A Nanostructured SERS Switch Based on Molecular Beacon-Controlled Assembly of Gold Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
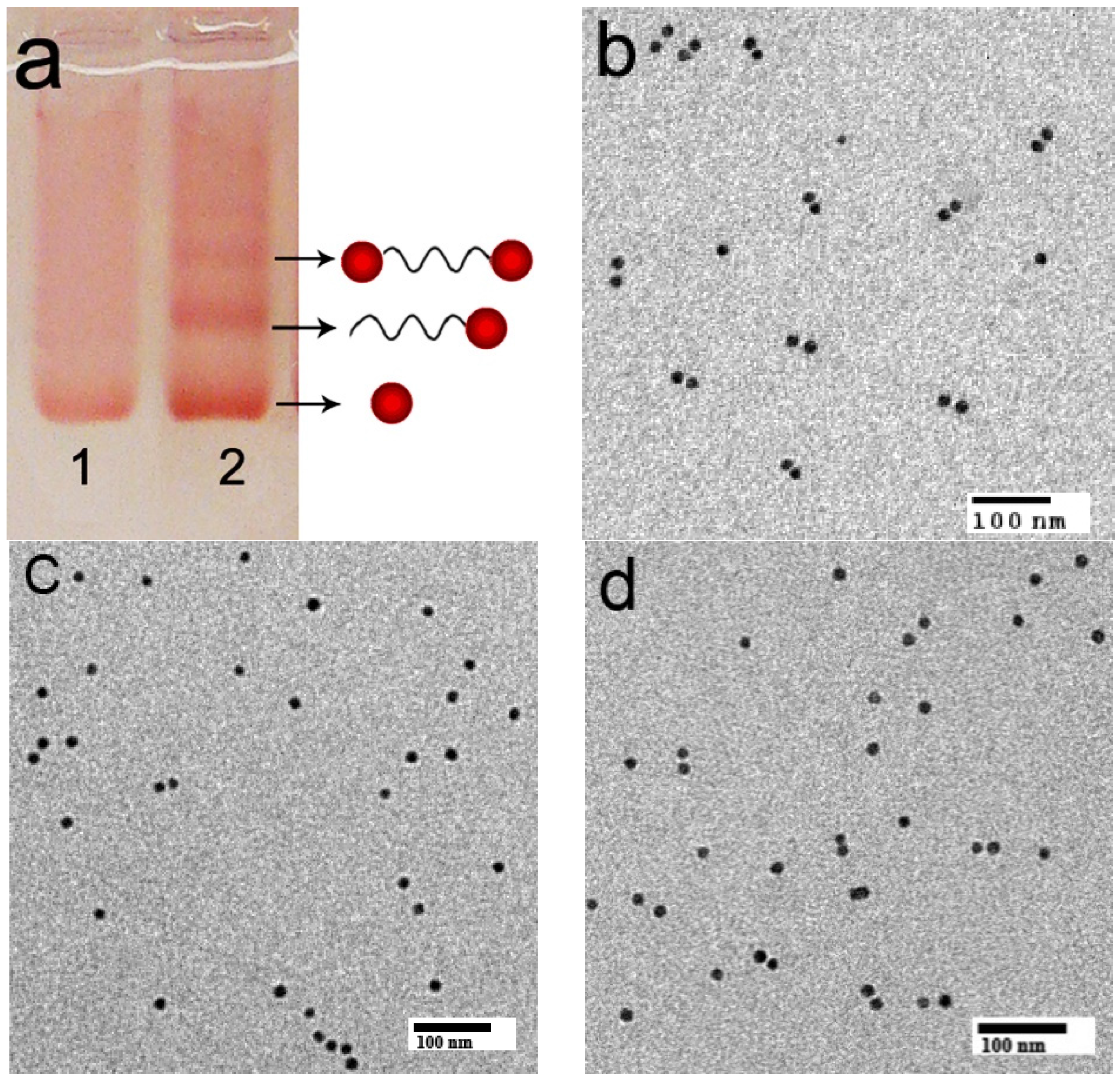
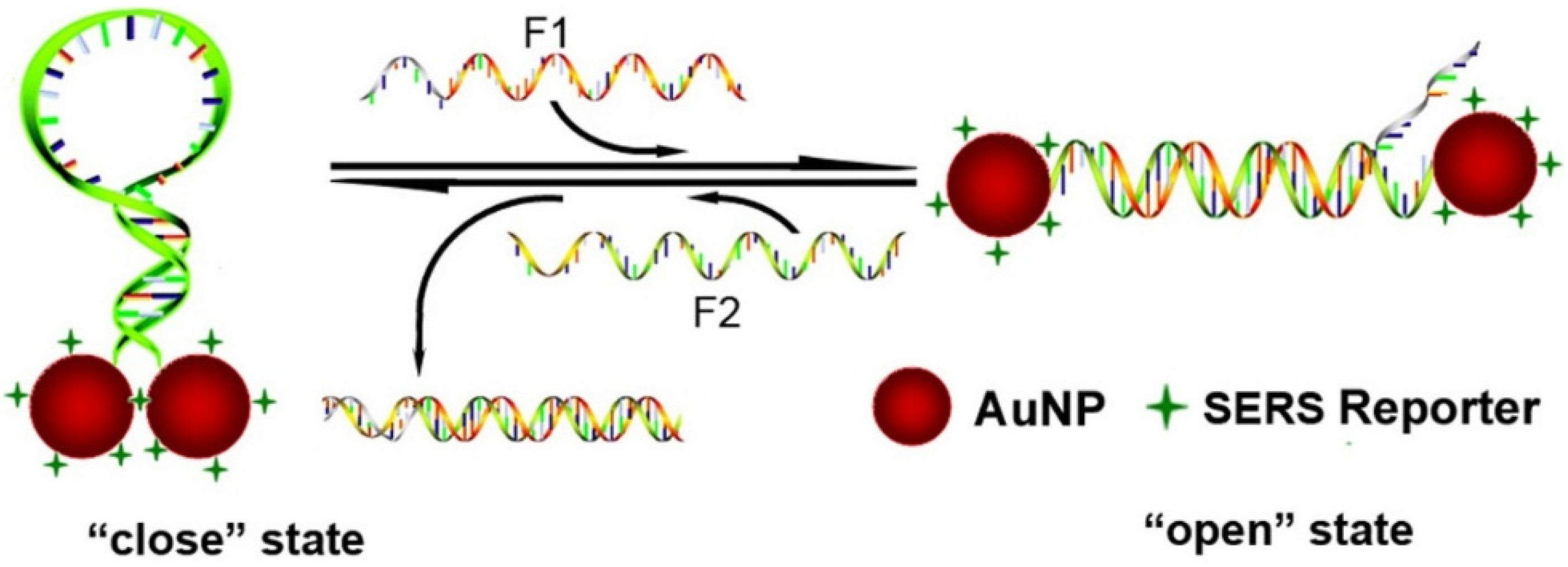
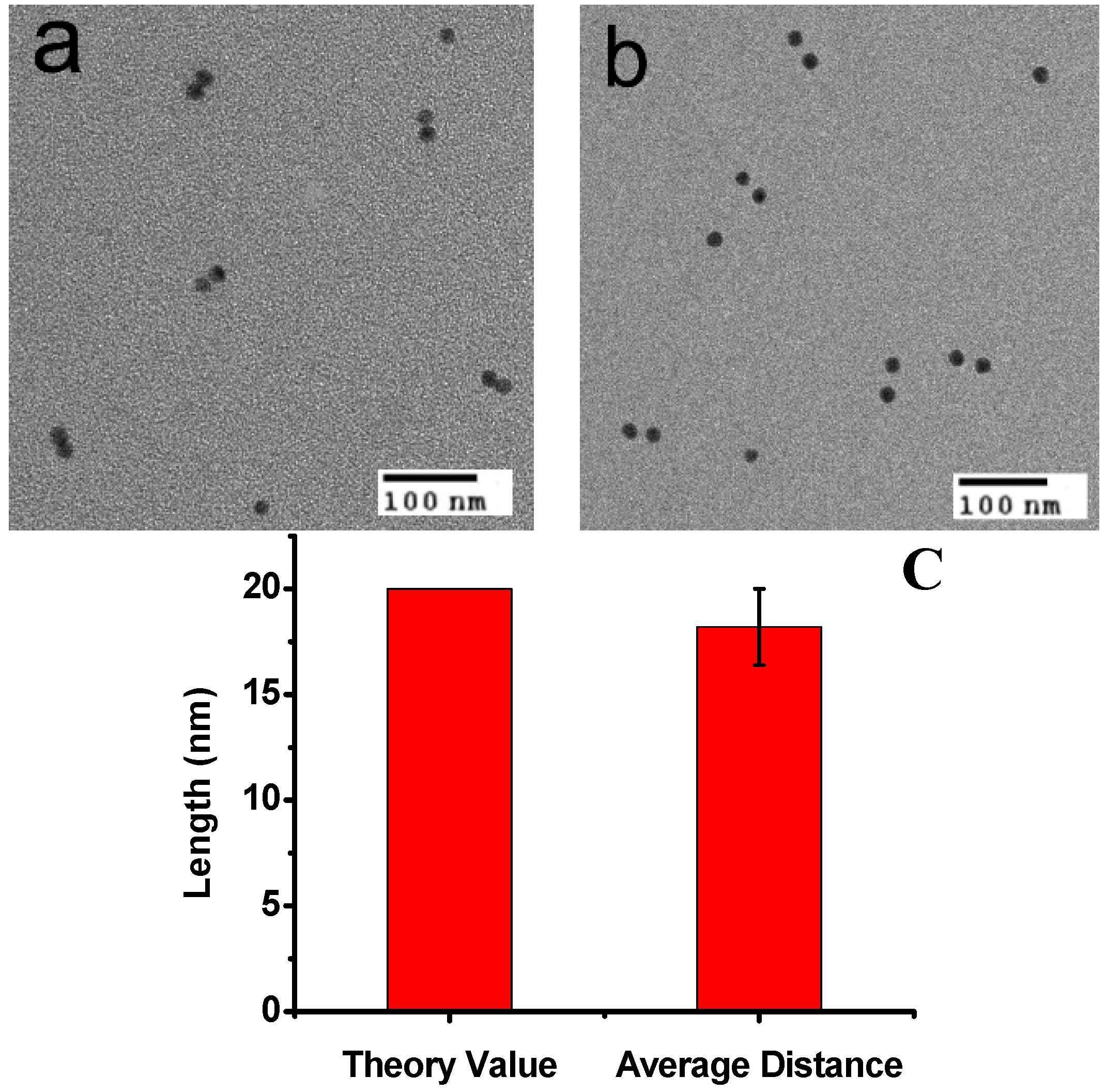


3. Experimental Section
3.1. Chemicals and Materials
3.2. Experiment Procedure
3.3. Characterization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Xu, P.; Lee, J.; Ma, K.; Choi, C.; Jin, S.H.; Wang, J.; Cha, J.N. Enhanced Raman Signals from Switchable Nanoparticle Probes. Chem. Commun. 2013, 49, 8994–8996. [Google Scholar] [CrossRef] [PubMed]
- Bi, L.Y.; Rao, Y.Y.; Tao, Q.; Dong, J.; Su, T.; Liu, F.J.; Qian, W.P. Fabrication of large-scale gold nanoplate films as highly active SERS substrates for label-free DNA detection. Biosens. Bioelectron. 2013, 43, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Yin, H.H.; Xu, L.G.; Wu, X.L.; Kuang, H.; Wang, L.B.; Xu, C.L. Ultrasensitive aptamer-based SERS detection of PSAs by heterogeneous satellite nanoassemblies. Chem. Commun. 2014, 50, 9737–9740. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.; Thompson, D.G.; Smith, W.E.; Faulds, K. Control of enhanced Raman scattering using a DNA-based assembly process of dye-coded nanoparticles. Nat. Nanotechnol. 2008, 3, 548–551. [Google Scholar] [CrossRef] [PubMed]
- Li, J.F.; Huang, Y.F.; Ding, Y.; Yang, Z.L.; Li, S.B.; Zhou, X.S.; Fan, F.R.; Zhang, W.; Zhou, Z.Y.; Wu, D.Y.; et al. Shell-isolated nanoparticle-enhanced Raman spectroscopy. Nature 2010, 464, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Kneipp, J.; Kneipp, H.; Kneipp, K. SERS—A single-molecule and nanoscale tool for bioanalytics. Chem. Soc. Rev. 2008, 37, 1052–1060. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.M.; Nie, S.M. Single-molecule and single-nanoparticle SERS: From fundamental mechanisms to biomedical applications. Chem. Soc. Rev. 2008, 37, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Banholzer, M.J.; Millstone, J.E.; Qin, L.; Mirkin, C.A. Rationally designed nanostructures for surface-enhanced Raman spectroscopy. Chem. Soc. Rev. 2008, 37, 885–897. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.J.; Wu, Y.Y.; Zhang, W.; Li, N.; Tang, B. A sensitive SERS assay for detecting proteins and nucleic acids using a triple-helix molecular switch for cascade signal amplification. Chem. Commun. 2014, 50, 9409–9412. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.G.; Chen, Y.; Wang, T.T.; Ma, Z.F.; Su, Z.M. Monodispersed gold nanorods embedded silica particles as novel Raman labels for biosensing. Adv. Funct. Mater. 2008, 18, 355–361. [Google Scholar] [CrossRef]
- Wen, Y.Q.; Wang, W.Q.; Zhang, Z.L.; Xu, L.P.; Du, H.W.; Zhang, X.J.; Song, Y.L. Controllable and reproducible construction of a SERS substrate and its sensing applications. Nanoscale 2013, 5, 523–526. [Google Scholar] [CrossRef] [PubMed]
- O'Steen, M.R.; Cornett, E.M.; Kolpashchikov, D.M. Nuclease-containing media for resettable operation of DNA logic gates. Chem. Commun. 2015, 51, 1429–1431. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Nangreave, J.; Liu, Y.; Yan, H. Structural DNA Nanotechnology: State of the Art and Future Perspective. J. Am. Chem. Soc. 2014, 136, 11198–11211. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.C.; Yang, Z.Q.; Liu, D.S. DNA Nanotechnology Based on i-Motif Structures. Acc. Chem. Res. 2014, 47, 1853–1860. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.Y.; Song, Y.H.; Wei, G.; Zhang, W.X.; Li, Z.; Dong, W.-F. Self-assembly of λ-DNA networks/Ag nanoparticles: Hybrid architecture and active-SERS substrate. J. Colloid Interface Sci. 2008, 317, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Wang, L.; Liu, Z.G.; Song, Y.H.; Sun, L.L.; Yang, T.; Li, Z. DNA-Network-Templated Self-Assembly of Silver Nanoparticles and Their Application in Surface-Enhanced Raman Scattering. J. Phys. Chem. B 2005, 109, 23941–23947. [Google Scholar] [CrossRef] [PubMed]
- Mastroianni, A.J.; Claridge, S.A.; Alivisatos, A.P. Pyramidal and chiral groupings of gold nanocrystals assembled using DNA scaffolds. J. Am. Chem. Soc. 2009, 131, 8455–8459. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Deng, D.W.; Xue, J.Q.; Qu, L.Z.; Achilefu, S.; Gu, Y.Q. Quantum dots based molecular beacons for in vitro and in vivo detection of MMP-2 on tumor. Biosens. Bioelectron. 2014, 61, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Yurke, B.; Turberfield, A.J.; Mills, A.P., Jr.; Simmel, F.C.; Neumann, J.L. A DNA-fuelled molecular machine made of DNA. Nature 2000, 406, 605–608. [Google Scholar] [PubMed]
