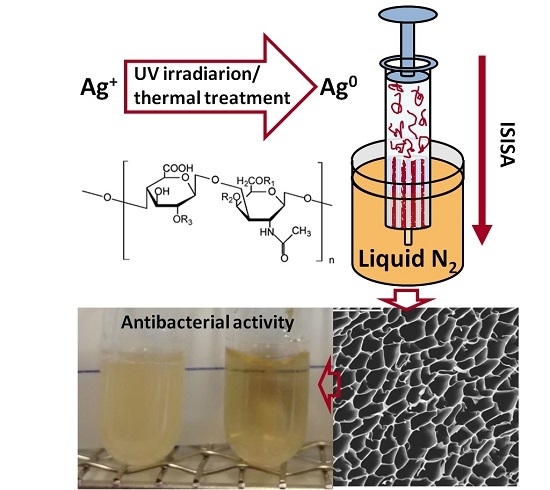
Green Synthesis of Hierarchically Structured Silver-Polymer Nanocomposites with Antibacterial Activity
Abstract
:1. Introduction
2. Results and Discussion
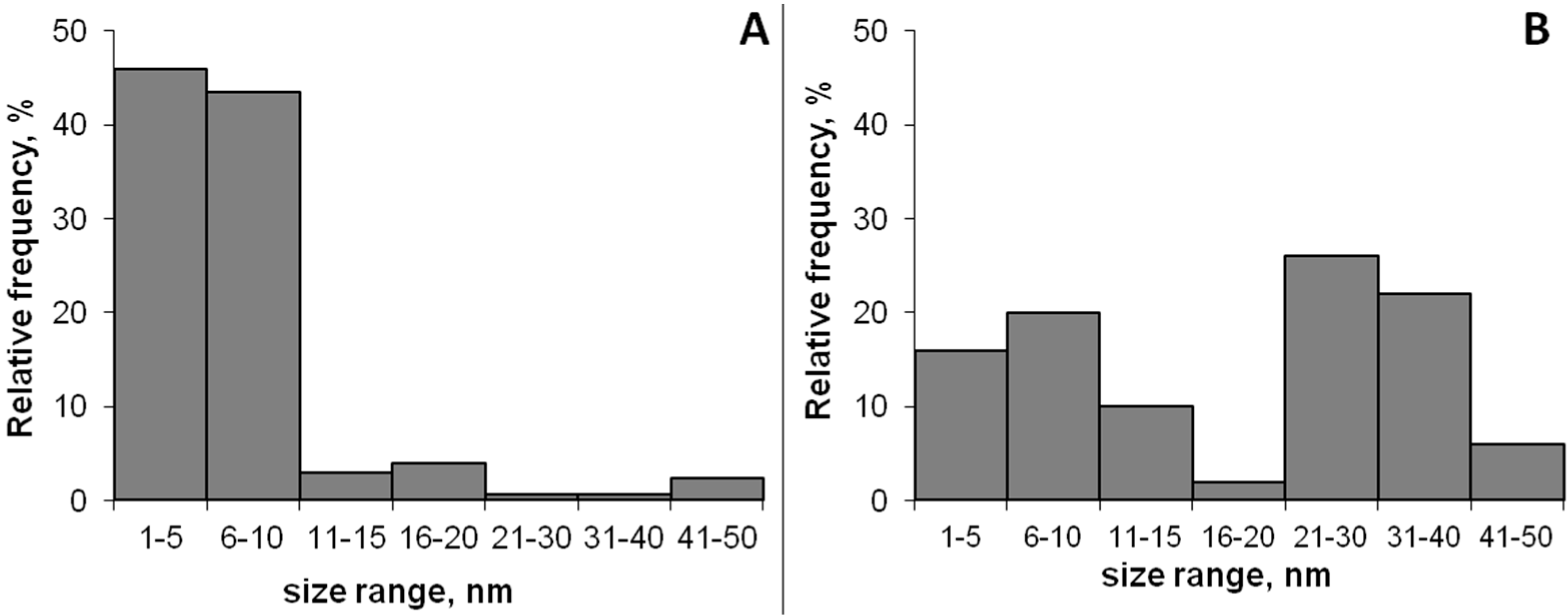
2.1. AgNPs Production and Characterization
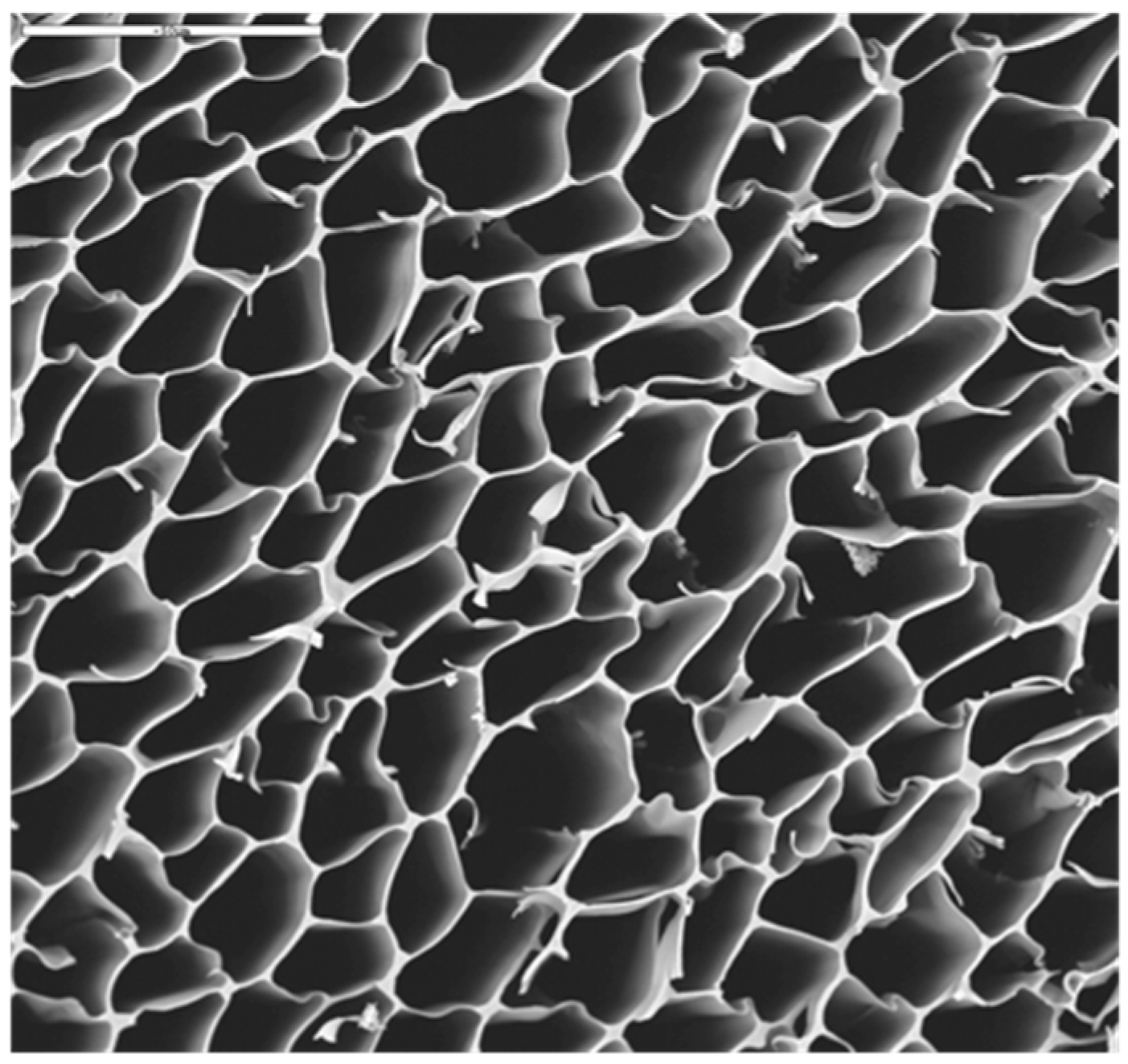
2.2. AgNPs-CS Scaffold Preparation and Characterization
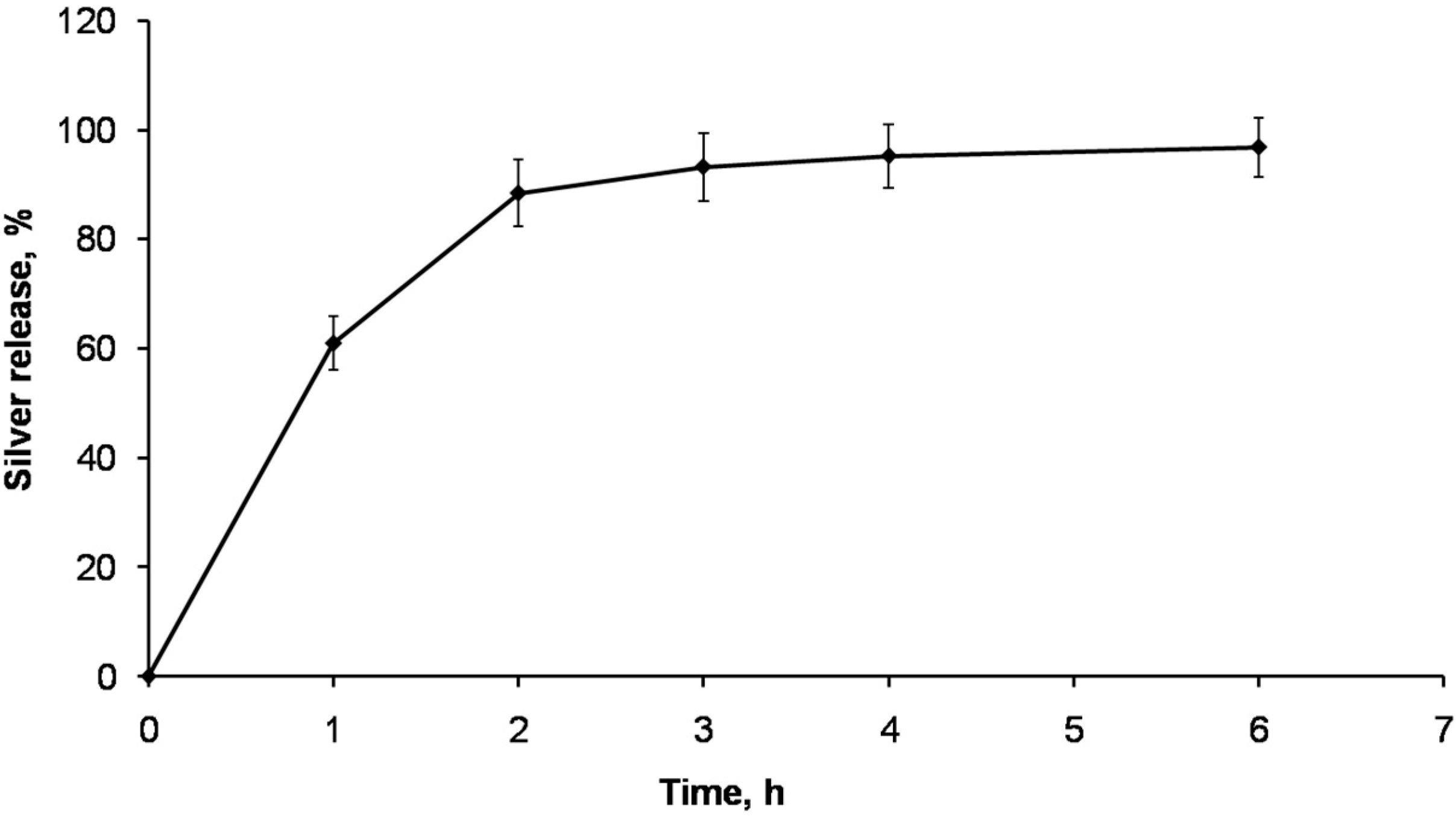
2.3. Silver Release from AgNPs-CS Crosslinked Scaffolds
2.4. AgNPs-CS Antimicrobial Activity
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Preparation of Ag Nanoparticles in Chondroitin Sulfate Solution by Thermal Treatment
3.2.2. Preparation of Ag Nanoparticles in chondroitin Sulfate Solution by UV-Irradiation
3.2.3. Preparation of AgNPs-Chondroitin Sulfate and AgNO3-Chondroitin Sulfate Scaffolds
3.2.4. Sample Characterization
3.2.5. Silver Release
3.2.6. Antibacterial Activity
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AgNPs | Silver nanoparticles |
| CS | Chondroitin sulphate |
| TEM | Transmission electron microscopy |
| MWCNTs | Multi-walled carbon nanotubes |
| XPS | X-ray photoelectron spectroscopy |
| SAED | selected-area electron diffraction |
| ISISA | ice-segregation-induced self-assembly |
| SEMPBS | Scanning electron microscopyPhosphate saline buffer |
| MIC | Minimum inhibitory concentration |
| LB | Lysogeny broth medium |
| HDI | Hexamethylenediisocyanate |
References
