Non-Enzymatic Glucose Biosensor Based on CuO-Decorated CeO2 Nanoparticles
Abstract
:1. Introduction
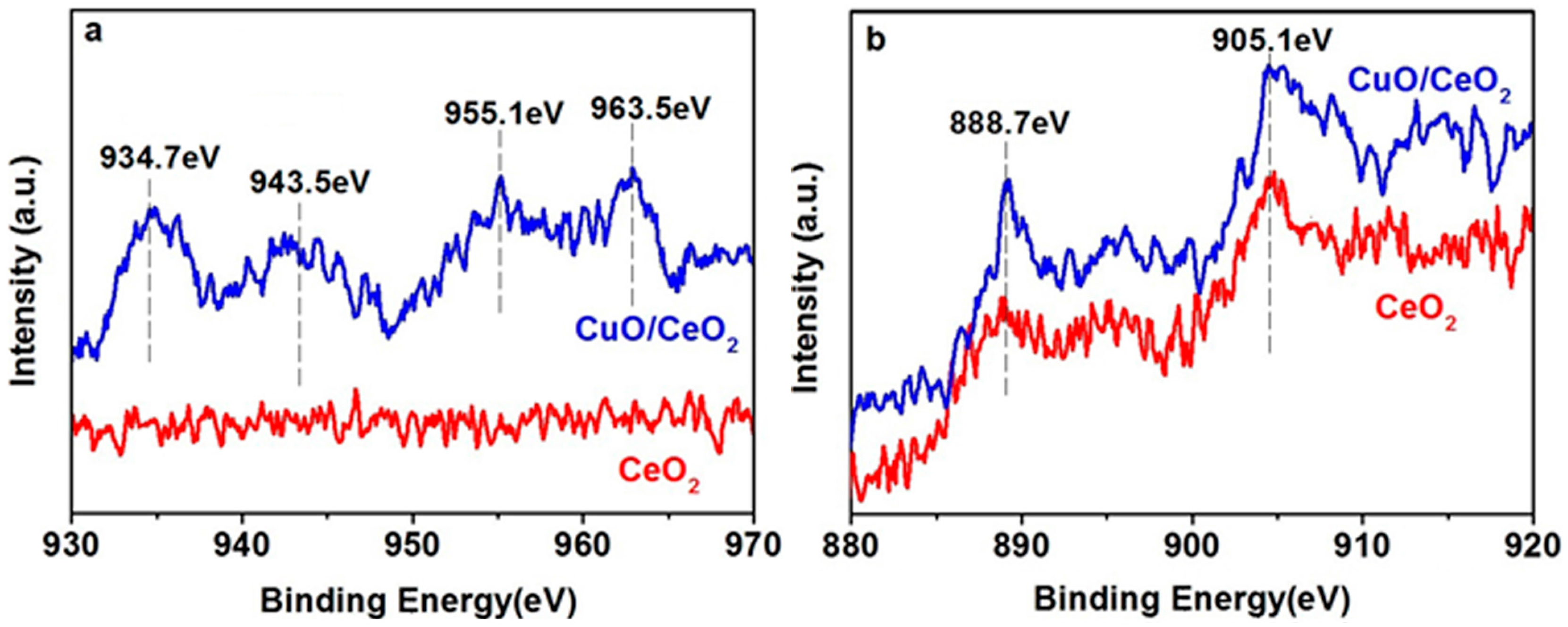
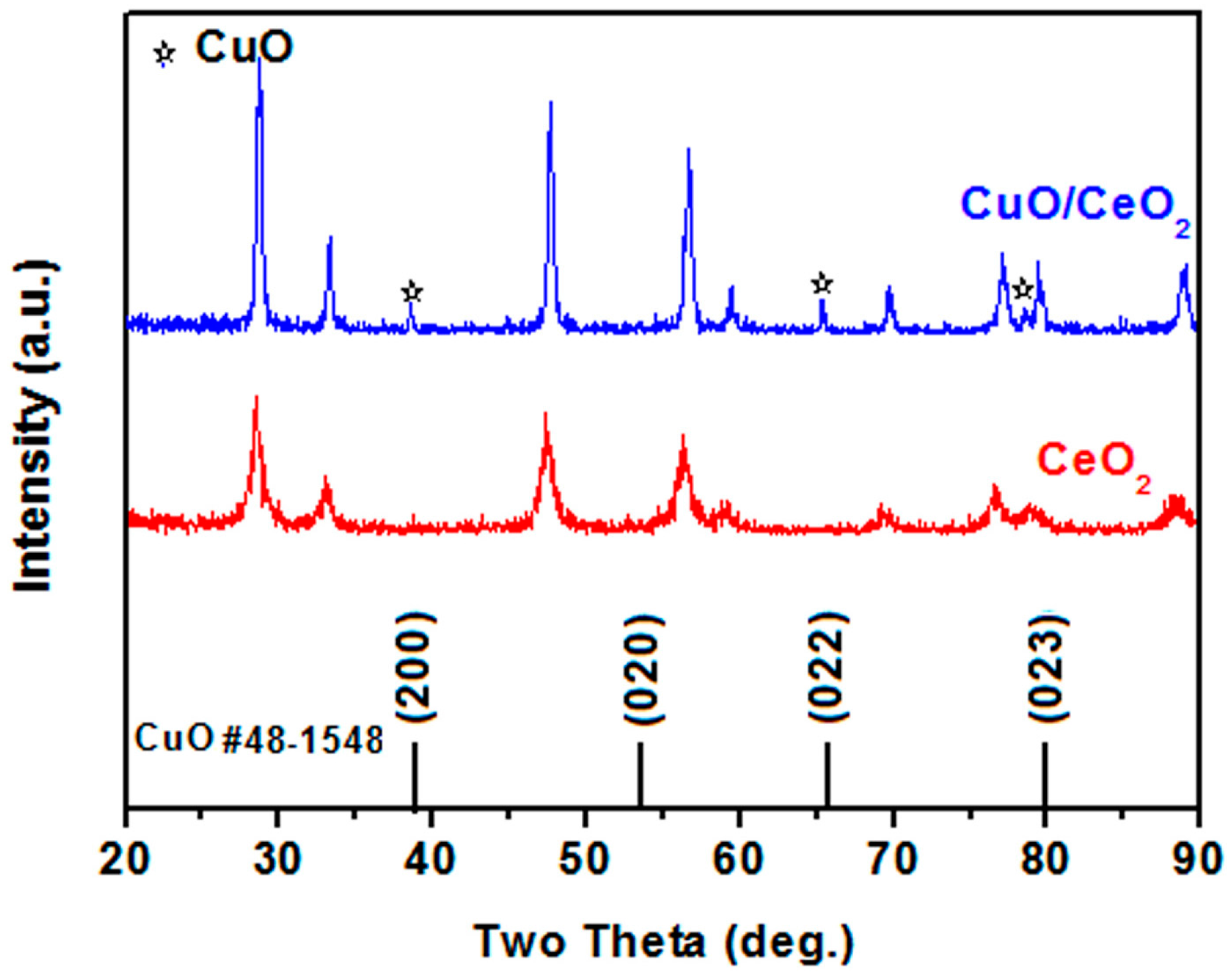
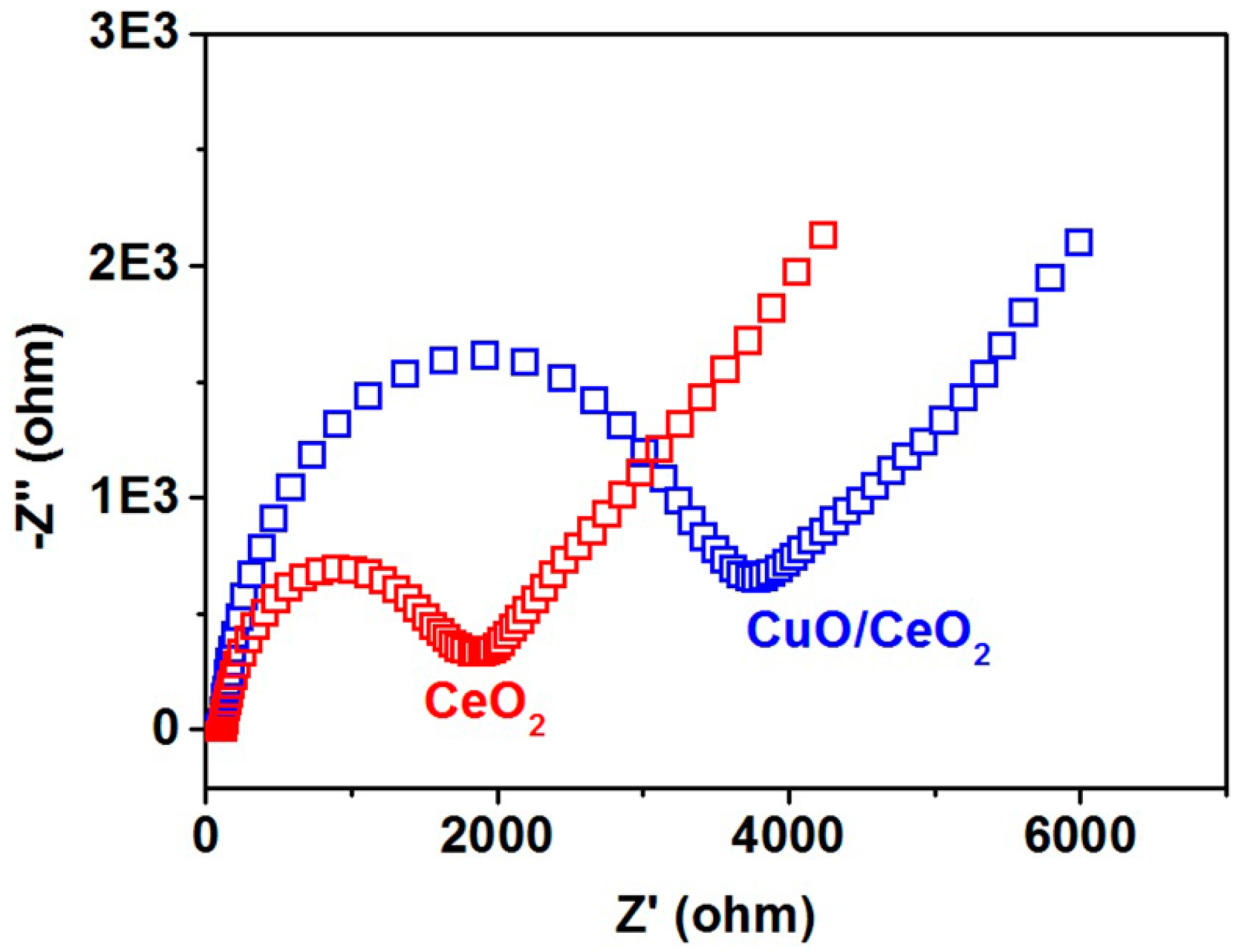
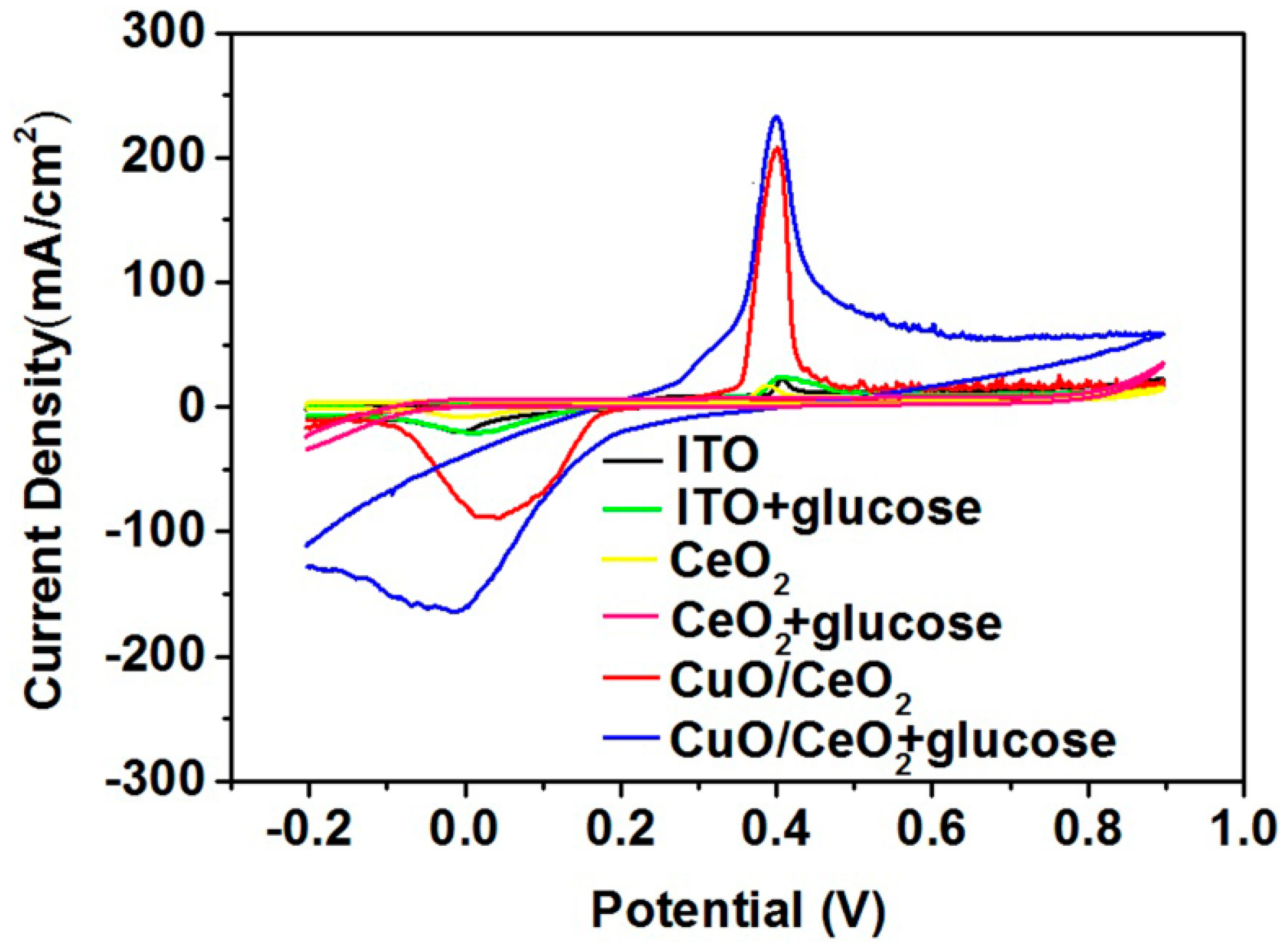
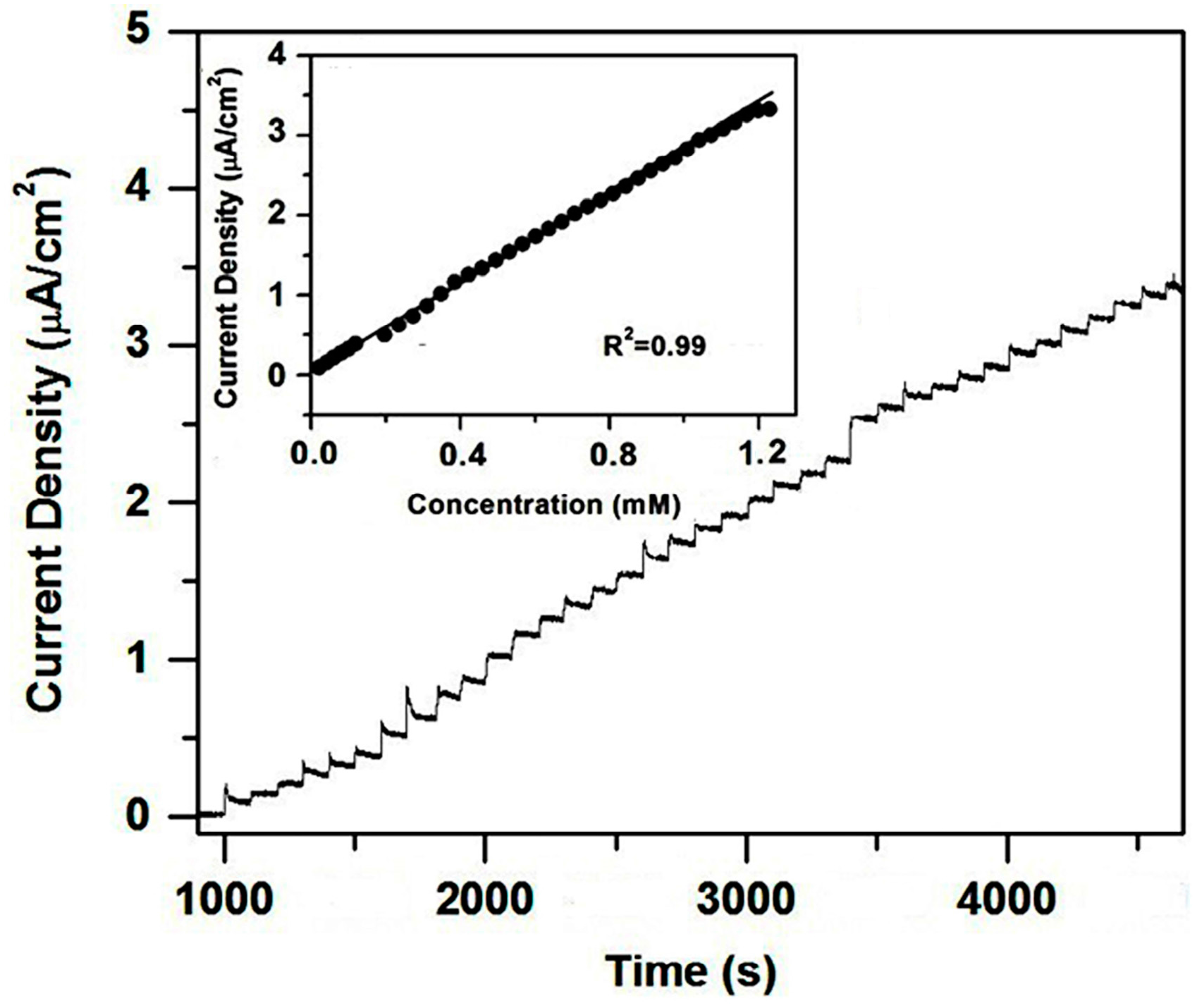
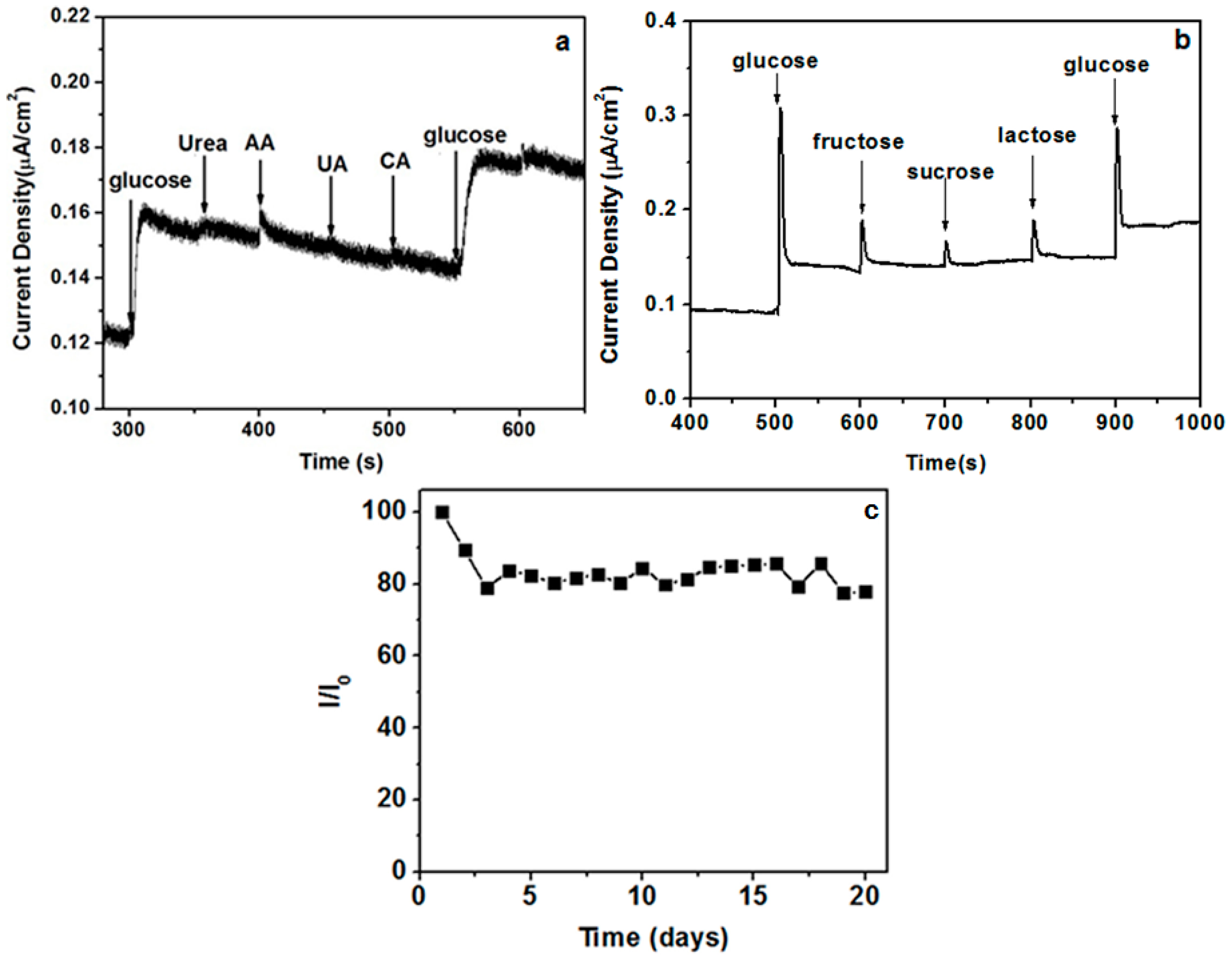
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ameen, S.; Akhtar, M.S.; Shin, H.S. Nanocages-augmented aligned polyaniline nanowires as unique platform for electrochemical non-enzymatic glucose biosensor. Appl. Catal. A 2016, 517, 21–29. [Google Scholar] [CrossRef]
- Aggidis, A.G.A.; Newman, J.D.; Aggidis, G.A. Investigating pipeline and state of the art blood glucose biosensors to formulate next steps. Biosens. Bioelectron. 