Targeting and Photodynamic Killing of Cancer Cell by Nitrogen-Doped Titanium Dioxide Coupled with Folic Acid
Abstract
:1. Introduction
2. Results
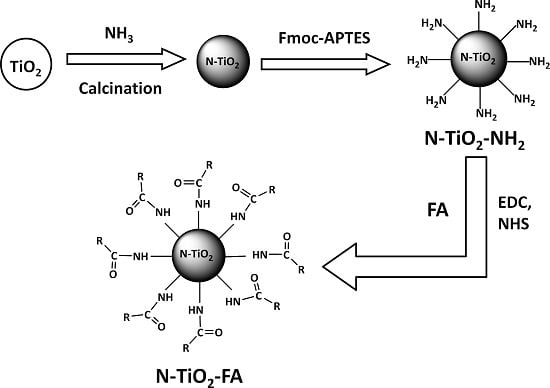
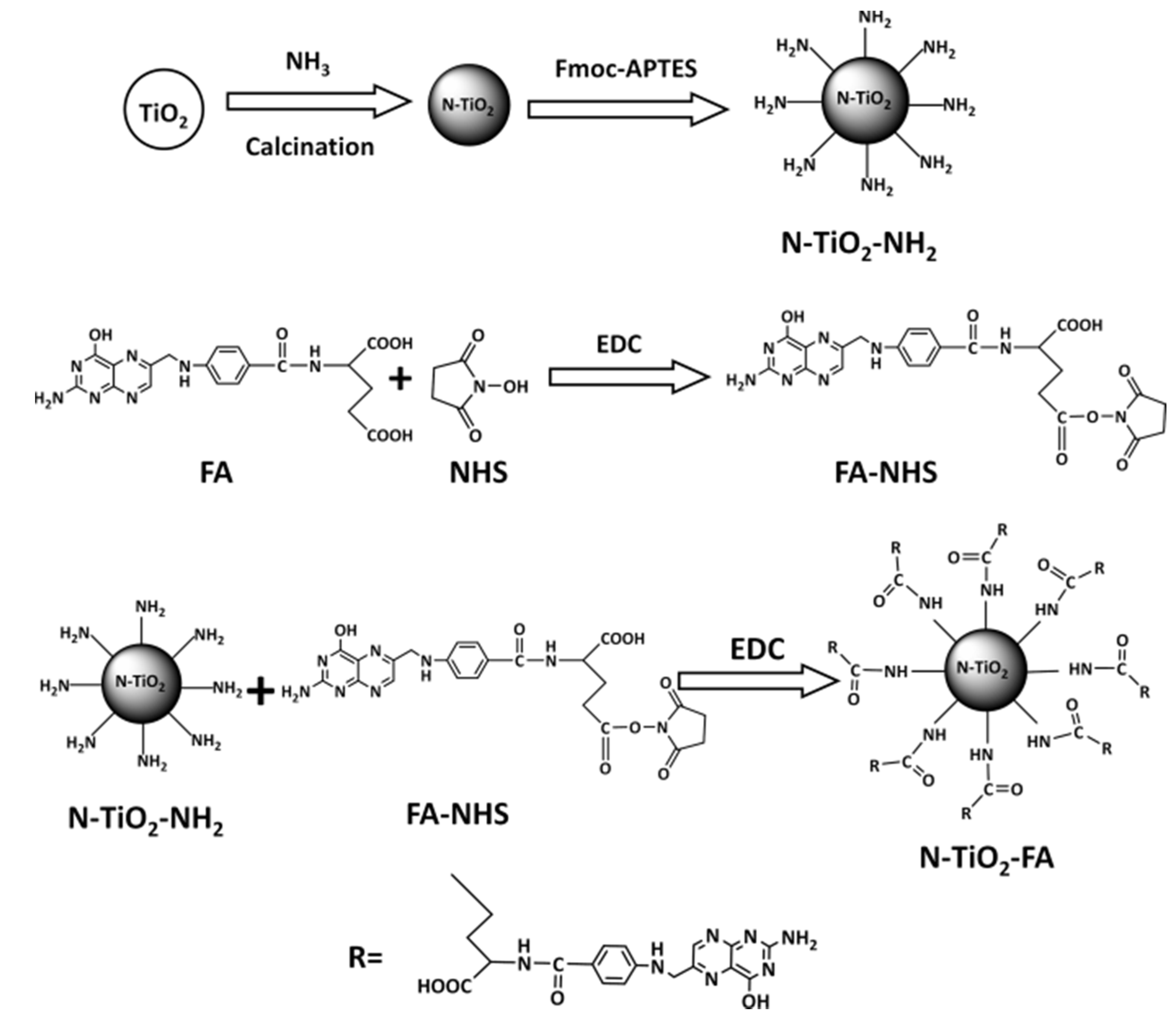
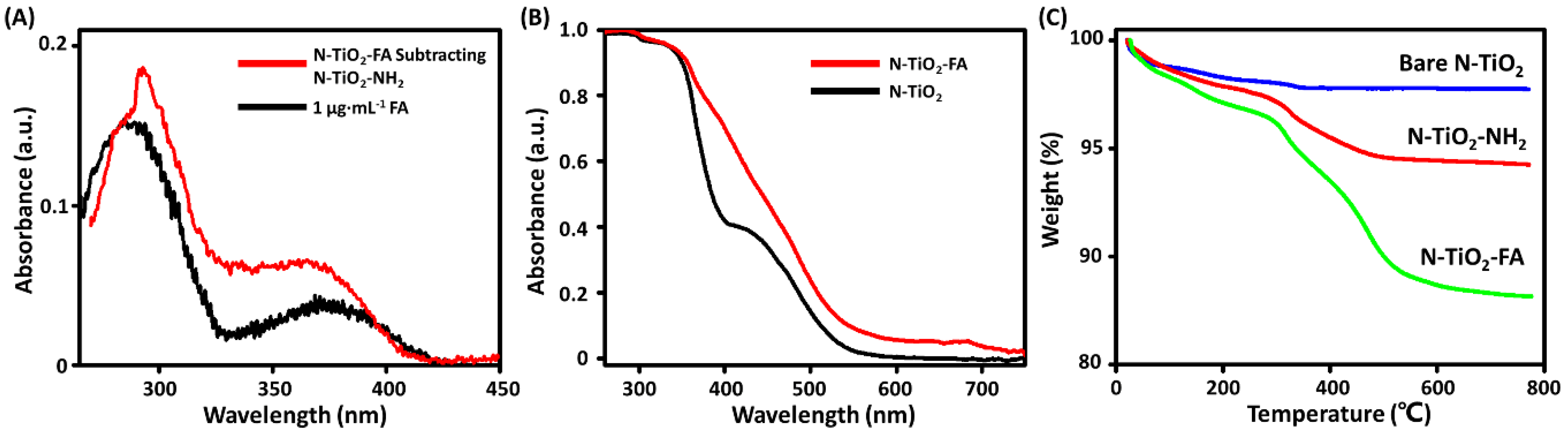
2.1. Preparation and Characterization of N-TiO2-FA
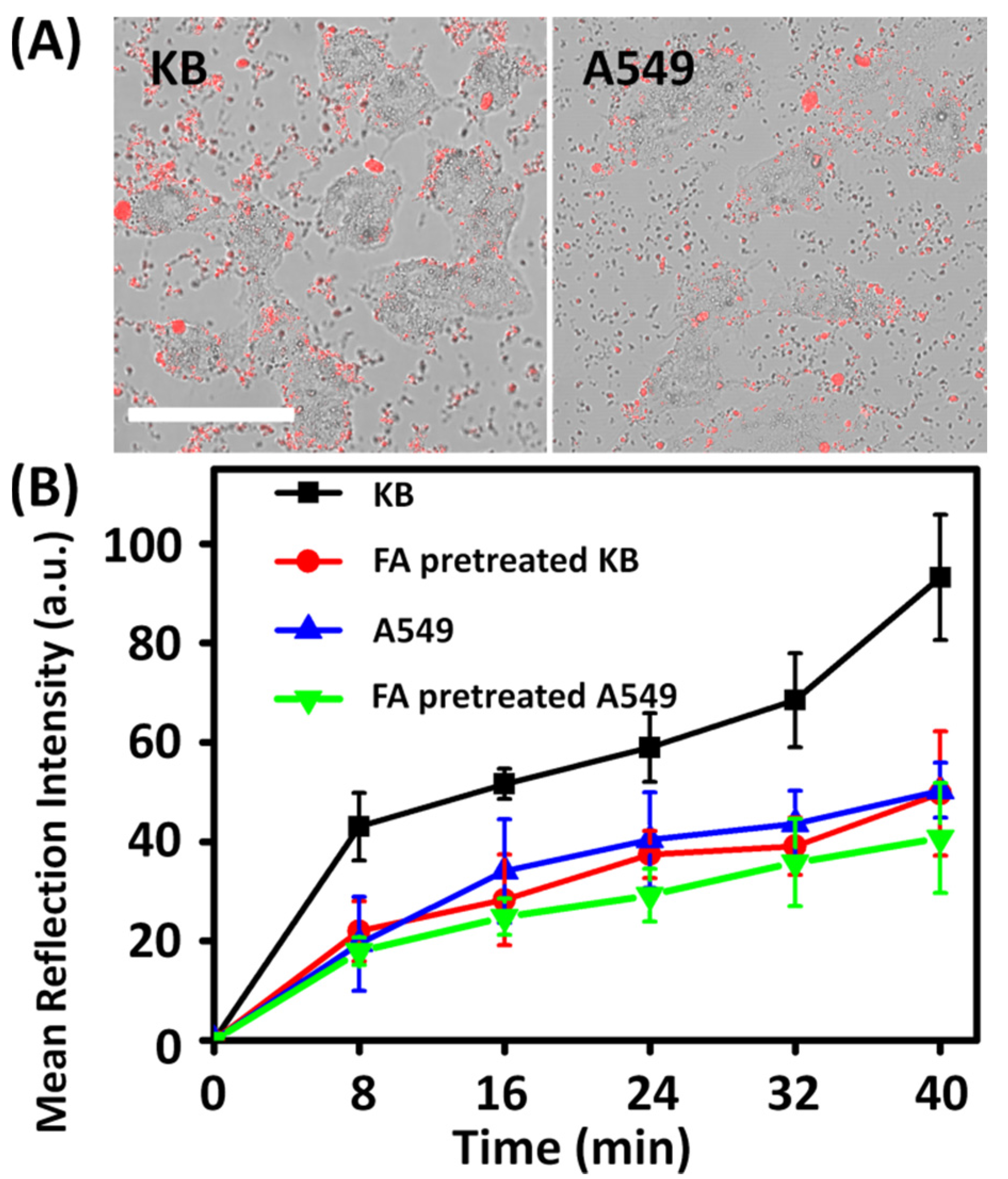
2.2. Cellular Uptake
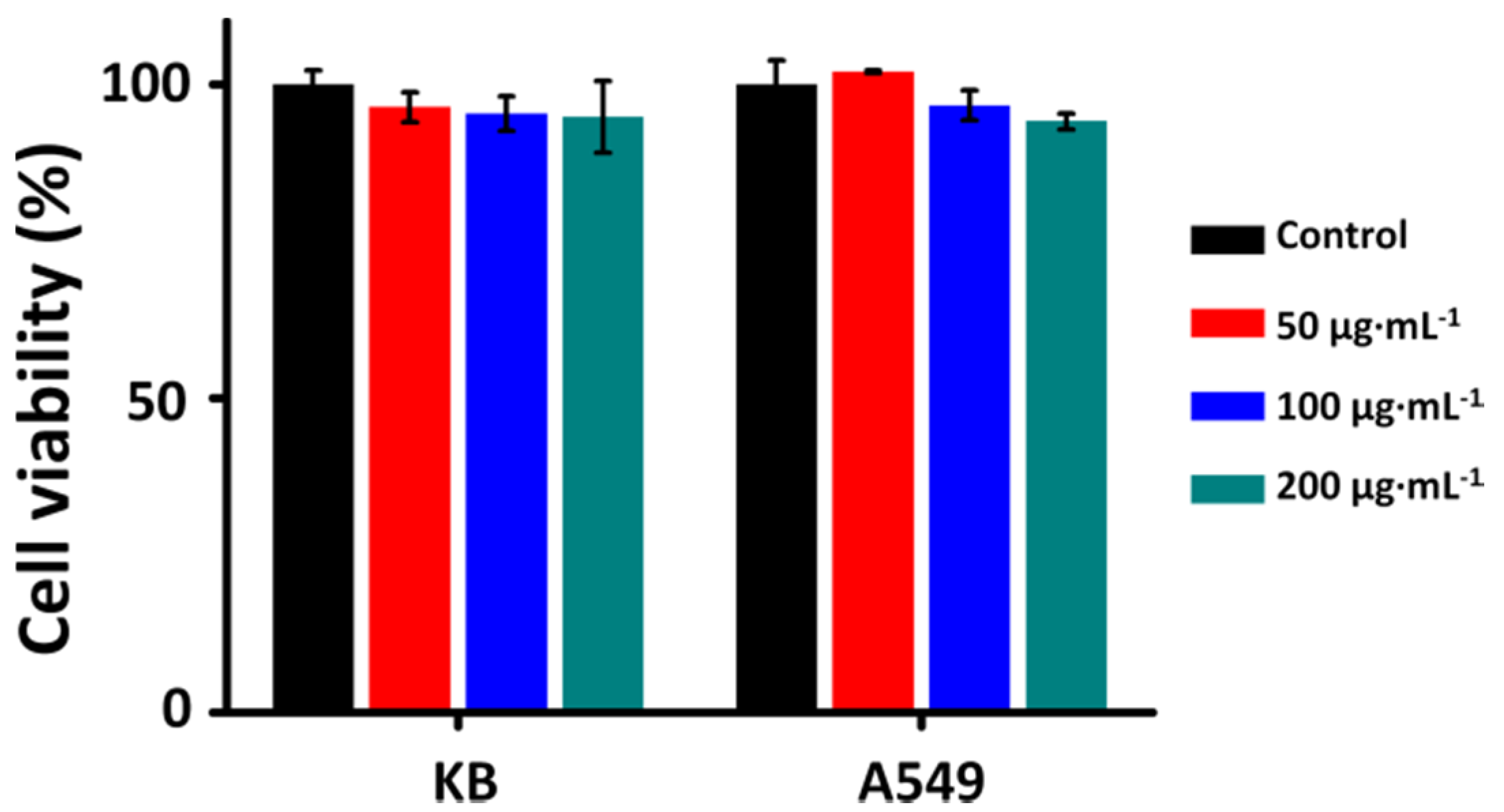
2.3. Cytotoxicity of N-TiO2-FA
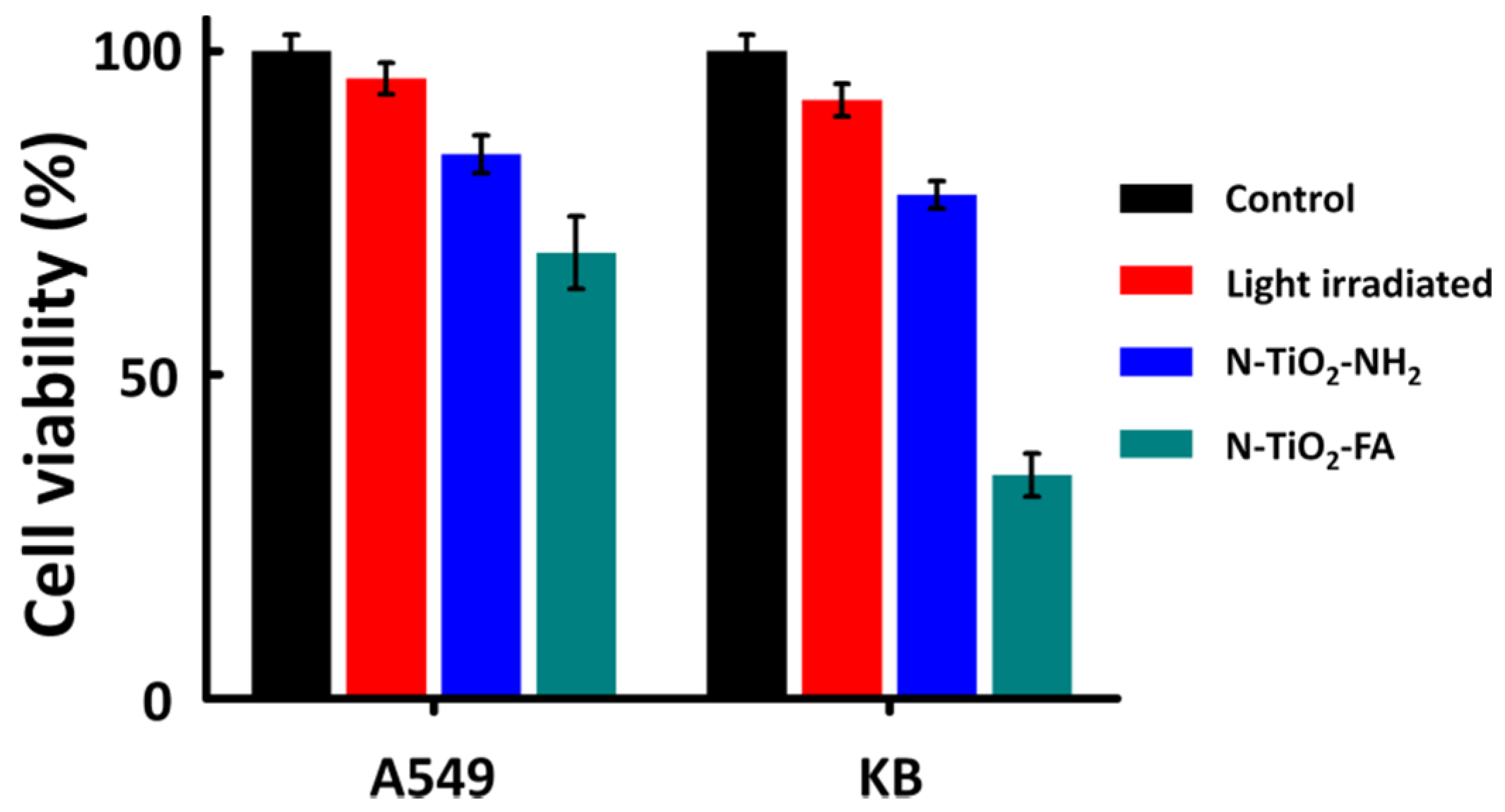
2.4. Targeting and Photokilling Effect on Cancer Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of N-TiO2-FA
4.3. Characterization of Samples
4.4. Cell Culture
4.5. Cellular Uptake Study of N-TiO2-FA
4.6. Measurements of Photokilling Effect and Cytotoxicity
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic therapy. J. Natl. Cancer Inst. 1998, 90, 889–905. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Kamkaew, A.; Lim, S.H.; Lee, H.B.; Kiew, L.V.; Chung, L.Y.; Burgess, K.; Burgess, K. BODIPY dyes in photodynamic therapy. Chem. Soc. Rev. 2013, 42, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Wang, S.J.; Huang, P.; Wang, Z.; Chen, S.H.; Niu, G.; Li, W.W.; He, J.; Cui, D.X.; Lu, G.M. Photosensitizer-loaded