- Daniel, M.C.; Astruc, D. Gold nanoparticles: Assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef] [PubMed]
- Raveendran, P.; Fu, J.; Wallen, S.L. Completely “green” synthesis and stabilization of metal nanoparticles. J. Am. Chem. Soc. 2003, 125, 13940–13941. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Li, M.; Dujardin, E.; Girard, C.; Mann, S. One-Dimensional Plasmon Coupling by Facile Self-Assembly of Gold Nanoparticles into Branched Chain Networks. Adv. Mater. 2005, 17, 2553–2559. [Google Scholar] [CrossRef]
- Lopes, A.; Jaeger, H.M. Hierarchical self-assembly of metal nanostructures on diblock copolymer scaffolds. Nature 2001, 414, 735–738. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Li, Y. Colloidal carbon spheres and their core/shell structures with noble-metal nanoparticles. Angew. Chem. Int. Ed. 2004, 43, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Du, J.; Han, B.; Liu, Z.; Jiang, T.; Zhang, Z. Sonochemical formation of single-crystalline gold nanobelts. Angew. Chem. Int. Ed. 2006, 45, 1116–1119. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Gonzalez, B.; Salgueiriño-Maceira, V.; Garcia-Santamaria, F.; Liz-Marzan, L.M. Fully accessible gold nanoparticles within ordered macroporous solids. Nano Lett. 2002, 2, 471–473. [Google Scholar] [CrossRef]