2015, 74, 243–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saei, A.A.; Dolatabadi, J.E.N.; Najafi-Marandi, P.; Abhari, A.; Guardia, M. Electrochemical biosensors for glucose based on metal nanoparticles. Trends Anal. Chem. 2013, 42, 216–227. [Google Scholar] [CrossRef]
- Wang, L.; Chen, X.; Liu, C.; Yang, W. Non-enzymatic acetylcholine electrochemical biosensor based on flower-like NiAl layered double hydroxides decorated with carbon dots. Sens. Actuators B 2016, 233, 199–205. [Google Scholar] [CrossRef]
- Yang, J.; Liang, X.; Cui, L.; Liu, H.; Xie, J.; Liu, W. A novel non-enzymatic glucose sensor based on Pt3Ru1 alloy nanoparticles with high density of surface defects. Biosens. Bioelectron. 2016, 80, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Ahammad, A.J.S.; Jin, J.H.; Ahn, S.J.; Lee, J.J. A comprehensive review of glucose biosensors based on nanostructured metal-oxides. Sensors 2010, 10, 4855–4886. [Google Scholar] [CrossRef] [PubMed]
- Shearer, C.J.; Cherevan, A.; Eder, D. Application and future challenges of functional nanocarbon hybrids. Adv. Mater. 2014, 26, 2295–2318. [Google Scholar] [CrossRef] [PubMed]
- Patil, D.; Dung, N.Q.; Jung, H.; Ahn, S.Y.; Jang, D.M.; Kim, D. Enzymatic glucose biosensor based on CeO2 nanorods synthesized by non-isothermal precipitation. Biosens. Bioelectron. 2012, 31, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.Y.; Xu, L.; Song, J.; Xing, R.Q.; Xu, S.; Liu, D.L. Ultrasensitive non-enzymatic glucose sensor based on three-dimensional network of ZnO-CuO hierarchical nanocomposites by electrospinning. Sci. Rep. 2014, 4, 7382. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Gao, Q.; Chen, Z. Preparation of mesoporous copper cerium bimetal oxides with high performance for catalytic oxidation of carbon monoxide. Appl. Cata. B 2008, 81, 236–243. [Google Scholar] [CrossRef]
