Biosynthesis of Metal Nanoparticles: Novel Efficient Heterogeneous Nanocatalysts
Abstract
:1. Introduction
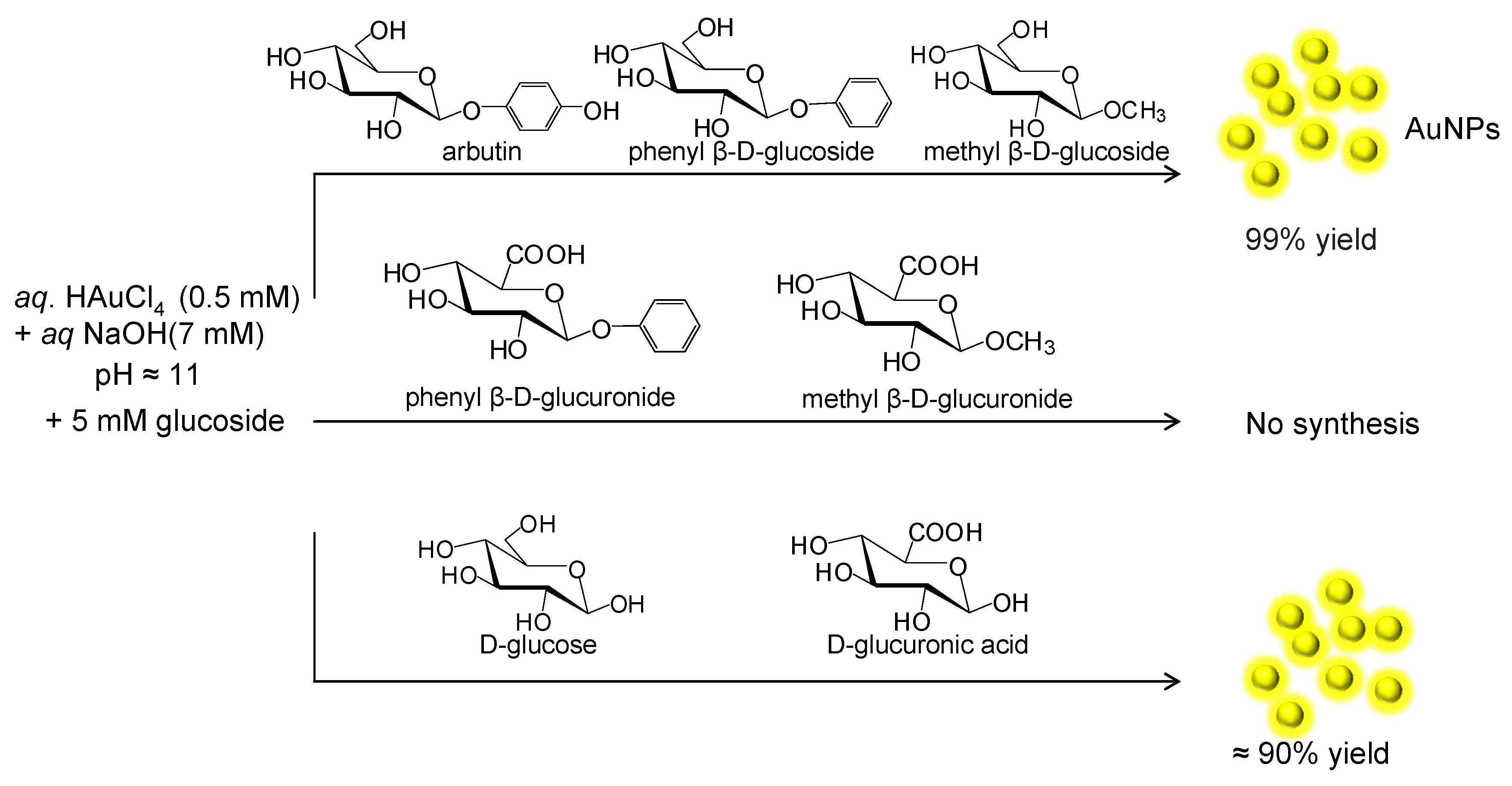
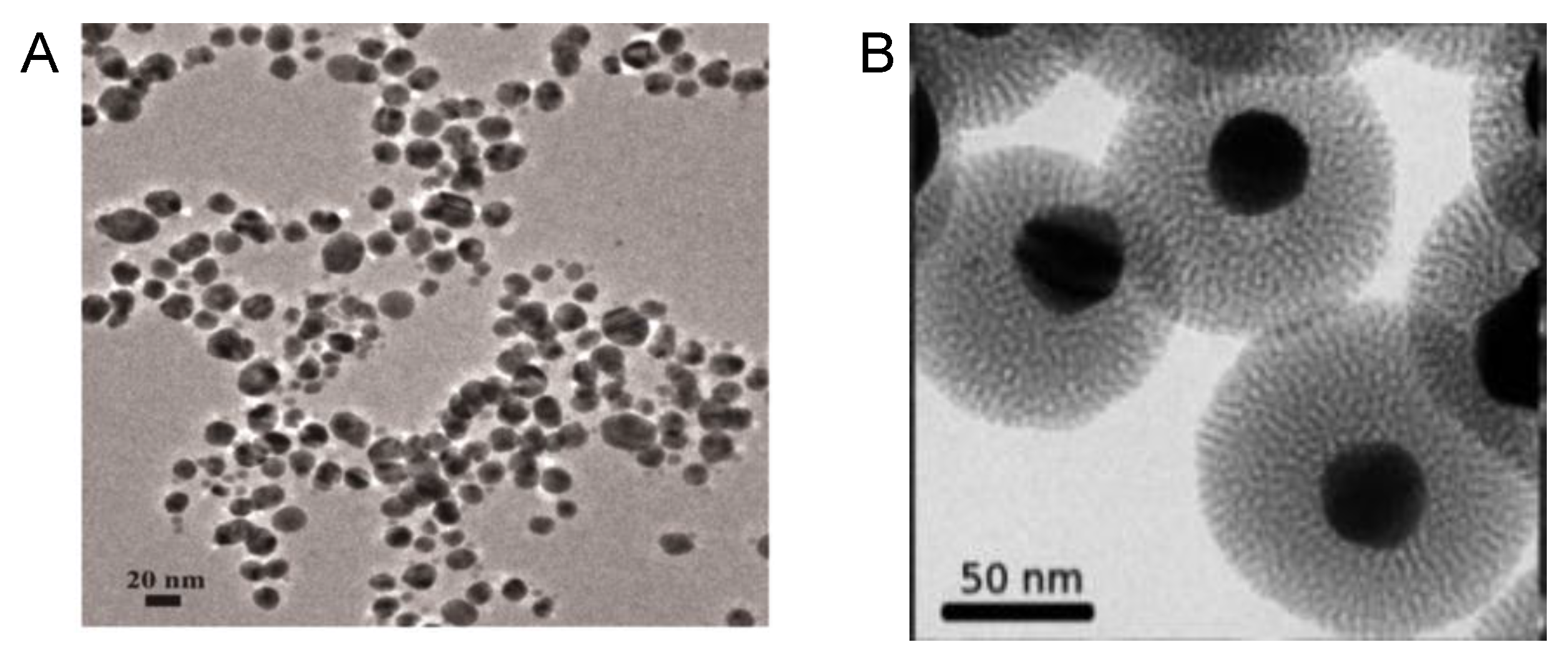
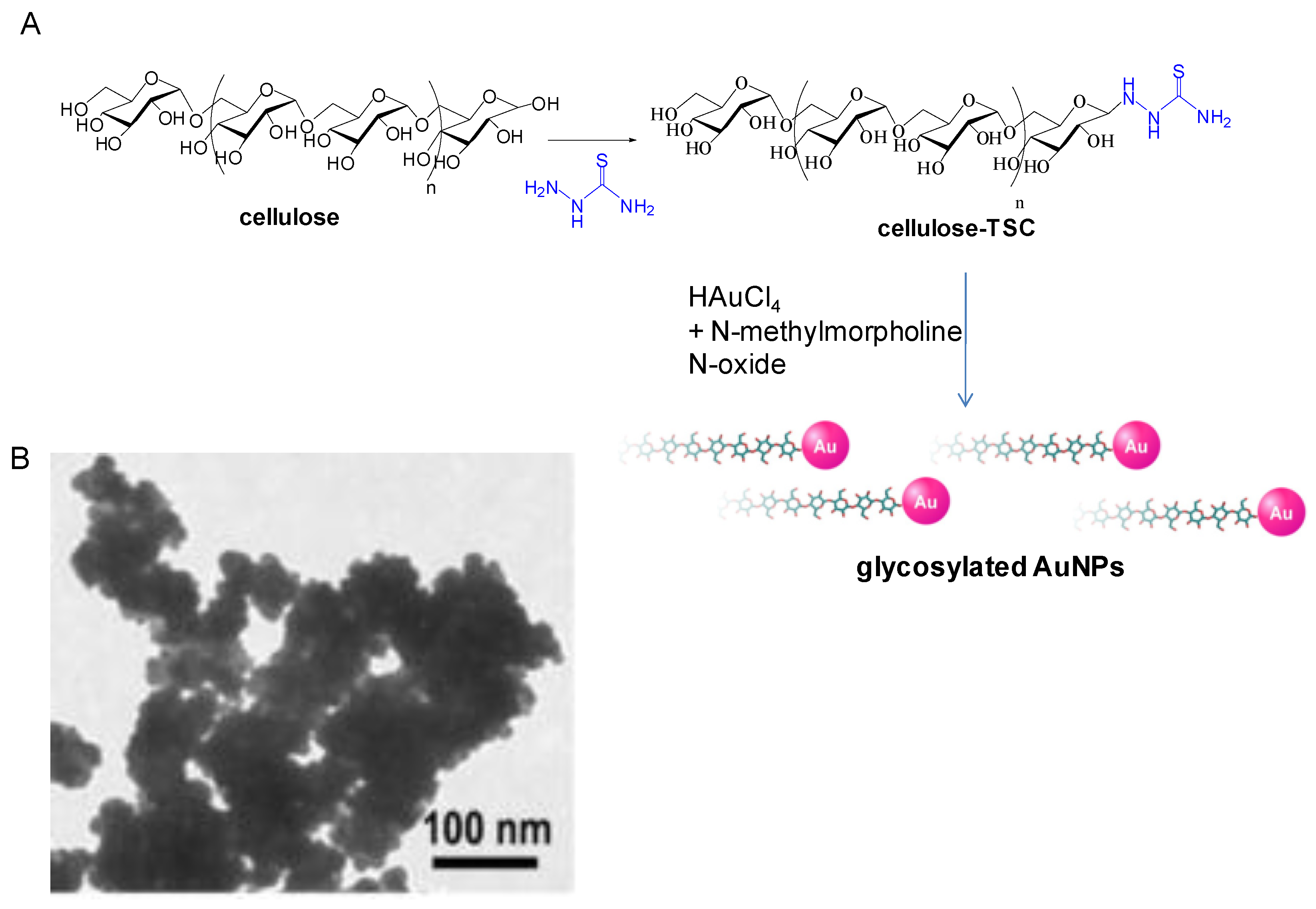
2. Synthesis of Metal Nanoparticles Induced by Glucosides
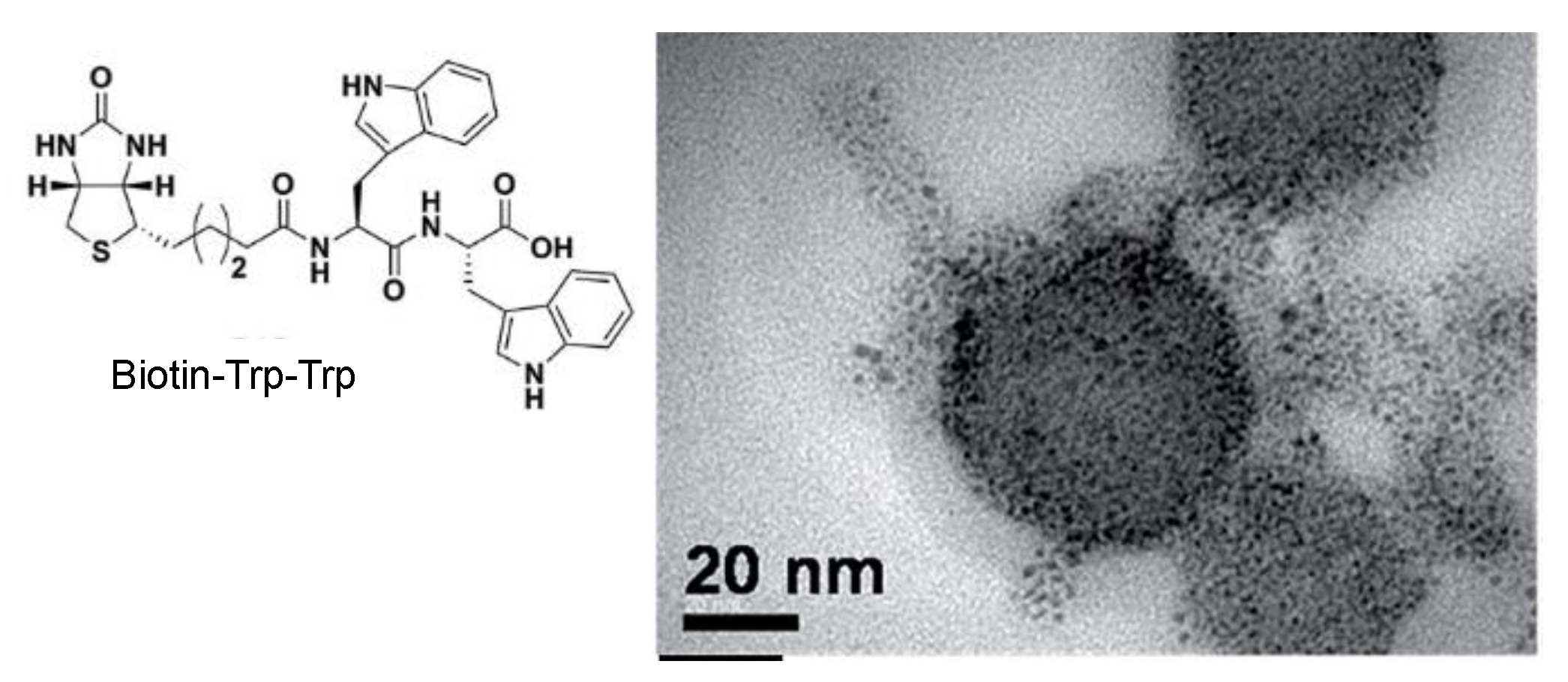
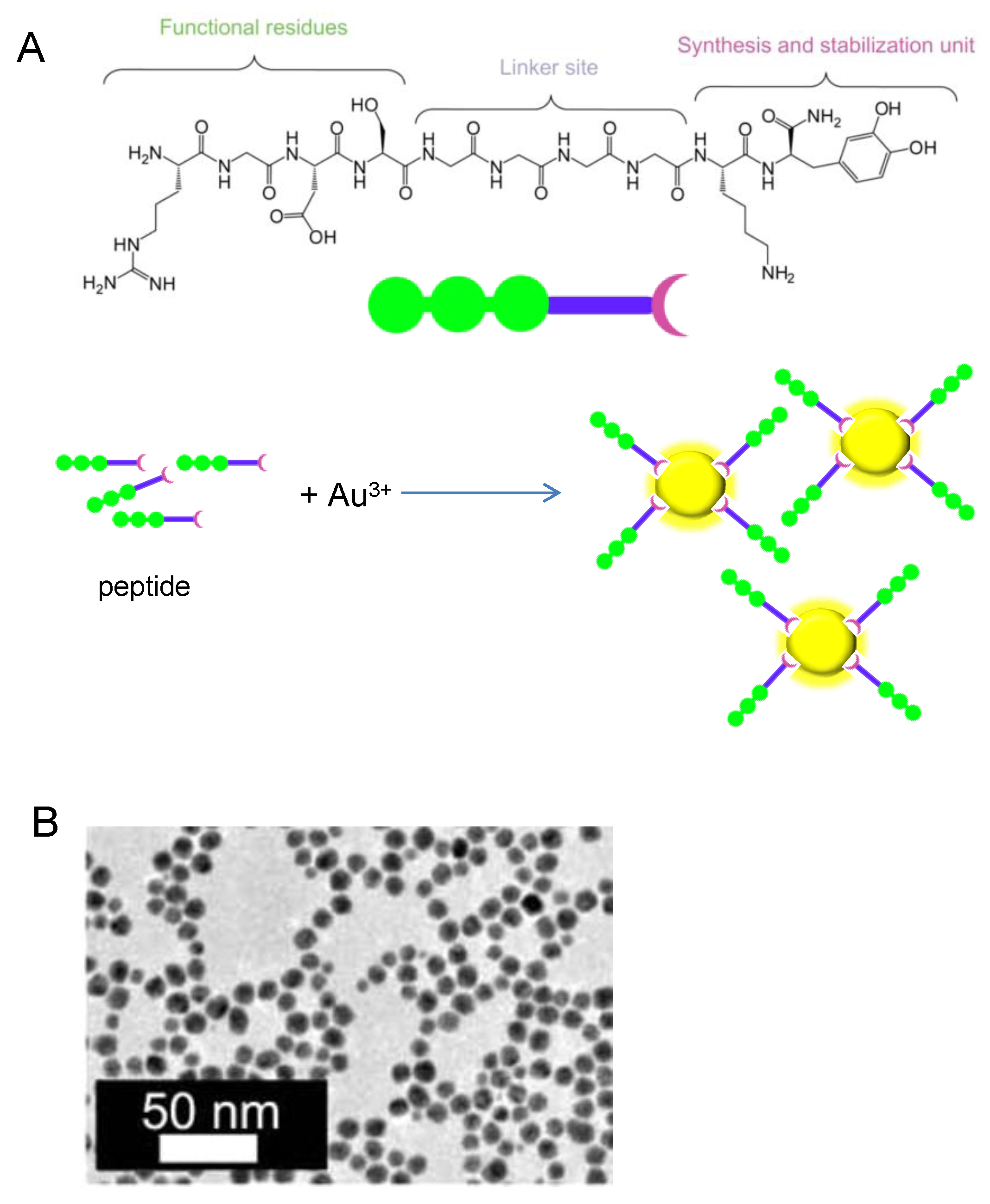
3. Biosynthesis of Metal Nanoparticles by Peptides
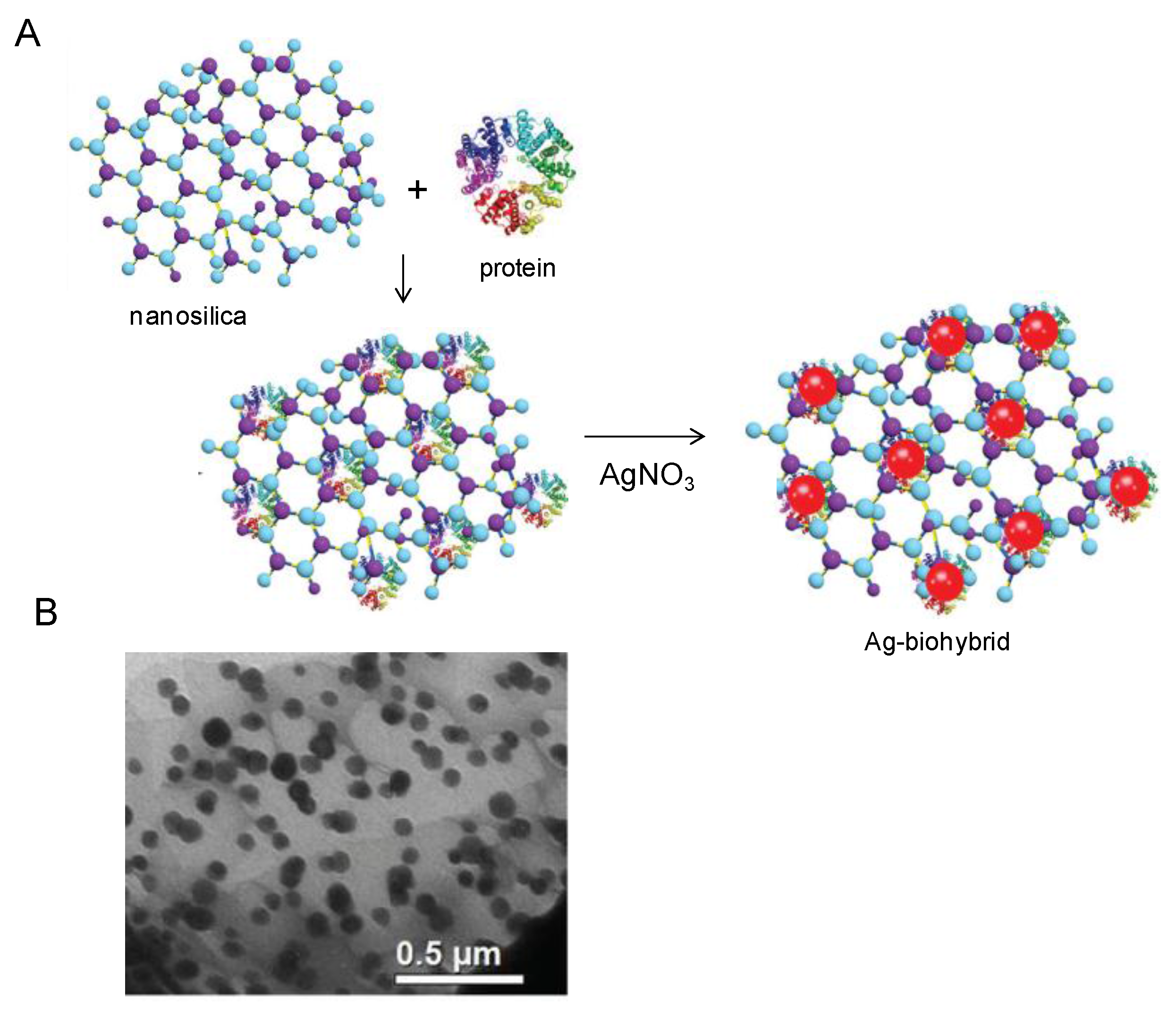
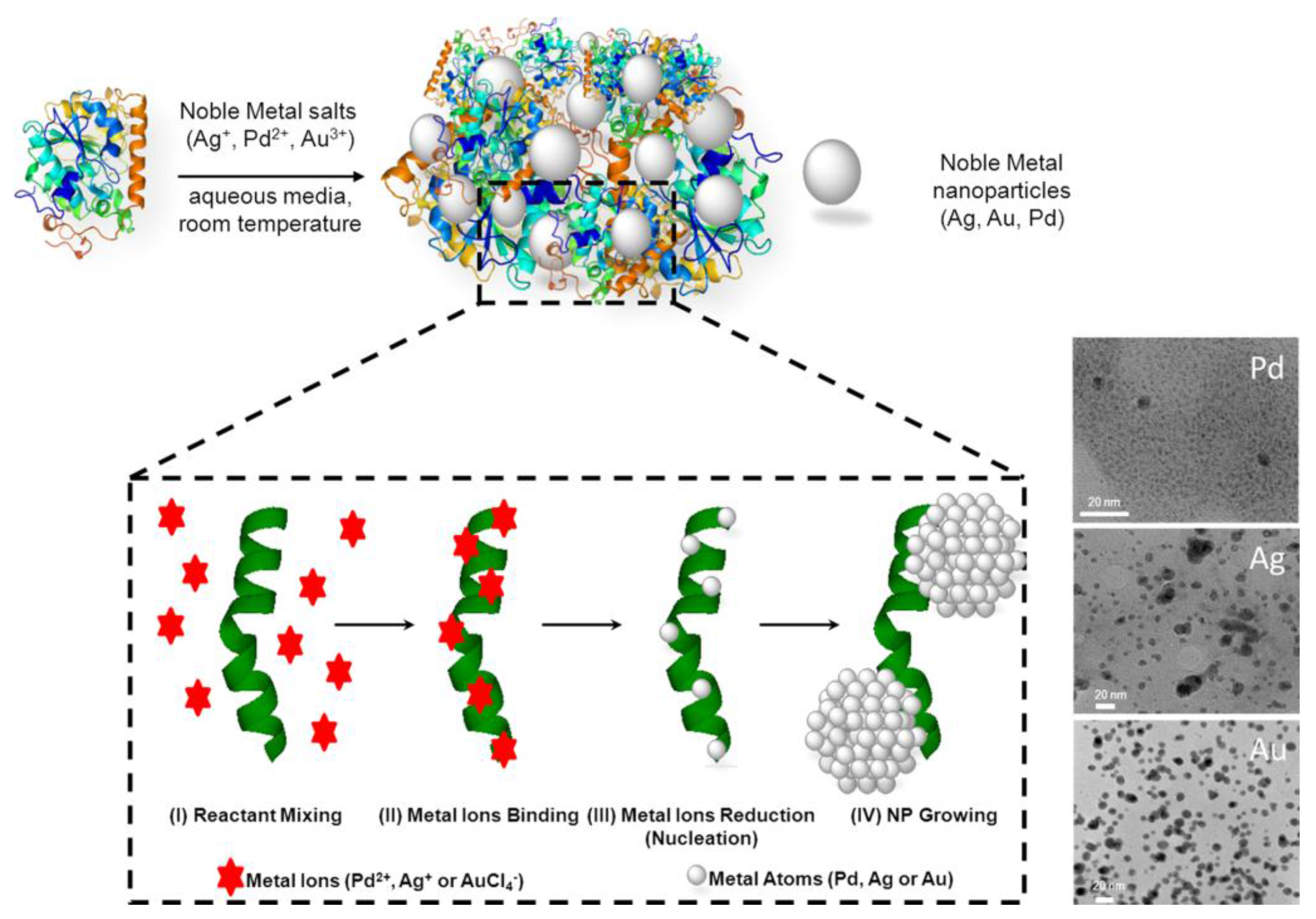
4. Bio-Inspired Synthesis of Nanoparticles by Proteins
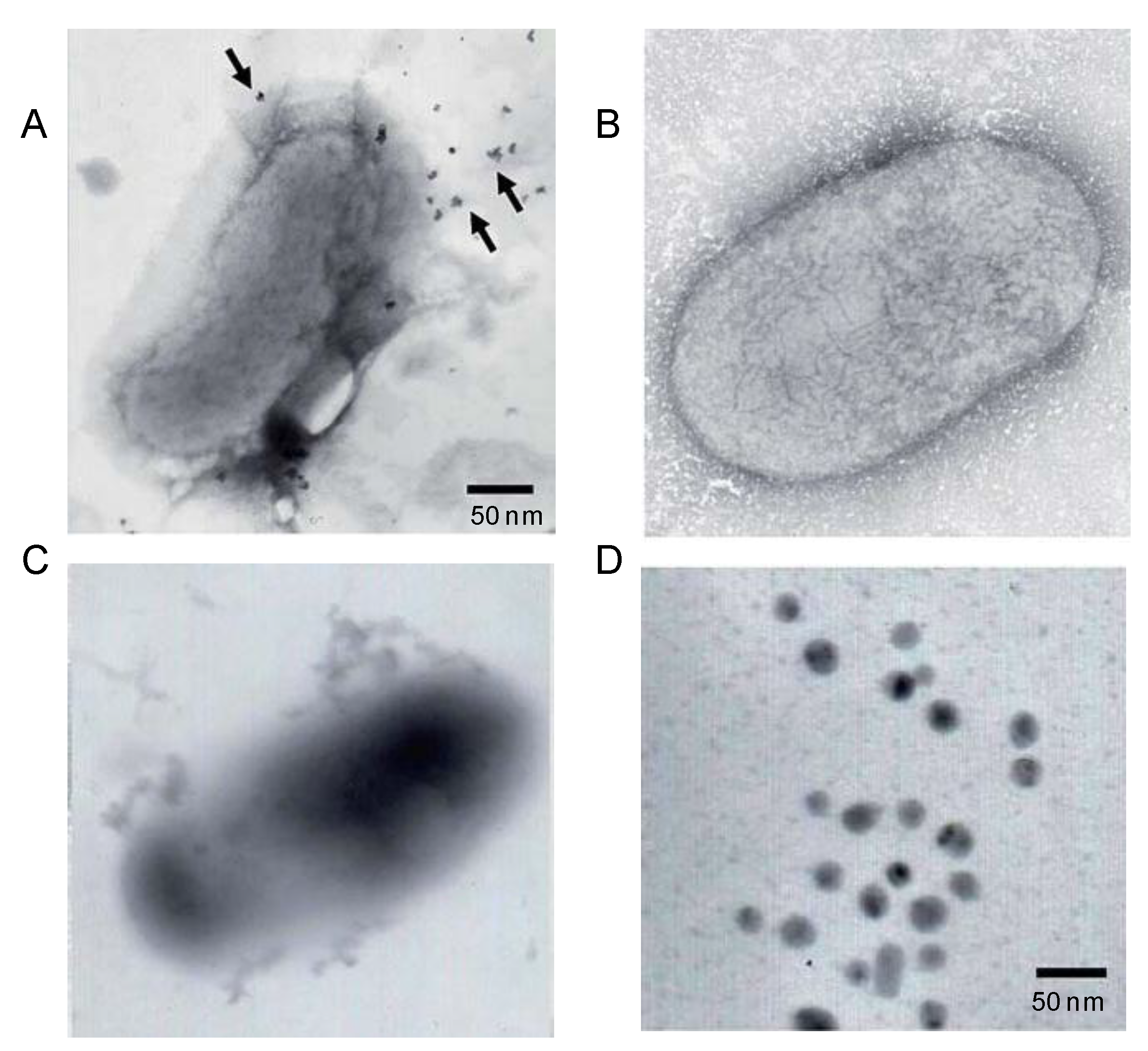
5. Biosynthesis of Metal Nanoparticles by Microorganisms
6. Biosynthesis of Metal Nanoparticles by Plant Extracts
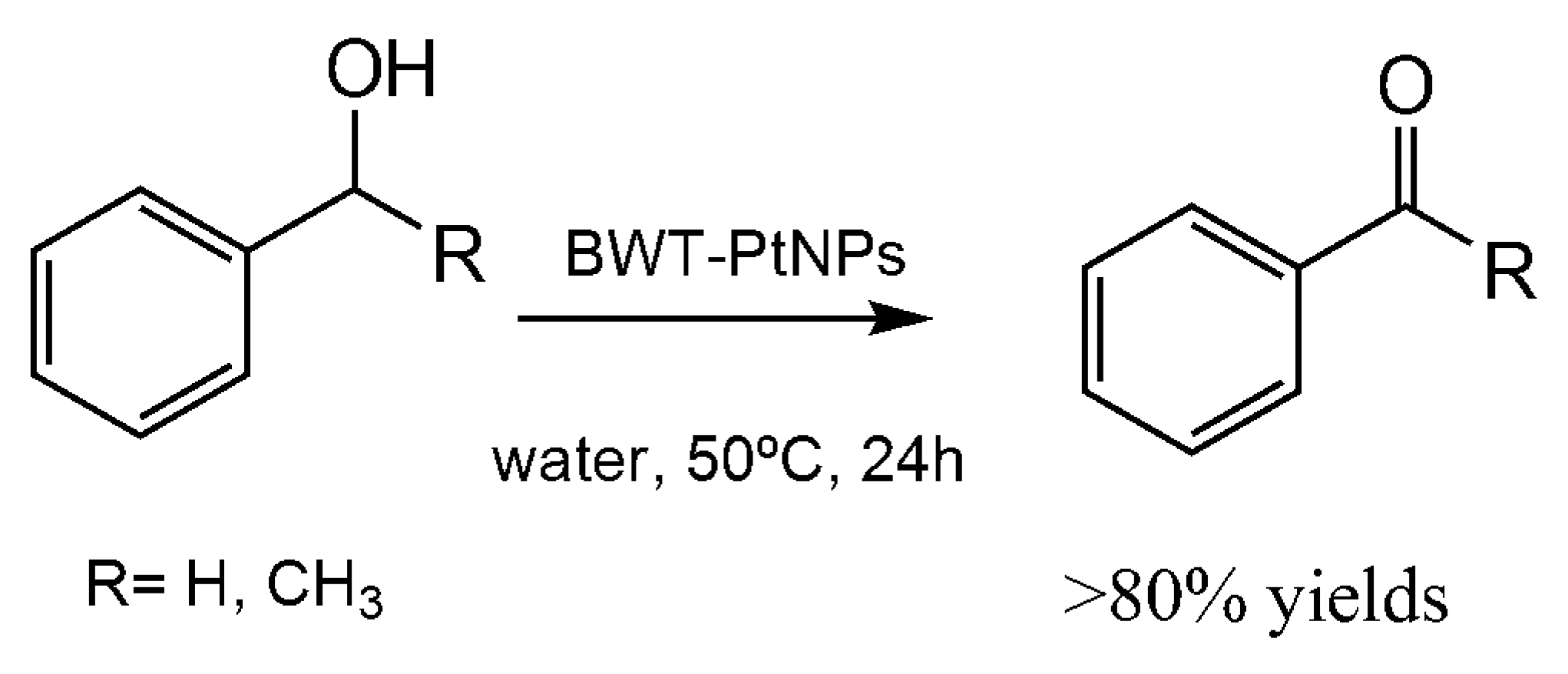
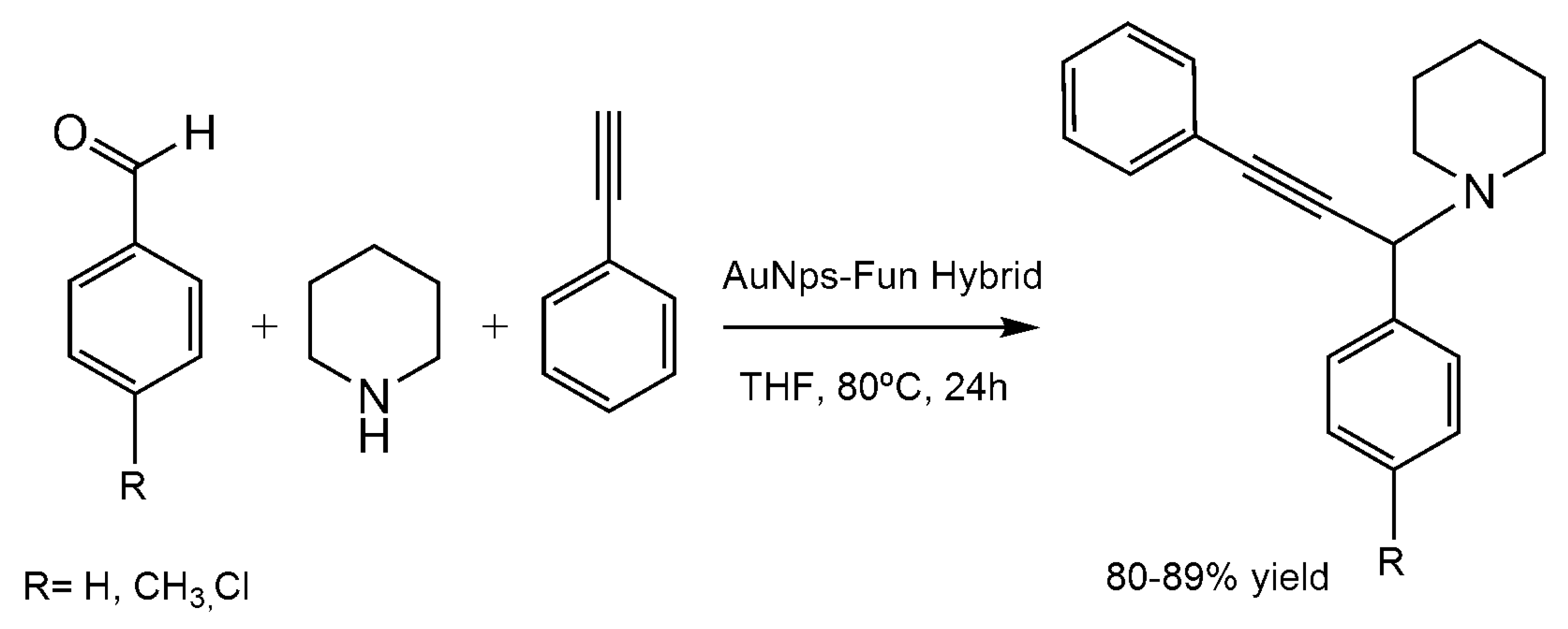
7. Application of the Biosynthesized Metallic NPs as Nanocatalysts
8. Conclusions
Acknowledgments
Conflicts of Interest
References
- Zhang, F.; Nangreave, J.; Liu, Y.; Yan, H. Structural DNA nanotechnology: State of the art and future perspective. J. Am. Chem. Soc. 2014, 136, 11198–11211. [Google Scholar] [CrossRef] [PubMed]
- Mo, R.; Jiang, T.; Di, J.; Tai, W.; Gu, Z. Emerging micro- and nanotechnology based synthetic approaches for insulin delivery. Chem. Soc. Rev. 2014, 43, 3595–3629. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zheng, Y.; Munro, C.J.; Ji, Y.; Braunschweig, A.B. Carbohydrate nanotechnology: Hierarchical assembly using nature’s other information carrying biopolymers. Curr. Opin. Biotechnol. 2015, 34, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Wang, Y.; Liu, B.; Yang, Y. Metallic nanocatalysis: An accelerating seamless integration with nanotechnology. Small 2015, 11, 268–289. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Gao, X.; Liu, D.; Chen, X. Gold Nanoparticles for in Vitro Diagnostics. Chem. Rev. 2015, 115, 10575–10636. [Google Scholar] [CrossRef] [PubMed]