- De Geest, B.G.; Skirtach, A.G.; Mamedov, A.A.; Antipov, A.A.; Kotov, N.A.; De Smedt, S.C.; Sukhorukov, G.B. Ultrasound-triggered release from multilayered capsules. Small 2007, 3, 804–808. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Al-Thabaiti, S.A.; Obaid, A.Y.; Al-Youbi, A.O. Preparation and characterization of silver nanoparticles by chemical reduction method. Coll. Surf. B Biointerfaces 2011, 82, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Ifuku, S.; Tsuji, M.; Morimoto, M.; Saimoto, H.; Yano, H. Preparation of gold nanoparticles loaded chitin nanofiber composite. Biomacromolecules 2009, 10, 2714–2717. [Google Scholar] [PubMed]
- Wei, D.; Sun, W.; Qian, W.; Ye, Y.; Ma, X. The synthesis of chitosan-based silver nanoparticles and their antibacterial activity. Carbohydr. Res. 2009, 344, 2375–2382. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Yang, X. Synthesis of polysaccharide-stabilized gold and silver nanoparticles: A green method. Carbohydr. Res. 2004, 339, 2627–2631. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Hung, Y.; Chen, C.; Liu, C.; Young, J. Green synthesis of chondroitin sulfate-capped silver nanoparticles: Characterization and surface modification. Carbohydr. Polym. 2014, 110, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.; Han, Y. Chondroitin sulfate-based biomaterials for tissue engineering. Turk. J. Biol. 2016, 40, 290–299. [Google Scholar] [CrossRef]
- Serrano, M.C.; Nardecchia, S.; García-Rama, C.; Ferrer, M.L.; Collazos-Castro, J.E.; del Monte, F.; Gutiérrez, M.C. Chondroitin sulphate-based 3D scaffolds containing MWCNTs for nervous tissue repair. Biomaterials 2014, 35, 1543–1551. [Google Scholar] [CrossRef] [PubMed]