gold vesicles with strong plasmonic coupling effect for imaging-guided photothermal/photodynamic therapy. ACS Nano 2013, 7, 5320–5329. [Google Scholar] [CrossRef] [PubMed]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’shea, K. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Shi, H.; Magaye, R.; Castranova, V.; Zhao, J.S. Titanium dioxide nanoparticles: A review of current toxicological data. Part. Fibre Toxicol. 2013, 10. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Mora-Seró, I.; De Angelis, F.; Bisquert, J.; Wang, P. Titanium dioxide nanomaterials for photovoltaic applications. Chem. Rev. 2014, 114, 10095–10130. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Yi, S.G.; Lin, L.W.; Huang, H.; Peng, T.; Liu, Z.T.; Liu, Z.P.; Pei, D.N.; Miao, X.Y.; Wen, Y. Anticancer Effect of Photodynamic Therapy with Photosan-Loaded Titanium Dioxide Nanoparticles on Panc-1 Pancreatic Cancer Cells In Vitro. Sci. Adv. Mat. 2016, 8, 1145–1153. [Google Scholar] [CrossRef]
- Zhang, S.C.; Yang, D.J.; Jing, D.W.; Liu, H.W.; Liu, L.; Jia, Y.; Gao, M.H.; Guo, L.J.; Huo, Z.Y. Enhanced photodynamic therapy of mixed phase TiO2 (B)/anatase nanofibers for killing of HeLa cells. Nano Res. 2014, 7, 1659–1669. [Google Scholar] [CrossRef]
- Yin, Z.F.; Wu, L.; Yang, H.G.; Su, Y.H. Recent progress in biomedical applications of titanium dioxide. Phys. Chem. Chem. Phys. 2013, 15, 4844–4858. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Xie, J.; Li, Z.; Chen, M.; Wang, P.N.; Chen, L.; Mi, L. Enhancement of the photokilling effect of aluminum phthalocyanine in photodynamic therapy by conjugating with nitrogen-doped TiO2 nanoparticles. Colloids Surf. B 2015, 130, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Tokuoka, Y.; Yamada, M.; Kawashima, N.; Miyasaka, T.; Miyasaka, T. Anticancer effect of dye-sensitized TiO2 nanocrystals by polychromatic visible light irradiation. Chem. Lett. 2006, 35, 496–497. [Google Scholar] [CrossRef]
- Li, Z.; Pan, X.; Wang, T.; Wang, P.N.; Chen, J.Y.; Mi, L. Comparison of the killing effects between nitrogen-doped and pure TiO2 on HeLa cells with visible light irradiation. Nanoscale Res. Lett. 