Nickel Based Electrospun Materials with Tuned Morphology and Composition
Abstract
:1. Introduction
2. Results and Discussion
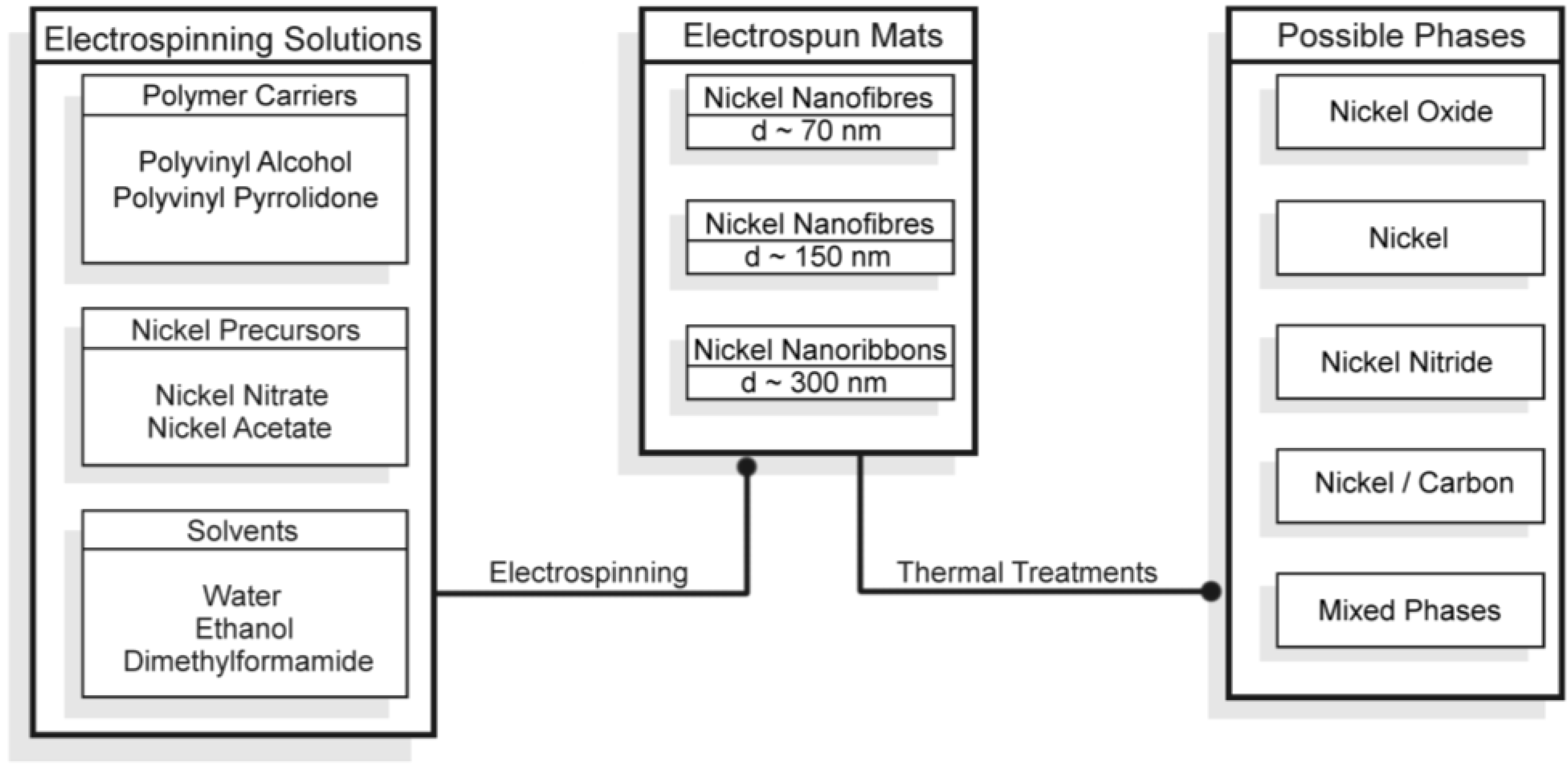
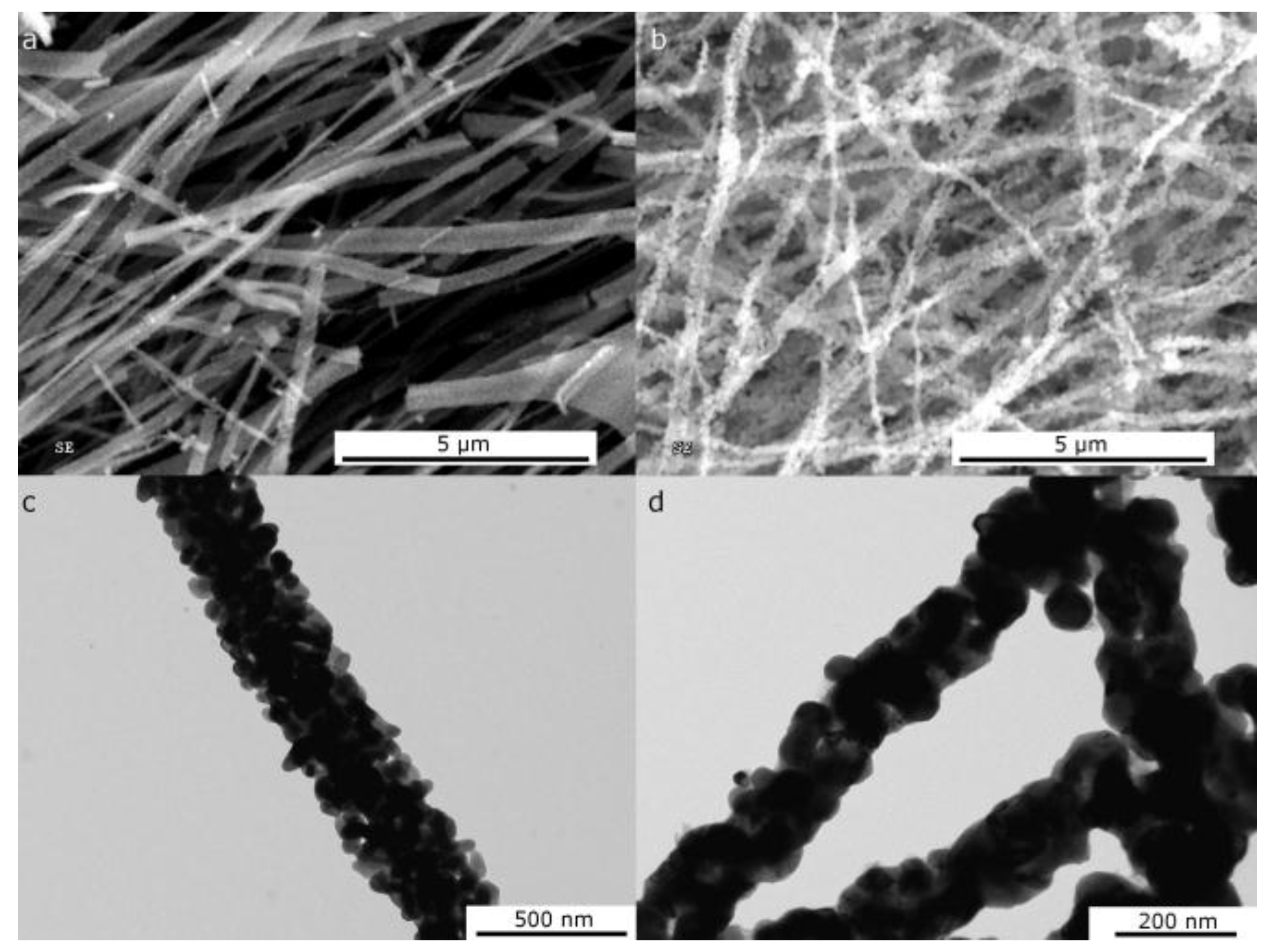
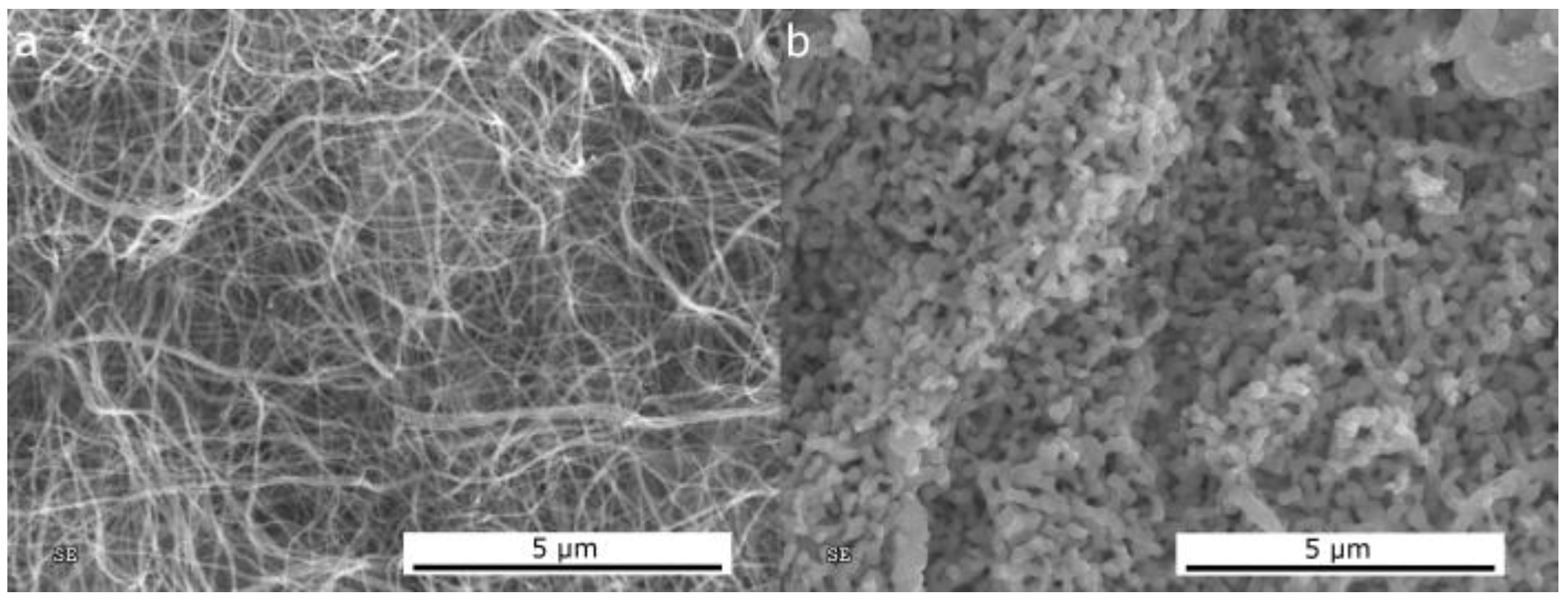
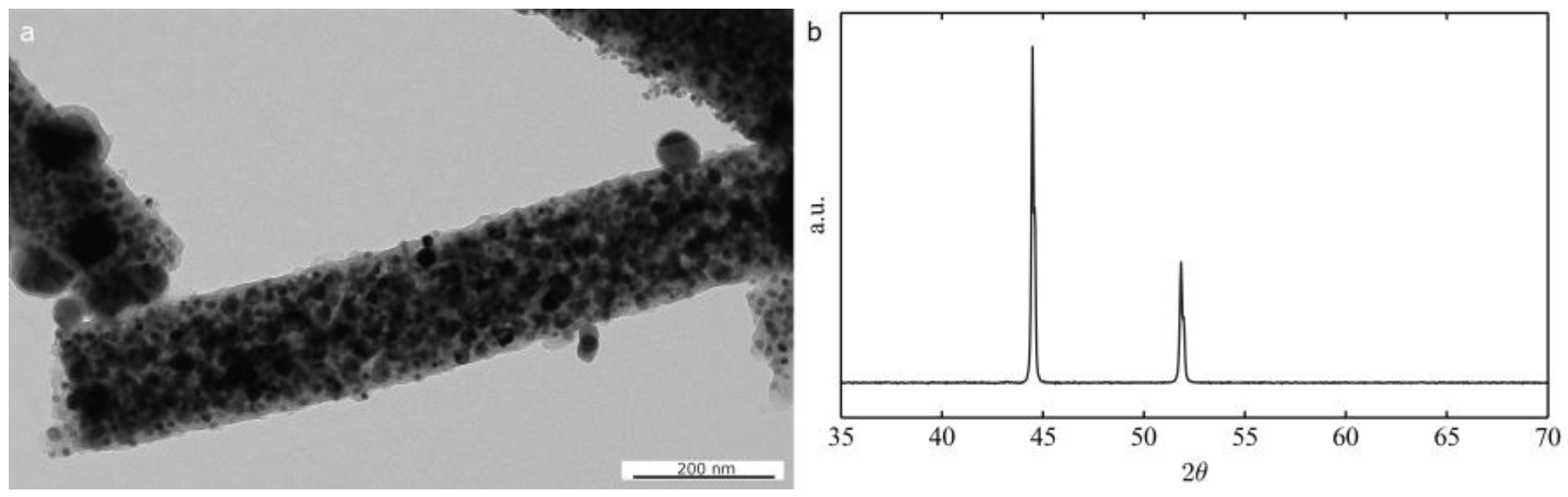
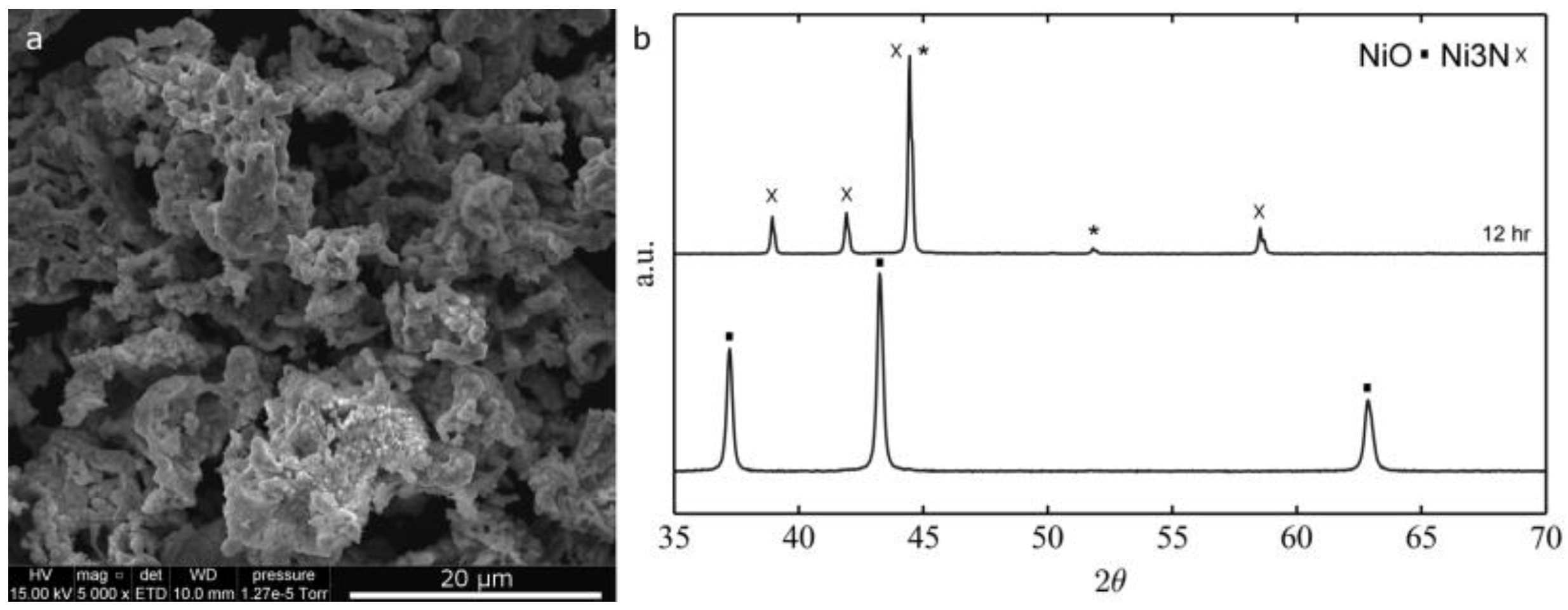
2.1. Morphological and Structural Characterization of the Ni-Based Electrospun Materials
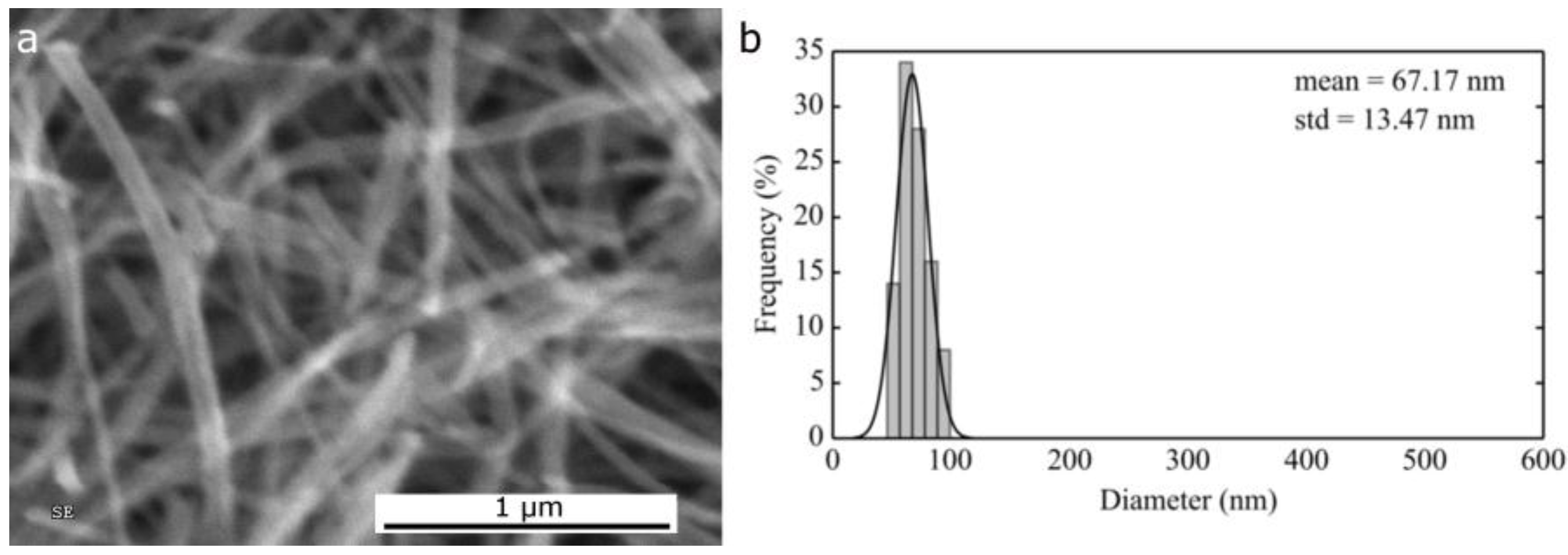
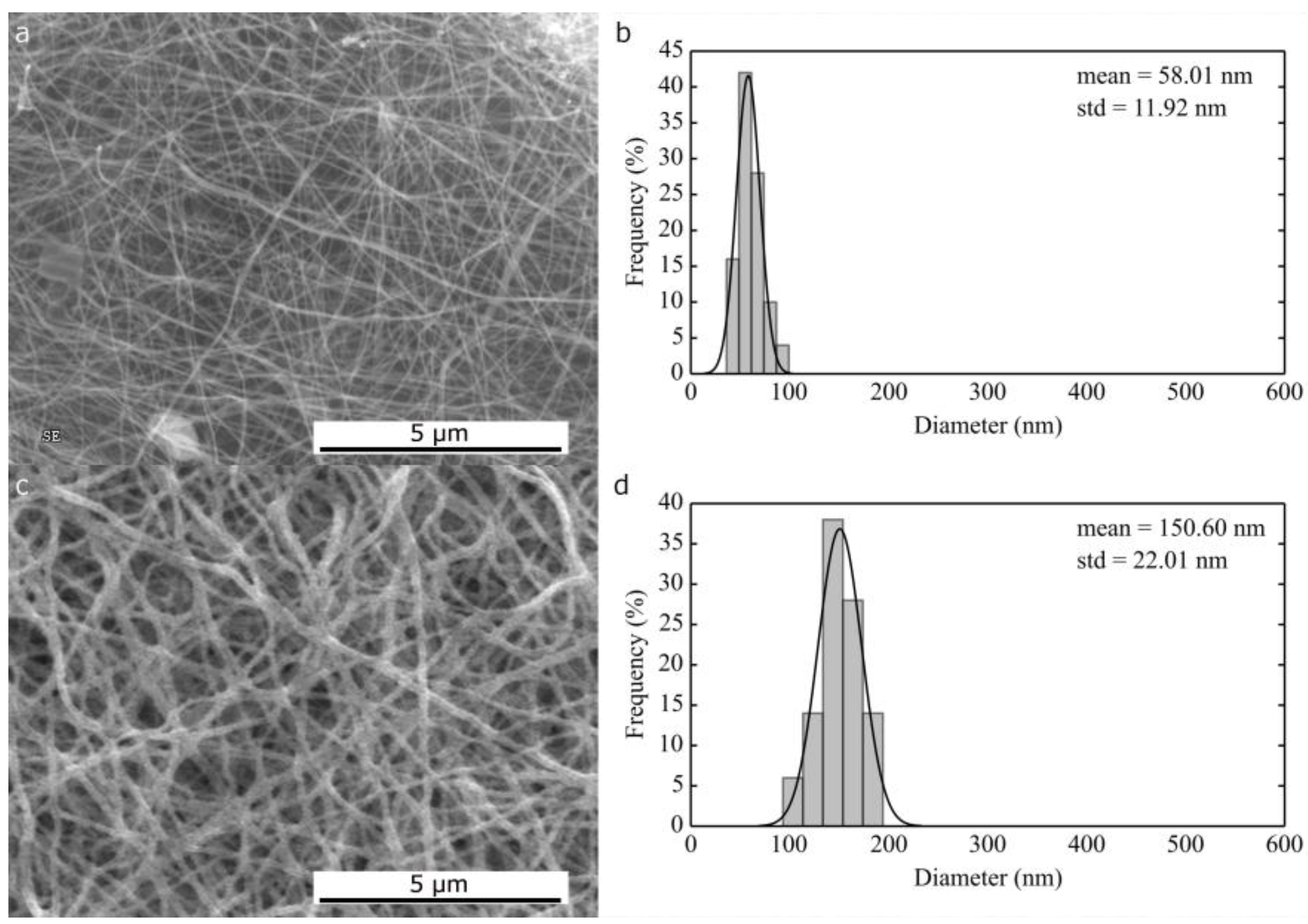
2.1.1. Morphological Differences of Different Electrospinning Based NiO Synthesis
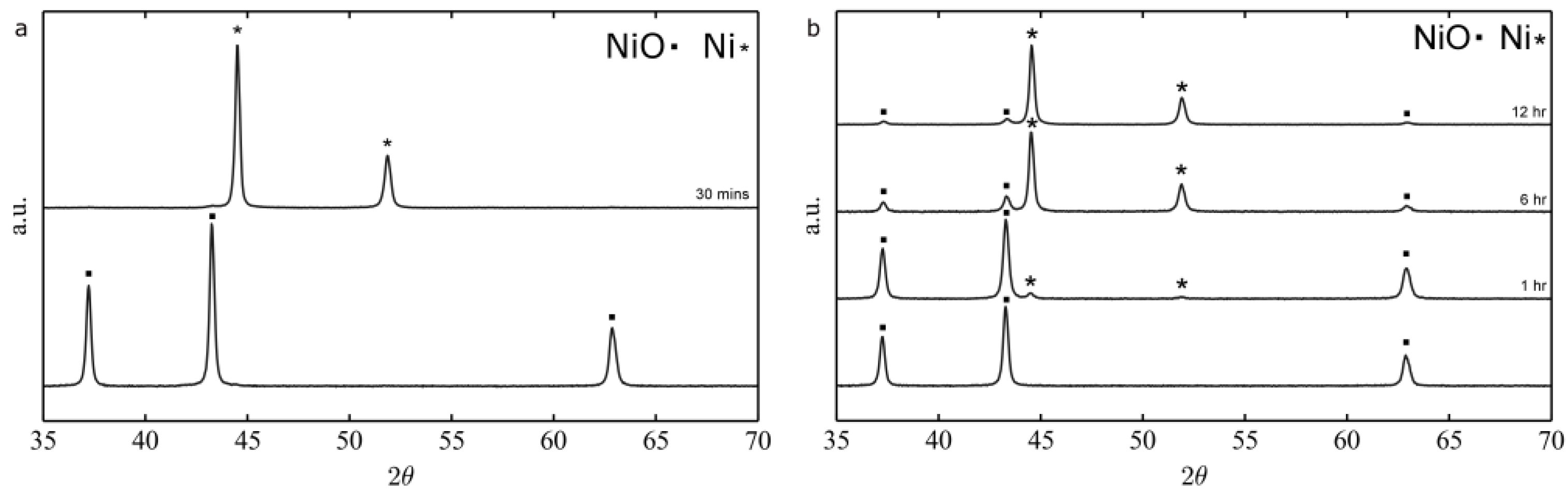
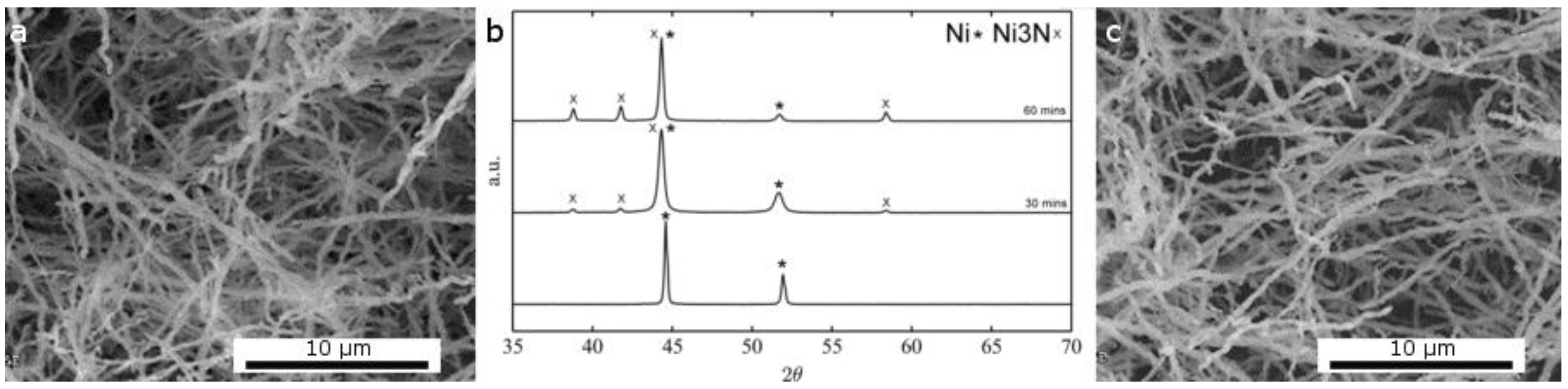