- DaCosta, M.V.; Doughan, S.; Han, Y.; Krull, U.J. Lanthanide upconversion nanoparticles and applications in bioassays and bioimaging: A review. Anal. Chim. Act. 2014, 832, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Sadat, M.E.; Dunn, A.W.; Mast, D.B. Photo-fluorescent and magnetic properties of iron oxide nanoparticles for biomedical applications. Nanoscale 2015, 7, 8209–8232. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M.S. Immunoengineering: How nanotechnology can enhance cancer immunotherapy. Cell 2015, 161, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.-Y.; Gao, Z.-W.; Yang, K.-F.; Zhang, W.-Q.; Xu, L.-W. Nanosilver as a new generation of silver catalysts in organic transformations for efficient synthesis of fine chemicals. Catal. Sci. Technol. 2015, 5, 2554–2574. [Google Scholar] [CrossRef]
- Filice, M.; Palomo, J.M. Cascade reactions catalyzed by bionanostructures. ACS Catal. 2014, 4, 1588–1598. [Google Scholar] [CrossRef]
- Serna, P.; Corma, A. Transforming nano metal nonselective particulates into chemoselective catalysts for hydrogenation of substituted nitrobenzenes. ACS Catal. 2015, 5, 7114–7121. [Google Scholar] [CrossRef]
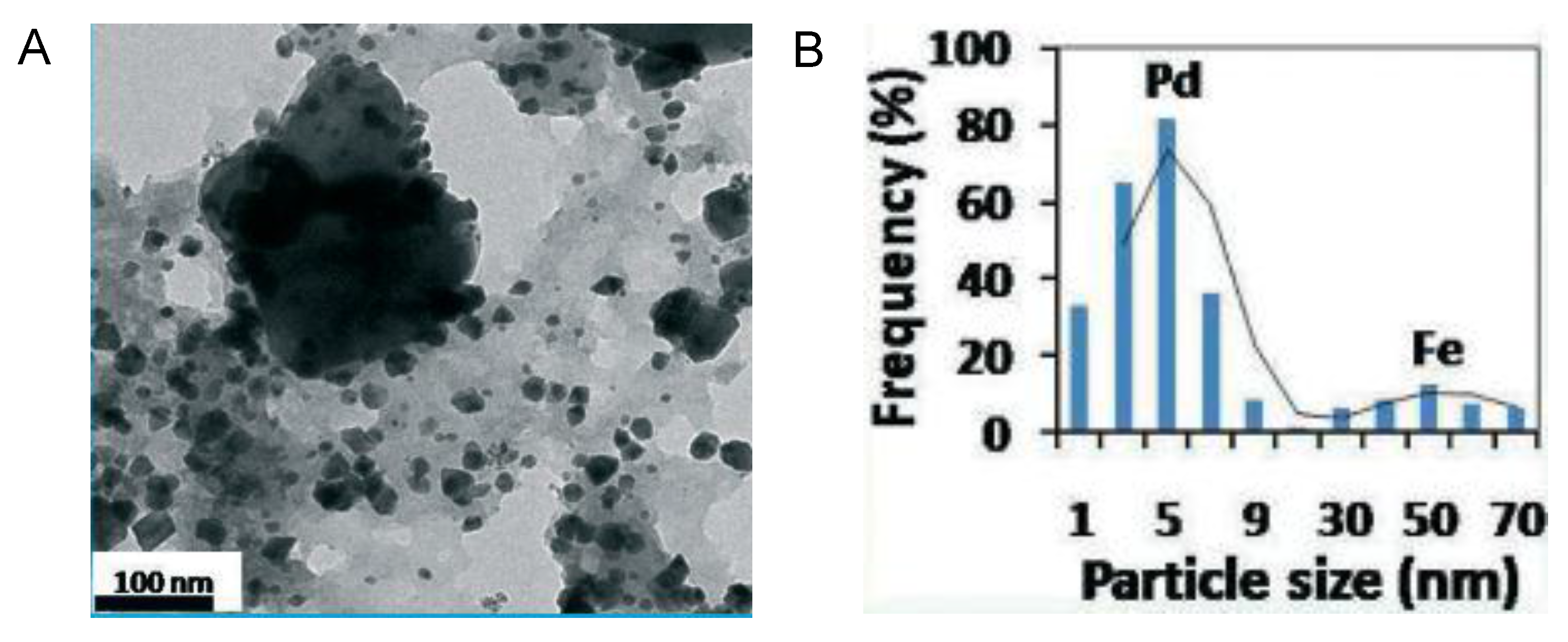
- Mishra, K.; Basavegowda, N.; Lee, Y.R. Biosynthesis of Fe, Pd, and Fe–Pd bimetallic nanoparticles and their application as recyclable catalysts for [3 + 2] cycloaddition reaction: A comparative approach. Catal. Sci. Technol. 2015, 5, 2612–2621. [Google Scholar] [CrossRef]
- Aditya, T.; Pal, A.; Pal, T. Nitroarene reduction: A trusted model reaction to test nanoparticle catalysts. Chem. Commun. 2015, 51, 9410–9431. [Google Scholar] [CrossRef] [PubMed]