- Khadse, V.R.; Thakur, S.; Patil, K.R.; Patil, P. Humidity-sensing studies of cerium oxide nanoparticles synthesizedby non-isothermal precipitation. Sens. Actuators B 2014, 203, 229–238. [Google Scholar] [CrossRef]
- Wrobel, R.; Suchorski, Y.; Becker, S.; Weiss, H. Cerium oxide layers on the Cu (111) surface: Substrate-mediated redox properties. Surf. Sci. 2008, 602, 436–442. [Google Scholar] [CrossRef]
- Hoa, D.H.; Quy, N.Y.; Jung, H.; Kim, D.; Kim, H.; Hong, S.K. Synthesis of porous CuO nanowires and its application to hydrogen detection. Sens. Actuators B 2010, 146, 266–272. [Google Scholar] [CrossRef]
- Wang, W.; Li, Z.; Zheng, W.; Yang, J.; Zhang, H.; Wang, C. Electrospun palladium (IV)-doped copper oxide composite nanofibersfor non-enzymatic glucose sensors. Electrochem. Commun. 2009, 11, 1811–1814. [Google Scholar] [CrossRef]
- Luo, L.; Zhu, L.; Wang, Z. Nonenzymatic amperometric determination of glucose by CuO nanocubes-grapheme nanocomposite modified electrode. Bioelectrochemistry 2012, 88, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Dung, N.Q.; Patil, D.; Jung, H.; Kim, J.; Kim, D. NiO-decorated single-walled carbon nanotubes for high-performance nonenzymatic glucose sensing. Sens. Actuators B 2013, 183, 381–387. [Google Scholar] [CrossRef]
- Ansari, A.A.; Solanki, P.R.; Malhotra, B.D. Sol-gel derived nanostructured cerium oxide film for glucose sensor. Appl. Phys. Lett. 2008, 92, 263901. [Google Scholar] [CrossRef]
- Sriparna, D.; Mohanty, S.; Nayak, S.K. Nano-CeO2 decorated graphene based chitosan nanocomposites as enzymatic biosensing platform: Fabrication and cellular biocompatibility assessment. Bioprocess. Biosyst. Eng. 2015, 38, 1671–1683. [Google Scholar]