2.1.2. Thermal Treatment Effect on Crystalline Structure and Grain Morphology
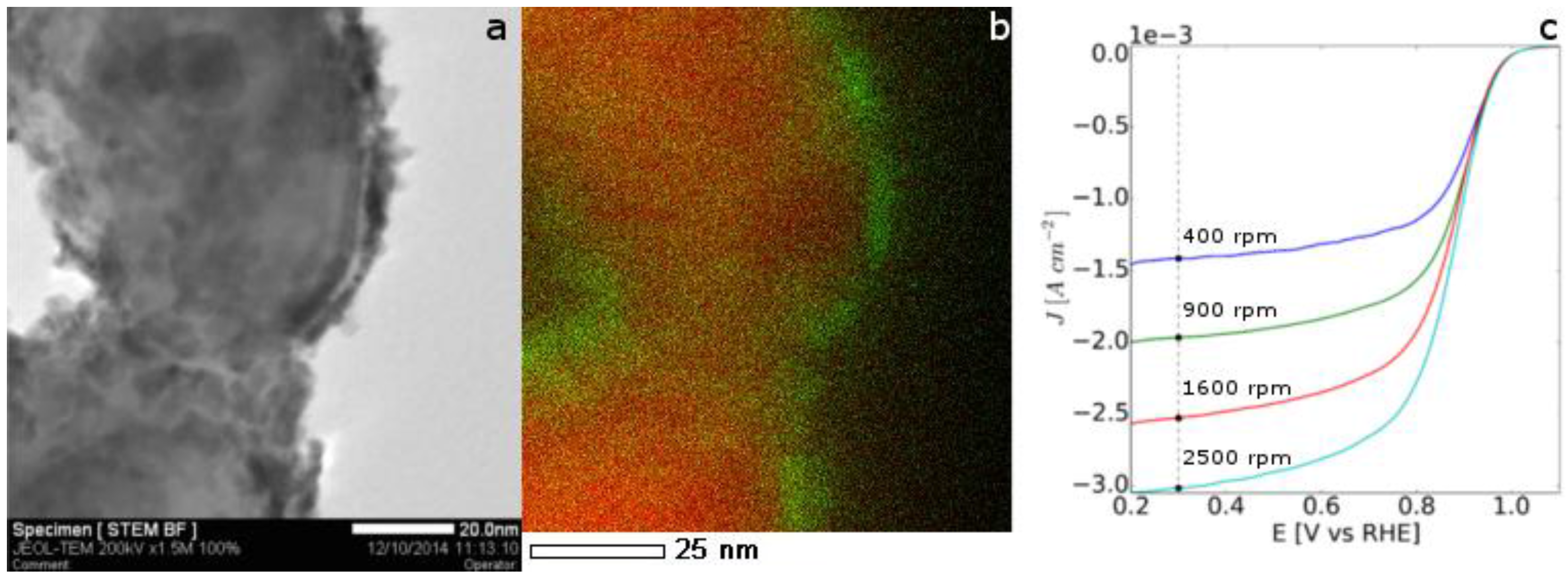
2.2. Application of Nickel Electrospun Nanofibers in Fuel Cells
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Ni-Based Nanofibers
3.3. Characterization of Ni-Based Nanofibers
3.4. Pt Deposition on to Nickel Nanofibres
3.5. Characterisation of Ni@Pt Nanofibres
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, Z.; Li, M.; Bian, Z.; Kathiraser, Y.; Kawi, S. Design of highly stable and selective core/yolk–shell nanocatalysts—A review. Appl. Catal. B 2016, 188, 324–341. [Google Scholar] [CrossRef]