- MubarakAli, D.; Gopinath, V.; Rameshbabu, N.; Thajuddin, N. Synthesis and characterization of CdS nanoparticles using C-phycoerythrin from the marine cyanobacteria. Mater. Lett. 2012, 74, 8–11. [Google Scholar] [CrossRef]
- Saldan, I.; Semenyuk, Y.; Marchuk, I.; Reshetnyak, O. Chemical synthesis and application of palladium nanoparticles. J. Mat. Sci. 2015, 50, 2337–2354. [Google Scholar] [CrossRef]
- Gutiérrez, L.; Costo, R.; Grüttner, C.; Westphal, F.; Gehrke, N.; Heinke, D.; Fornara, A.; Pankhurst, Q.A.; Johansson, C.; Veintemillas-Verdaguer, S.; et al. Synthesis methods to prepare single- and multi-core iron oxide nanoparticles for biomedical applications. Dalton Trans. 2015, 44, 2943–2952. [Google Scholar] [CrossRef] [PubMed]
- Shervani, Z.; Yamamoto, Y. Carbohydrate-directed synthesis of silver and gold nanoparticles: Effect of the structure of carbohydrates and reducing agents on the size and morphology of the composites. Carbohydr. Res. 2011, 346, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Yokota, S.; Kitaoka, T.; Opietnik, M.; Rosenau, T.; Wariishi, H. Synthesis of gold nanoparticles for in situ conjugation with structural carbohydrates. Angew. Chem. Int. Ed. 2008, 47, 9866–9869. [Google Scholar] [CrossRef] [PubMed]
- Engelbrekt, C.; Sørensen, K.H.; Zhang, J.; Welinder, A.C.; Jensen, P.S.; Ulstrup, J. Green synthesis of gold nanoparticles with starch–glucose and application in bioelectrochemistry. J. Mater. Chem. 2009, 19, 7839–7847. [Google Scholar] [CrossRef]
- Care, A.; Bergquist, P.L.; Sunna, A. Solid-binding peptides: Smart tools for nanobiotechnology. Trends Biotechnol. 2015, 33, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.N.; Lee, J.Y.; Wang, D.I.C. Uncovering the design rules for peptide synthesis of metal nanoparticles. J. Am. Chem. Soc. 2010, 132, 5677–5686. [Google Scholar] [CrossRef] [PubMed]