- Gougis, M.; Pereira, A.; Ma, D.L.; Mohamedi, M. Simultaneous deposition of cerium oxide and gold nanostructures-characterization and analytical properties toward glucose electro-oxidation and sensing. RSC Adv. 2014, 4, 39955–39961. [Google Scholar] [CrossRef]
- Gougis, M.; Tabet-Aoul, A.; Ma, D.L.; Mohamedi, M. Nanostructured cerium oxide catalyst support: Effects of morphology on the electroactivity of gold toward oxidative sensing of glucose. Microchim. Acta 2014, 181, 1207–1214. [Google Scholar] [CrossRef]


| Working Electrode | Detection Limit (μM) | Sensitivity (μA mM−1cm−2) | References |
|---|---|---|---|
| GOx a/CeO2/Au | 12.0 | 0.051 | [17] |
| GOx/CeO2/ITO | 100 | 0.165 | [8] |
| GOx/CeO2/graphene/FTO b | 2 | 7.198 | [18] |
| CeO2/Au/CPE c | 10 | 57.5 | [19] |
| CeO2/Au | 10 | 44 | [20] |
| CuO/CeO2/ITO | 10 | 2.77 | This work |
| Spiked (μM) | Found (μM) | Recovery (%) |
|---|---|---|
| 0 | 50.7 | - |
| 25 | 74.3 | 94.4 |
| 50 | 98.7 | 96.0 |
| 75 | 122.3 | 95.5 |
| 100 | 153.3 | 102.6 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, P.; Li, Y.; Zhang, J.; Li, W. Non-Enzymatic Glucose Biosensor Based on CuO-Decorated CeO2 Nanoparticles. Nanomaterials 2016, 6, 159. https://doi.org/10.3390/nano6090159
Guan P, Li Y, Zhang J, Li W. Non-Enzymatic Glucose Biosensor Based on CuO-Decorated CeO2 Nanoparticles. Nanomaterials. 2016; 6(9):159. https://doi.org/10.3390/nano6090159
Chicago/Turabian StyleGuan, Panpan, Yongjian Li, Jie Zhang, and Wei Li. 2016. "Non-Enzymatic Glucose Biosensor Based on CuO-Decorated CeO2 Nanoparticles" Nanomaterials 6, no. 9: 159. https://doi.org/10.3390/nano6090159





