N-doped TiO2 Nanotubes as an Effective Additive to Improve the Catalytic Capability of Methanol Oxidation for Pt/Graphene Nanocomposites
Abstract
:1. Introduction
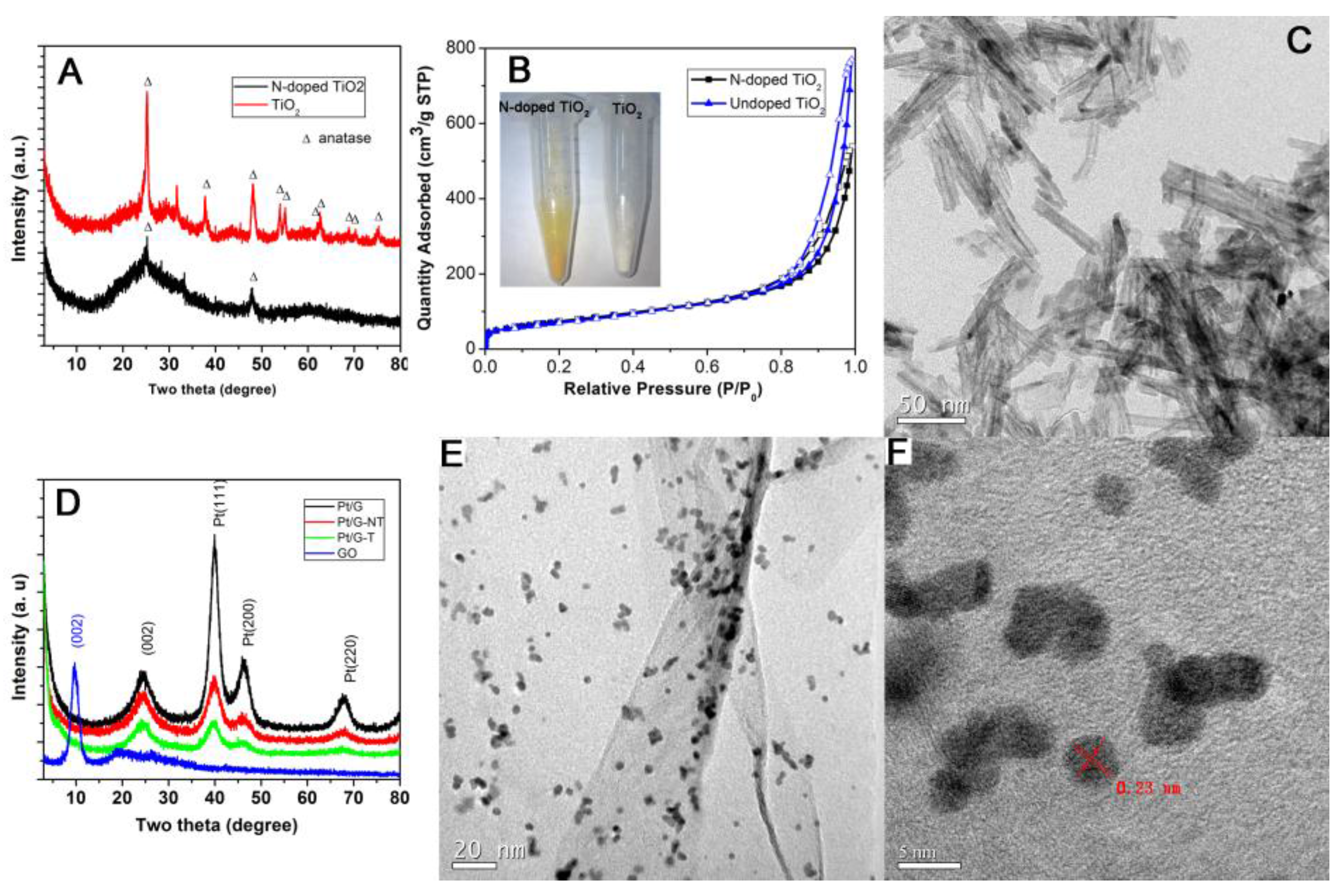
2. Results and Discussion
3. Experimental Section
3.1. Method
3.2. Characterization
3.3. Electrochemical Measurements
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Girishkumar, G.; Vinodgopal, K.; Kamat, P.V. Carbon nanostructures in portable fuel cells: Single-walled carbon nanotube electrodes for methanol oxidation and oxygen reduction. J. Phys. Chem. B 2004, 108, 19960–19966. [Google Scholar] [CrossRef]
- Pandolfo, A.; Hollenkamp, A. Carbon properties and their role in supercapacitors. J. Power Sources 2006, 157, 11–27. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhao, X. Carbon–based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, Z.; Huang, Y.; Ma, Y.; Wang, C.; Chen, M.; Chen, Y. Supercapacitor devices based on graphene materials. J. Phys. Chem. C 2009, 113, 13103–13107. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Capacitive energy storage in nanostructured carbon-electrolyte systems. Acc. Chem. Res. 2012, 46, 1094–1103. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Song, C.; Zhang, L.; Zhang, J.; Wang, H.; Wilkinson, D.P. A review of anode catalysis in the direct methanol fuel cell. J. Power Sources 2006, 155, 95–110. [Google Scholar] [CrossRef]
- Zhu, C.; Du, D.; Eychmüller, A.; Lin, Y. Engineering ordered and nonordered porous noble metal nanostructures: Synthesis, assembly, and their applications in electrochemistry. Chem. Rev. 2015, 115, 8896–8943. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liang, C.; Zhou, W.; Qiu, J.; Zhou, Z.; Sun, G.; Xin, Q. Preparation and characterization of multiwalled carbon nanotube–supported platinum for cathode catalysts of direct methanol fuel cells. J. Phys. Chem. B 2003, 107, 6292–6299. [Google Scholar] [CrossRef]
- Arico, A.S.; Bruce, P.; Scrosati, B.; Tarascon, J.M.; van Schalkwijk, W. Nanostructured materials for advanced energy conversion and storage devices. Nat. Mater. 2005, 4, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tang, L.; Li, J. Preparation and electrochemical performance for methanol oxidation of Pt/graphene nanocomposites. Electrochem. Commun. 2009, 11, 846–849. [Google Scholar] [CrossRef]
- Jang, H.D.; Kim, S.K.; Chang, H.; Choi, J.-H.; Cho, B.-G.; Jo, E.H.; Choi, J.-W.; Huang, J. Three-dimensional crumpled graphene-based platinum-gold alloy nanoparticle composites as superior electrocatalysts for direct methanol fuel cells. Carbon 2015, 93, 869–877. [Google Scholar] [CrossRef]