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Jańczyk, A.; Wolnicka-Głubisz, A.; Urbanska, K.; Stochel, G.; Macyk, W. Photocytotoxicity of platinum (IV)-chloride surface modified TiO2 irradiated with visible light against murine macrophages. J. Photochem. Photobiol. B 2008, 92, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Parker, N.; Turk, M.J.; Westrick, E.; Lewis, J.D.; Low, P.S.; Leamon, C.P. Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal. Biochem. 2005, 338, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Hilgenbrink, A.R.; Low, P.S. Folate receptor-mediated drug targeting: From therapeutics to diagnostics. J. Pharm. Sci. 2005, 94, 2135–2146. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Li, J.; Li, C.; Zhao, S.; Li, N.; Wang, Y.; Wei, F.; Chen, L.; Huang, Y. Folic acid-conjugated graphene–ZnO nanohybrid for targeting photodynamic therapy under visible light irradiation. J. Mater. Chem. B 2013, 1, 5003–5013. [Google Scholar] [CrossRef]
- Huang, P.; Bao, L.; Zhang, C.; Lin, J.; Luo, T.; Yang, D.; He, M.; Li, Z.; Gao, G.; Gao, B. Folic acid-conjugated silica-modified gold nanorods for X-ray/CT imaging-guided dual-mode radiation and photo-thermal therapy. Biomaterials 2011, 32, 9796–9809. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.Y.; Lee, W.C. Killing of cancer cell line by photoexcitation of folic acid-modified titanium dioxide nanoparticles. J. Photochem. Photobiol. A 2009, 204, 148–153. [Google Scholar] [CrossRef]
- Li, Z.; Mi, L.; Wang, P.N.; Chen, J. Study on the visible-light-induced photokilling effect of nitrogen-doped TiO2 nanoparticles on cancer cells. Nanoscale Res. Lett. 2011, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Pelton, R.; Brook, M.A. Biotinylation of TiO2 nanoparticles and their conjugation with streptavidin. Langmuir 2007, 23, 5630–5637. [Google Scholar] [CrossRef] [PubMed]
- Hermanson, G. Bioconjugate Techniques; Acad Press: San Diego, CA, USA; New York, NY, USA; Boston, MA, USA, 1996; pp. 173–177. [Google Scholar]
- Yoo, H.S.; Park, T.G. Folate-receptor-targeted delivery of doxorubicin nano-aggregates stabilized by doxorubicin–PEG–folate conjugate. J. Control. Release 2004, 100, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Pan, X.; Wang, M.; Ma, J.; Fei, Y.; Wang, P.N.; Mi, L. The role of surface modification for TiO2 nanoparticles in cancer cells. Colloids