- Liu, C.J.; Ye, J.; Jiang, J.; Pan, Y. Progresses in the preparation of coke resistant Ni-based catalyst for steam and CO2 reforming of methane. ChemCatChem 2011, 3, 529–541. [Google Scholar] [CrossRef]
- Cai, H.; Liu, L.; Chen, Q.; Lu, P.; Dong, J. Ni-polymer nanogel hybrid particles: A new strategy for hydrogen production from the hydrolysis of dimethylamine-borane and sodium borohydride. Energy 2016, 99, 129–135. [Google Scholar] [CrossRef]
- Cai, W.; Liu, W.; Han, J.; Wang, A. Enhanced hydrogen production in microbial electrolysis cell with 3D self-assembly nickel foam-graphene cathode. Biosens. Bioelectron. 2016, 80, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Wang, D.Y.; Chen, C.C.; Hwang, B.J.; Dai, H. A mini review on nickel-based electrocatalysts for alkaline hydrogen evolution reaction. Nano Res. 2016, 9, 28–46. [Google Scholar] [CrossRef]
- Xing, Z.; Li, Q.; Wang, D.; Yang, X.; Sun, X. Self-supported nickel nitride as an efficient high-performance three-dimensional cathode for the alkaline hydrogen evolution reaction. Electrochim. Acta 2016, 191, 841–845. [Google Scholar] [CrossRef]
- Shalom, M.; Ressnig, D.; Yang, X.; Clavel, G.; Fellinger, T.P.; Antonietti, M. Nickel nitride as an efficient electrocatalyst for water splitting. J. Mater. Chem. A 2015, 3, 8171–8177. [Google Scholar] [CrossRef]
- Lee, M.J.; Hong, S.K.; Choi, B.H.; Hwang, H.J. Fabrication and performance of solid oxide fuel cell anodes