- Chen, D.; Tang, L.H.; Li, J.H. Graphene-based materials in electrochemistry. Chem. Soc. Rev. 2010, 39, 3157–3180. [Google Scholar] [CrossRef]
- Xiong, B.; Zhou, Y.; Zhao, Y.; Wang, J.; Chen, X.; O’Hayre, R.; Shao, Z. The use of nitrogen-doped graphene supporting Pt nanoparticles as a catalyst for methanol electrocatalytic oxidation. Carbon 2013, 52, 181–192. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, Y.K.; Lu, J.M.; Tian, X.H.; Zhu, H.X.; Liu, J.B. Single-step synthesis of PtRu/N-doped graphene for methanol electrocatalytic oxidation. Electrochim. Acta 2014, 120, 439–451. [Google Scholar] [CrossRef]
- Zhao, S.L.; Yin, H.J.; Du, L.; Yin, G.P.; Tang, Z.Y.; Liu, S.Q. Three dimensional N-doped graphene/PtRu nanoparticle hybrids as high performance anode for direct methanol fuel cells. J. Mater. Chem. A 2014, 2, 3719–3724. [Google Scholar] [CrossRef]
- Kakati, N.; Maiti, J.; Lee, S.H.; Jee, S.H.; Viswanathan, B.; Yoon, Y.S. Anode catalysts for direct methanol fuel cells in acidic media: Do we have any alternative for Pt or Pt–Ru? Chem. Rev. 2014, 114, 12397–12429. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Kuai, L.; Geng, B. CeO2/rGO/Pt sandwich nanostructure: rGO–enhanced electron transmission between metal oxide and metal nanoparticles for anodic methanol oxidation of direct methanol fuel cells. Nanoscale 2012, 4, 5738–5743. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.-Z.; Gu, D.-M.; Wang, Z.-B.; Qu, W.-L.; Yin, G.-P.; Qian, K.-J. Effects of anatase TiO2 with different particle sizes and contents on the stability of supported Pt catalysts. J. Power Sources 2011, 196, 8207–8215. [Google Scholar] [CrossRef]
- Xia, B.Y.; Wu, H.B.; Chen, J.S.; Wang, Z.; Wang, X.; Lou, X.W. Formation of Pt-TiO2-rGO 3-phase junctions with significantly enhanced electro-activity for methanol oxidation. Phys. Chem. Chem. Phys. 2012, 14, 473–476. [Google Scholar] [CrossRef] [PubMed]
- Ramadoss, A.; Kim, S.J. Facile preparation and electrochemical characterization of graphene/ZnO nanocomposite for supercapacitor applications. Mater. Chem. Phys. 2013, 140, 405–411. [Google Scholar] [CrossRef]
- Di Valentin, C.; Pacchioni, G.; Selloni, A.; Livraghi, S.; Giamello, E. Characterization of paramagnetic species in N-doped TiO2 powders by EPR spectroscopy and DFT calculations. J. Phys. Chem. B 2005, 109, 11414–11419. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Huang, B.; Lu, J.; Wang, Z.; Qin, X.; Zhang, X.; Dai, Y.; Whangbo, M.-H. Hydrogenated titania: Synergy of surface modification and morphology improvement for enhanced photocatalytic activity. Chem. Commun. 2012, 48, 5733–5735. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Burda, C. Photoelectron spectroscopic investigation of nitrogen-doped titania nanoparticles. J. Phys. Chem. B 2004, 108, 15446–15449. [Google Scholar] [CrossRef]