- Filice, M.; Marciello, M.; Morales, M.P.; Palomo, J.M. Synthesis of heterogeneous enzyme-metal nanoparticle biohybrids in aqueous media and their applications in C–C bond formation and tandem catalysis. Chem. Commun. 2013, 49, 6876–6878. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.K.; Chisti, Y.; Banerjee, U.C. Synthesis of metallic nanoparticles using plant extracts. Biotechnol. Adv. 2013, 31, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Chinnadayyala, S.R.; Santhosh, M.; Singh, N.K.; Goswami, P. Alcohol oxidase protein mediated in situ synthesized and stabilized gold nanoparticles for developing amperometric alcohol biosensor. Biosen. Bioelec. 2015, 69, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Hulkoti, N.I.; Taranath, T.C. Biosynthesis of nanoparticles using microbes—A review. Colloids Surf. B 2014, 121, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Mashwani, Z.-U.-R.; Khan, T.; Khan, M.A.; Nadhman, A. Synthesis in plants and plant extracts of silver nanoparticles with potent antimicrobial properties: Current status and future prospects. App. Microb. Biotechnol. 2015, 99, 9923–9934. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.A.; Hassan, S.A.; Knittel, L.L.; Balbo, A.; Aronova, M.A.; Brown, P.H.; Schuck, P.; Leapman, R.D. Biointeractions of ultrasmall glutathione-coated gold nanoparticles: Effect of small size variations. Nanoscale 2016, 8, 6577–6588. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Park, S.; Hong, S.; Ha, M.W.; Park, H.-G.; Lee, H.-J.; Park, Y.; Park, Y. Synthesis of gold nanoparticles with glycosides: Synthetic trends based on the structures of glycones and aglycones. Carbohydr. Res. 2014, 386, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Raman, R.P.; Parthiban, S.; Srinithya, B.; Vinod, V.; Savarimuthu, K.; Anthony, P.; Sivasubramanian, A.; Muthuraman, M.S. Biogenic silver nanoparticles synthesis using the extract of the medicinal plant Clerodendron serratum and its in vitro antiproliferative Activity. Mat. Lett. 2015, 160, 400–403. [Google Scholar] [CrossRef]