Surf. B 2016, 143, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Cao, S.; Tie, X.; Qiu, B.; Wu, A.; Zheng, Z. Induction of cytotoxicity by photoexcitation of TiO2 can prolong survival in glioma-bearing mice. Mol. Biol. Rep. 2011, 38, 523–530. [Google Scholar] [CrossRef] [PubMed]
- You, D.G.; Deepagan, V.G.; Um, W.; Jeon, S.; Son, S.; Chang, H.; Yoon, H.I.; Cho, Y.W.; Swierczewska, M.; Lee, S.; et al. ROS-generating TiO2 nanoparticles for non-invasive sonodynamic therapy of cancer. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhao, Z.; Lv, Y.; Fan, H.; Bai, H.; Meng, H.; Long, Y.; Fu, T.; Zhang, X.; Tan, W. Gold nanorod-photosensitizer conjugate with extracellular pH-driven tumor targeting ability for photothermal/photodynamic therapy. Nano Res. 2014, 7, 1291–1301. [Google Scholar] [CrossRef]
- Khlebtsov, N.; Dykman, L. Biodistribution and toxicity of engineered gold nanoparticles: A review of in vitro and in vivo studies. Chem. Soc. Rev. 2011, 40, 1647–1671. [Google Scholar] [CrossRef] [PubMed]