from core-shell structured Ni/yttria-stabilized zirconia (YSZ) powders. Ceram. Int. 2016, 42, 10110–10115. [Google Scholar] [CrossRef]
- Alia, S.M.; Larsen, B.A.; Pylypenko, S.; Cullen, D.A.; Diercks, D.R.; Neyerlin, K.C.; Kocha, S.S.; Pivovar, B.S. Platinum-coated nickel nanowires as oxygen-reducing electrocatalysts. ACS Catal. 2014, 4, 1114–1119. [Google Scholar] [CrossRef]
- Han, B.; Carlton, C.E.; Kongkanand, A.; Kukreja, R.S.; Theobald, B.R.; Gan, L.; O’Malley, R.; Strasser, P.; Wagner, F.T.; Shao-Horn, Y. Record activity and stability of dealloyed bimetallic catalysts for proton exchange membrane fuel cells. Energy Environ. Sci. 2015, 8, 258–266. [Google Scholar] [CrossRef]
- Tian, X.; Luo, J.; Nan, H.; Zou, H.; Chen, R.; Shu, T.; Li, X.; Li, Y.; Song, H.; Liao, S.; et al. Transition Metal Nitride Coated with Atomic Layers of Pt as a Low-Cost, Highly Stable Electrocatalyst for the Oxygen Reduction Reaction. J. Am. Chem. Soc. 2016, 138, 1575–1583. [Google Scholar] [CrossRef] [PubMed]
- Kuttiyiel, K.A.; Sasaki, K.; Choi, Y.; Su, D.; Liu, P.; Adzic, R.R. Nitride Stabilized PtNi Core—Shell Nanocatalyst for high Oxygen Reduction Activity. Nano Lett. 2012, 12, 6266–6271. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.; Giles, S.A.; Zheng, J.; Jenness, G.R.; Caratzoulas, S.; Vlachos, D.G.; Yan, Y. Nickel supported on nitrogen-doped carbon nanotubes as hydrogen oxidation reaction catalyst in alkaline electrolyte. Nat. Commun. 