- Saint-Cricq, P.; Wang, J.; Sugawara-Narutaki, A.; Shimojima, A.; Okubo, T. A new synthesis of well-dispersed, core–shell Ag@SiO mesoporous nanoparticles using amino acids and sugars. J. Mater. Chem. B 2013, 1, 2451–2454. [Google Scholar] [CrossRef]
- Kitaoka, T.; Yokota, S.; Opietnik, M.; Rosenau, T. Synthesis and bio-applications of carbohydrate–gold nanoconjugates with nanoparticle and nanolayer forms. Mat. Sci. Eng. C 2011, 31, 1221–1229. [Google Scholar] [CrossRef]
- Mishra, N.K.; Kumar, V.; Joshi, K.B. Fabrication of gold nanoparticles on biotin-ditryptophan scaffold for plausible biomedical applications. RSC Adv. 2015, 5, 64387–64394. [Google Scholar] [CrossRef]
- Kracht, S.; Messerer, M.; Lang, M.; Eckhardt, S.; Lauz, M.; Grobty, B.; Fromm, K.M.; Giese, B. Electron Transfer in Peptides: On the Formation of Silver Nanoparticles. Angew. Chem. Int. Ed. 2015, 54, 2912–2916. [Google Scholar] [CrossRef] [PubMed]
- Gulsuner, H.U.; Ceylan, H.; Guler, M.O.; Tekinay, A.B. Multi-domain short peptide molecules for in situ synthesis and biofunctionalization of gold nanoparticles for integrin-targeted cell uptake. ACS Appl. Mater. Interfaces 2015, 7, 10677–10683. [Google Scholar] [CrossRef] [PubMed]
- Belser, K.; Slenters, T.V.; Ofumbidzai, C.; Upert, G.; Mirolo, L.; Fromm, K.M.; Wennermers, H. Silver nanoparticle formation in different sizes induced by peptides identified within split-and-mix libraries. Angew. Chem. Int. Ed. 2009, 48, 3661–3664. [Google Scholar] [CrossRef] [PubMed]
- Tomizaki, K.-Y.; Kubo, S.; Ahn, S.-A.; Satake, M.; Imai, T. Biomimetic alignment of zinc oxide nanoparticles along a peptide nanofiber. Langmuir 2012, 28, 13459–13466. [Google Scholar] [CrossRef] [PubMed]