- Jang, B.; Choi, Y. Photosensitizer-conjugated gold nanorods for enzyme-activatable fluorescence imaging and photodynamic therapy. Theranostics 2012, 2, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Jang, B.; Park, J.Y.; Tung, C.H.; Kim, I.H.; Choi, Y. Gold nanorod−photosensitizer complex for near-infrared fluorescence imaging and photodynamic/photothermal therapy in vivo. ACS Nano 2011, 5, 1086–1094. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, J.; Pan, X.; Wang, M.; Yao, L.; Liang, X.; Ma, J.; Fei, Y.; Wang, P.-N.; Mi, L. Targeting and Photodynamic Killing of Cancer Cell by Nitrogen-Doped Titanium Dioxide Coupled with Folic Acid. Nanomaterials 2016, 6, 113. https://doi.org/10.3390/nano6060113
Xie J, Pan X, Wang M, Yao L, Liang X, Ma J, Fei Y, Wang P-N, Mi L. Targeting and Photodynamic Killing of Cancer Cell by Nitrogen-Doped Titanium Dioxide Coupled with Folic Acid. Nanomaterials. 2016; 6(6):113. https://doi.org/10.3390/nano6060113
Chicago/Turabian StyleXie, Jin, Xiaobo Pan, Mengyan Wang, Longfang Yao, Xinyue Liang, Jiong Ma, Yiyan Fei, Pei-Nan Wang, and Lan Mi. 2016. "Targeting and Photodynamic Killing of Cancer Cell by Nitrogen-Doped Titanium Dioxide Coupled with Folic Acid" Nanomaterials 6, no. 6: 113. https://doi.org/10.3390/nano6060113