- Im, A.R.; Kim, J.Y.; Kim, H.S.; Cho, S.; Park, Y.; Kim, Y.S. Wound healing and antibacterial activities of chondroitin sulfate- and acharan sulfate-reduced silver nanoparticles. Nanotechnology 2013, 24, 395102. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.G.; Xiao, F.; Wang, C.-W.; Lee, Y.-I.; Xue, Q.; Chen, X.; Qian, D.J.; Hao, J.; Jiang, J. One-step synthesis of silver nanoparticles at the air–water interface using different methods. Nanotechnology 2008, 19. [Google Scholar] [CrossRef] [PubMed]
- Henglein, A. Colloidal Silver Nanoparticles: Photochemical preparation and interaction with O2, CCl4, and some metal ions. Chem. Mater. 1998, 10, 444–450. [Google Scholar] [CrossRef]
- Fromm, K.M. Give silver a shine. Nat. Chem. 2011, 3, 178. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.; Pradhan, A.; Pakstis, L.; Pochan, D.J.; Shah, S.I.J. Synthesis and antibacterial properties of silver nanoparticles. J. Nanosci. Nanotechnol. 2005, 5, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, K.N.; Mhatre, S.S.; Parikh, R.Y. Biological synthesis of metallic nanoparticles. Nanomedicine 2010, 6, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, M.C.; Ferrer, M.L.; Del Monte, F. Ice-templated materials: Sophisticated structures exhibiting enhanced functionalities obtained after unidirectional freezing and ice-segregation- induced self-assembly. Chem. Mater. 2008, 20, 634–648. [Google Scholar] [CrossRef]