- Naik, R.R.; Stringer, S.J.; Agarwal, G.; Jones, S.E.; Stone, M.O. Biomimetic synthesis and patterning of silver nanoparticles. Nat. Mater. 2002, 1, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Gholami-Shabani, M.; Shams-Ghahfarokhi, M.; Gholami-Shabani, Z.; Akbarzadeh, A.; Riazi, G.; Ajdari, S.; Amani, A.; Razzaghi-Abyaneh, M. Enzymatic synthesis of gold nanoparticles using sulfite reductase purified from Escherichia coli: A green eco-friendly approach. Process Biochem. 2015, 50, 1076–1085. [Google Scholar] [CrossRef]
- Das, S.K.; Khan, M.R.; Guhab, A.K.; Naskar, N. Bio-inspired fabrication of silver nanoparticles on nanostructured silica: Characterization and application as a highly efficient hydrogenation catalyst. Green Chem. 2013, 15, 2548–2557. [Google Scholar] [CrossRef]
- Cuenca, T.; Filice, M.; Palomo, J.M. Palladium nanoparticles enzyme aggregate (PANEA) as efficient catalyst for Suzuki-Miyaura reaction in aqueous media. Enzyme Microb. Technol. 2016, in press. [Google Scholar] [CrossRef]
- Das, S.K.; Parandhaman, T.; Pentela, N.; Islam, A.K.M.M.; Mandal, A.B.; Mukherjee, M. Understanding the biosynthesis and catalytic activity of Pd, Pt, and Ag nanoparticles in hydrogenation and Suzuki coupling reactions at the nano−bio interface. J. Phys. Chem. C 2014, 118, 24623–24632. [Google Scholar] [CrossRef]
- Colombo, M.; Mazzucchelli, S.; Collico, V.; Avvakumova, S.; Pandolfi, L.; Corsi, F.; Porta, F.; Prosperi, D. Protein-assisted one-pot synthesis and biofunctionalization of spherical gold nanoparticles for selective targeting of cancer cells. Angew. Chem. Int. Ed. 2012, 51, 9272–9275. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.-S.; Kim, S.-J.; Choi, S.-J.; Kim, N.