- Elbaz, J.; Cecconello, A.; Fan, Z.Y.; Govorov, A.O.; Willner, I. Powering the programmed nanostructure and function of gold nanoparticles with catenated DNA machines. Nat. Commun. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.Y.; Lai, W.Q.; Chen, G.N.; Tang, D.P. A rolling circle amplification-based DNA machine for miRNA screening coupling catalytic hairpin assembly with DNAzyme formation. Chem. Commun. 2014, 50, 2935–2938. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.Q.; Xu, L.P.; Li, C.B.; Du, H.W.; Chen, L.F.; Su, B.; Zhang, Z.L.; Zhang, X.J.; Song, Y.L. DNA-based intelligent logic controlled release systems. Chem. Commun. 2012, 48, 8410–8412. [Google Scholar] [CrossRef] [PubMed]
- Serpell, C.J.; Edwardson, T.G.W.; Chidchob, P.; Carneiro, K.M.M.; Sleiman, H.F. Precision Polymers and 3D DNA Nanostructures: Emergent Assemblies from New Parameter Space. J. Am. Chem. Soc. 2014, 44, 15767–15774. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Fan, C.; Pei, H.; Shi, J.; Huang, Q. Smart Drug Delivery Nanocarriers with Self-Assembled DNA Nanostructures. Adv. Mater. 2013, 25, 4386–4396. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Sun, M.Z.; Xu, L.G.; Wang, L.B.; Kuang, H.; Xu, C.L. A SERS active gold nanostar dimer for mercury ion detection. Chem. Commun. 2013, 49, 4989–4991. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Jiao, A.L.; Yang, R.H.; Li, H.M.; Li, J.S.; Shi, M.L.; Ma, C.; Jiang, Y.; Deng, L.; Tan, W.H. Fabricating a Reversible and Regenerable Raman-Active Substrate with a Biomolecule-Controlled DNA Nanomachine. J. Am. Chem. Soc. 2012, 134, 19957–19960. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.L.; Wen, Y.Q.; Ma, Y.; Luo, J.; Zhang, X.Y.; Jiang, L.; Song, Y.L. Enhanced nanoparticle-oligonucleotide conjugates for DNA nanomachine controlled SERS switch. Appl. Phys. Lett. 2011, 98. [Google Scholar] [CrossRef]