- Aranaz, I.; Harris, R.; Navarro-García, F.; Heras, A.; Acosta, N. Chitosan based films as supports for dual antimicrobial release. Carbohydr. Polym. 2016, 146, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.; Jang, J. Antibacterial Properties of Novel Poly(methyl methacrylate) Nanofiber Containing Silver Nanoparticles. Langmuir 2008, 24, 2051–2056. [Google Scholar] [CrossRef] [PubMed]
- Venkatpurwar, V.; Pokharkar, V. Green synthesis of silver nanoparticles using marine polysaccharide: Study of in vitro antibacterial activity. Mater. Lett. 2011, 65, 999–1002. [Google Scholar] [CrossRef]
- Rai, M.; Yaday, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef] [PubMed]






© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hortigüela, M.J.; Yuste, L.; Rojo, F.; Aranaz, I. Green Synthesis of Hierarchically Structured Silver-Polymer Nanocomposites with Antibacterial Activity. Nanomaterials 2016, 6, 137. https://doi.org/10.3390/nano6080137
Hortigüela MJ, Yuste L, Rojo F, Aranaz I. Green Synthesis of Hierarchically Structured Silver-Polymer Nanocomposites with Antibacterial Activity. Nanomaterials. 2016; 6(8):137. https://doi.org/10.3390/nano6080137
Chicago/Turabian StyleHortigüela, María Jesús, Luis Yuste, Fernando Rojo, and Inmaculada Aranaz. 2016. "Green Synthesis of Hierarchically Structured Silver-Polymer Nanocomposites with Antibacterial Activity" Nanomaterials 6, no. 8: 137. https://doi.org/10.3390/nano6080137