-H.; Hakim, M.; Rothschild, A.; Kim, I.-D. Thin-walled SnO2 nanotubes functionalized with Pt and Au catalysts via the protein templating route and their selective detection of acetone and hydrogen sulfide molecules. Nanoscale 2015, 7, 16417–16426. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, A.B.; Namvar, F.; Moniri, M.; Md Tahir, P.; Azizi, S.; Mohamad, R. Nanoparticles biosynthesized by fungi and yeast: A review of their preparation, properties, and medical applications. Molecules 2015, 20, 16540–16565. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.; Mehboob, F.; Stams, A.J.M.; Mota, M.M.; Rijnaarts, H.H.M.; Alves, M.M. Metallic nanoparticles: Microbial synthesis and unique properties for biotechnological applications, bioavailability and biotransformation. Crit. Rev. Biotechnol. 2015, 35, 114–128. [Google Scholar] [CrossRef] [PubMed]
- Chitam, H.; Zhu, N.; Shang, R.; Shi, C.; Cui, J.; Sohoo, I.; Wu, P.; Cao, Y. Biorecovery of palladium as nanoparticles by Enterococcus faecalis and its catalysis for chromate reduction. Chem. Eng. J. 2016, 288, 246–254. [Google Scholar]
- Lloyd, J.R.; Byrne, J.M.; Coker, V.S. Biotechnological synthesis of functional nanomaterials. Curr. Opin. Biotechnol. 2011, 22, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y. Synthesis of nanoparticles by microorganisms and their application in enhancing microbiological reaction rates. Chemosphere 2011, 82, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.P.; Juang, C.P.; Morehart, K.; Allen, L. The removal of Cu (II) from dilute aqueous solutions by Saccharomyces cerevisiae. Water Res. 1990, 24, 433–439. [Google Scholar] [CrossRef]
- Bhargavaa, A.; Jaina, N.; Gangopadhyayb, S.; Panwara, J. Development of gold nanoparticle-fungal hybrid based heterogeneous interface for catalytic applications. Process Biochem. 2015, 50, 1293–1300. [Google Scholar] [CrossRef]
