google Scholar] [CrossRef] [PubMed]
- Allen, G.L.; Bayles, R.A.; Gile, W.W.; Jesser, W.A. Small, Particle Melting of Pure Metals. Thin Solid Films 1986, 144, 297–308. [Google Scholar] [CrossRef]
- Yu, H.K.; Dong, W.J.; Jung, G.H.; Lee, J.-L. Three-dimensional nanobranched indium-tin-oxide anode for organic solar cells. ACS Nano 2011, 5, 8026–8032. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.J.; Su, Y.K.; Wu, S.L. The effect of solvent on the etching of ITO electrode. Mater. Chem. Phys. 2004, 84, 146–150. [Google Scholar] [CrossRef]
- Maki, H.; Ikoma, T.; Sakaguchi, I.; Ohashi, N.; Haneda, H.; Tanaka, J.; Ichinose, N. Control of surface morphology of ZnO (000-1) by hydrochloric acid etching. Thin Solid Films 2002, 411, 91–95. [Google Scholar] [CrossRef]
- Lee, J.; Lee, A.; Yu, H.K. Graphene protected Ag nanowires: Blocking of surface migration for thermally stable and wide-range-wavelength transparent flexible electrodes. RSC Adv. 2016. revision submitted. [Google Scholar]
- Porter, D.A.; Easterling, K.E.; Sherif, M.Y. Phase Transformation in Metals and Alloys; CRC Press, Taylor & Francis: Boca Raton, FL, USA, 2009. [Google Scholar]
- Yu, H.K.; Balasubramanian, K.; Kim, K.; Lee, J.-L.; Maiti, M.; Ropers, C.; Krieg, J.; Kern, K.; Wodtke, A.M. Chemical vapor deposition of graphene grown on “peeled-off” epitaxial Cu(111) foil: A simple approach to improved properties. ACS Nano 2014, 8, 8636–8643. [Google Scholar] [CrossRef] [PubMed]
- Mattevi, C.; Kim, H.; Chhowalla, M. A review of chemical vapor deposition of graphene on copper. J. Mater. Chem. 2011, 21, 3324–3334. [Google Scholar] [CrossRef]
- Yu, H.K.; Baik, J.M.; Lee, J.-L. Design of interfacial layer to block chemical reaction for epitaxial ZnO growth on Si substrate. Cryst. Growth Des. 2011, 11, 2438–2443. [Google Scholar] [CrossRef]
- Yu, H.K.; Baik, J.M.; Lee, J.-L. Self-connected and habitually tilted piezoelectric nanorod array. ACS Nano 2011, 5, 8828–8833. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the Elastic Properties and Intrinsic Strength of Monolayer Graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H. Gas sensors using hierarchical and hollow oxide nanostructures: Overview. Sens. Actuators B 2009, 140, 319–336. [Google Scholar] [CrossRef]
© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.K. Copper Micro-Labyrinth with Graphene Skin: New Transparent Flexible Electrodes with Ultimate Low Sheet Resistivity and Superior Stability. Nanomaterials 2016, 6, 161. https://doi.org/10.3390/nano6090161
Yu HK. Copper Micro-Labyrinth with Graphene Skin: New Transparent Flexible Electrodes with Ultimate Low Sheet Resistivity and Superior Stability. Nanomaterials. 2016; 6(9):161. https://doi.org/10.3390/nano6090161
Chicago/Turabian StyleYu, Hak Ki. 2016. "Copper Micro-Labyrinth with Graphene Skin: New Transparent Flexible Electrodes with Ultimate Low Sheet Resistivity and Superior Stability" Nanomaterials 6, no. 9: 161. https://doi.org/10.3390/nano6090161