- Sakka, Y.; Ohno, S.; Uda, M. Oxidation and degradation of titanium nitride ultrafine powders exposed to air. J. Am. Chem. Soc. 1992, 75, 244–248. [Google Scholar] [CrossRef]
- Kim, J.-G.; Shi, D.; Kong, K.-J.; Heo, Y.-U.; Kim, J.H.; Jo, M.R.; Lee, Y.C.; Kang, Y.-M.; Dou, S.X. Structurally and electronically designed TiO2Nx nanofibers for lithium rechargeable batteries. ACS Appl. Mater. Interfaces 2013, 5, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-S.; Winter, A.; Chen, L.; Sun, Y.; Turchanin, A.; Feng, X.; Müllen, K. Three-dimensional nitrogen and boron co-doped graphene for high-performance all-solid-state supercapacitors. Adv. Mater. 2012, 24, 5130–5135. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Waller, G.; Liu, Y.; Liu, M.; Wong, C.P. Facile synthesis of nitrogen-doped graphene via pyrolysis of graphene oxide and urea, and its electrocatalytic activity toward the oxygen-reduction reaction. Adv. Energy Mater. 2012, 2, 884–888. [Google Scholar] [CrossRef]
- Søgaard, M.; Odgaard, M.; Skou, E.M. An improved method for the determination of the electrochemical active area of porous composite platinum electrodes. Solid State Ion. 2001, 145, 31–35. [Google Scholar] [CrossRef]
- Kang, Y.; Pyo, J.B.; Ye, X.; Gordon, T.R.; Murray, C.B. Synthesis, shape control, and methanol electro-oxidation properties of Pt–Zn alloy and Pt3Zn intermetallic nanocrystals. ACS Nano 2012, 6, 5642–5647. [Google Scholar] [CrossRef] [PubMed]
- Sanetuntikul, J.; Ketpang, K.; Shanmugam, S. Hierarchical nanostructured Pt8Ti-TiO2/C as an efficient and durable anode catalyst for direct methanol fuel cells. ACS Catal. 2015, 5, 7321–7327. [Google Scholar] [CrossRef]
- Shanmugam, S.; Gedanken, A. Carbon-coated anatase TiO2 nanocomposite as a high-performance electrocatalyst support. Small 2007, 3, 1189–1193. [Google Scholar] [CrossRef] [PubMed]
- Tamizhmani, G.; Capuano, G.A. Improved electrocatalytic oxygen reduction performance of platinum ternary alloy-oxide in solid-polymer-electrolyte fuel cells. J. Electrochem. Soc. 1994, 141, 968–975. [Google Scholar] [CrossRef]
- Zhu, J.; Zhao, X.; Xiao, M.; Liang, L.; Liu, C.; Liao, J.; Xing, W. The construction of nitrogen-doped graphitized carbon-TiO2 composite to improve the electrocatalyst for methanol oxidation. Carbon 2014, 72, 114–124. [Google Scholar] [CrossRef]