- Vetchinkina, E.P.; Loshchinina, E.A.; Burov, A.M.; Dykman, L.A.; Nikitina, V.E. Enzymatic formation of gold nanoparticles by submerged culture of the basidiomycete Lentinus edodes. J. Biotechnol. 2014, 182–183, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Kumari, M.; Pandey, S.; Chaudhry, V.; Gupta, K.C.; Nautiyal, C.S. Biocatalytic and antimicrobial activities of gold nanoparticles synthesized by Trichoderma sp. Bioresour. Technol. 2014, 166, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.-J.; Ouyang, C.-Y.; Ma, S.-Y.; Tsai, D.-Y.; Tsengand, H.-W.; Yeh, Y.-C. Biosynthesis and display of diverse metal nanoparticles by recombinant Escherichia coli. RSC Adv. 2014, 4, 58717–58719. [Google Scholar] [CrossRef]
- Mao, H.; Liao, Y.; Ma, J.; Zhao, S.L.; Huo, F.W. Water-soluble metal nanoparticles stabilized by plant polyphenols for improving the catalytic properties in oxidation of alcohols. Nanoscale 2016, 8, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Peshkov, V.A.; Pereshivko, O.P.; Van der Eycken, E.V. A walk around the A3-coupling. Chem. Soc. Rev. 2012, 41, 3790–3807. [Google Scholar] [CrossRef] [PubMed]



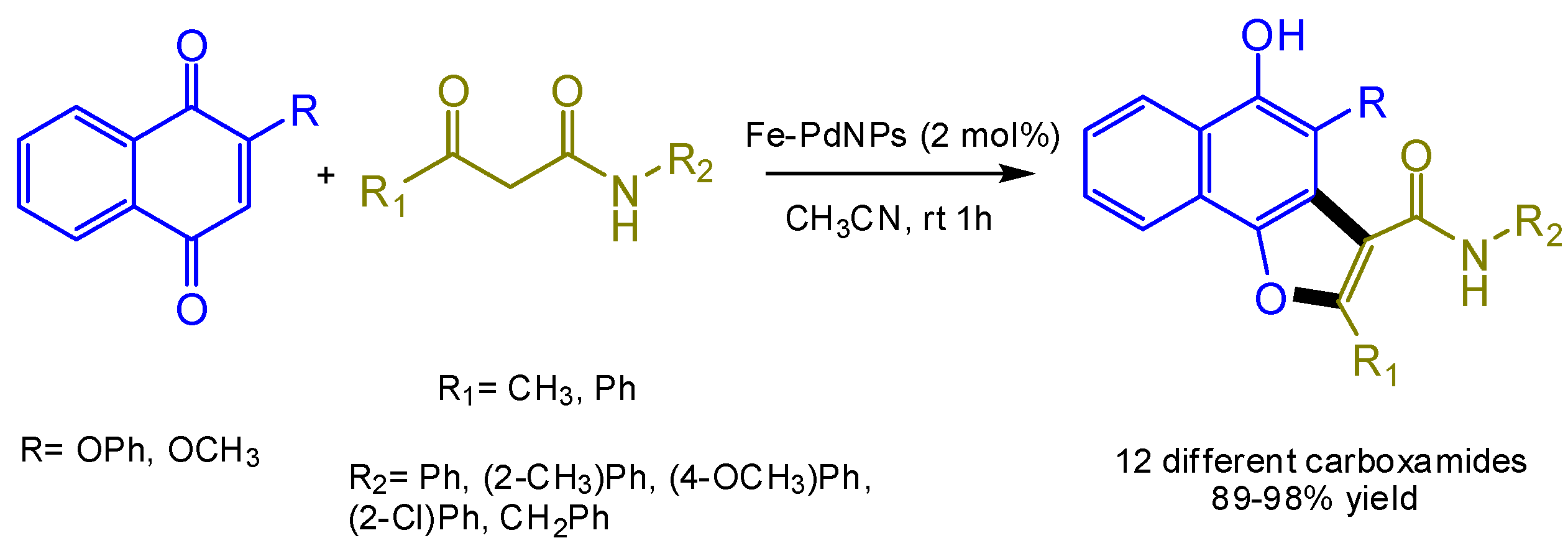

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palomo, J.M.; Filice, M. Biosynthesis of Metal Nanoparticles: Novel Efficient Heterogeneous Nanocatalysts. Nanomaterials 2016, 6, 84. https://doi.org/10.3390/nano6050084
Palomo JM, Filice M. Biosynthesis of Metal Nanoparticles: Novel Efficient Heterogeneous Nanocatalysts. Nanomaterials. 2016; 6(5):84. https://doi.org/10.3390/nano6050084
Chicago/Turabian StylePalomo, Jose M., and Marco Filice. 2016. "Biosynthesis of Metal Nanoparticles: Novel Efficient Heterogeneous Nanocatalysts" Nanomaterials 6, no. 5: 84. https://doi.org/10.3390/nano6050084