- Wen, Y.Q.; McLaughlin, C.K.; Lo, P.K.; Yang, H.; Sleiman, H.F. Stable Gold Nanoparticle Conjugation to Internal DNA Positions: Facile Generation of Discrete Gold Nanoparticle-DNA Assemblies. Bioconjug. Chem. 2010, 21, 1413–1416. [Google Scholar] [CrossRef] [PubMed]
- Letsinger, R.L.; Elghanian, R.; Viswanadham, G.; Mirkin, C.A. Use of a steroid cyclic disulfide anchor in constructing gold nanoparticle-oligonucleotide conjugates. Bioconjug. Chem. 2000, 11, 289–291. [Google Scholar] [CrossRef]
- Talley, C.E.; Jackson, J.B.; Oubre, C.; Grady, N.K.; Hollars, C.W.; Lane, S.M.; Huser, T.R.; Nordlander, P.; Halas, N.J. Surface-enhanced Raman scattering from individual Au nanoparticles and nanoparticle dimer substrates. Nano. Lett. 2005, 5, 1569–1574. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Cheng, Y.; Xu, L.; Du, H.; Zhang, P.; Wen, Y.; Zhang, X. A Nanostructured SERS Switch Based on Molecular Beacon-Controlled Assembly of Gold Nanoparticles. Nanomaterials 2016, 6, 24. https://doi.org/10.3390/nano6020024
Li Y, Cheng Y, Xu L, Du H, Zhang P, Wen Y, Zhang X. A Nanostructured SERS Switch Based on Molecular Beacon-Controlled Assembly of Gold Nanoparticles. Nanomaterials. 2016; 6(2):24. https://doi.org/10.3390/nano6020024
Chicago/Turabian StyleLi, Yansheng, Yaya Cheng, Liping Xu, Hongwu Du, Peixun Zhang, Yongqiang Wen, and Xueji Zhang. 2016. "A Nanostructured SERS Switch Based on Molecular Beacon-Controlled Assembly of Gold Nanoparticles" Nanomaterials 6, no. 2: 24. https://doi.org/10.3390/nano6020024