- Shanmugam, S.; Gedanken, A. Synthesis and electrochemical oxygen reduction of platinum nanoparticles supported on mesoporous TiO2. J. Phys. Chem. C 2009, 113, 18707–18712. [Google Scholar] [CrossRef]
- Tian, M.; Wu, G.; Chen, A. Unique electrochemical catalytic behavior of Pt nanoparticles deposited on TiO2 nanotubes. ACS Catal. 2012, 2, 425–432. [Google Scholar] [CrossRef]
- Gong, K.; Du, F.; Xia, Z.; Durstock, M.; Dai, L. Nitrogen-doped carbon nanotube arrays with high electrocatalytic activity for oxygen reduction. Science 2009, 323, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Geng, D.; Yang, S.; Zhang, Y.; Yang, J.; Liu, J.; Li, R.; Sham, T.-K.; Sun, X.; Ye, S.; Knights, S. Nitrogen doping effects on the structure of graphene. Appl. Surf. Sci. 2011, 257, 9193–9198. [Google Scholar] [CrossRef]
- Zhu, J.; Xiao, M.; Zhao, X.; Li, K.; Liu, C.; Xing, W. Nitrogen-doped carbon-graphene composites enhance the electrocatalytic performance of the supported Pt catalysts for methanol oxidation. Chem. Commun. 2014, 50, 12201–12203. [Google Scholar] [CrossRef] [PubMed]
- Kabbabi, A.; Faure, R.; Durand, R.; Beden, B.; Hahn, F.; Leger, J.M.; Lamy, C. In situ FTIRS study of the electrocatalytic oxidation of carbon monoxide and methanol at platinum-ruthenium bulk alloy electrodes. J. Electroanal. Chem. 1998, 444, 41–53. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Lv, X.-J. N-doped TiO2 nanotubes/N-doped graphene nanosheets composites as high performance anode materials in lithium-ion battery. J. Mater. Chem. A 2014, 2, 15473–15479. [Google Scholar] [CrossRef]
- Perera, S.D.; Mariano, R.G.; Vu, K.; Nour, N.; Seitz, O.; Chabal, Y.; Balkus, K.J., Jr. Hydrothermal synthesis of graphene-TiO2 nanotube composites with enhanced photocatalytic activity. ACS Catal. 2012, 2, 949–956. [Google Scholar] [CrossRef]


© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Li, Y.; Liu, S.; Zhang, L. N-doped TiO2 Nanotubes as an Effective Additive to Improve the Catalytic Capability of Methanol Oxidation for Pt/Graphene Nanocomposites. Nanomaterials 2016, 6, 40. https://doi.org/10.3390/nano6030040
Wang X, Li Y, Liu S, Zhang L. N-doped TiO2 Nanotubes as an Effective Additive to Improve the Catalytic Capability of Methanol Oxidation for Pt/Graphene Nanocomposites. Nanomaterials. 2016; 6(3):40. https://doi.org/10.3390/nano6030040
Chicago/Turabian StyleWang, Xiaohua, Yueming Li, Shimin Liu, and Long Zhang. 2016. "N-doped TiO2 Nanotubes as an Effective Additive to Improve the Catalytic Capability of Methanol Oxidation for Pt/Graphene Nanocomposites" Nanomaterials 6, no. 3: 40. https://doi.org/10.3390/nano6030040