2016, 7, 10141. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Ye, K.; Zhang, D.; Cheng, K.; Li, Y.; Wang, B.; Wang, G.; Cao, D. Methanol electrooxidation on flexible multi-walled carbon nanotube-modified sponge-based nickel electrode. J. Solid State Electrochem. 2015, 19, 3027–3034. [Google Scholar] [CrossRef]
- Mardanpour, M.M.; Yaghmaei, S. Characterization of a microfluidic microbial fuel cell as a power generator based on a nickel electrode. Biosens. Bioelectron. 2016, 79, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Shi, Y.; Luo, Y.; Wang, Y.; Cai, N. Carbon monoxide/carbon dioxide electrochemical conversion on patterned nickel electrodes operating in fuel cell and electrolysis cell modes. Int. J. Hydrogen Energy 2016, 41, 1–12. [Google Scholar] [CrossRef]
- Lim, R.J.; Xie, M.; Sk, M.A.; Lee, J.M.; Fisher, A.; Wang, X.; Lim, K.H. A review on the electrochemical reduction of CO2 in fuel cells, metal electrodes and molecular catalysts. Catal. Today 2014, 233, 169–180. [Google Scholar] [CrossRef]
- Alia, S.M.; Yan, Y.; Pivovar, B. Galvanic Displacement as a Route to Highly Active and Durable, Extended Surface Electrocatalysts. Catal. Sci. Technol. 2014, 4, 3589–3600. [Google Scholar] [CrossRef]
- Alia, S.M.; Pylypenko, S.; Dameron, A.; Neyerlin, K.C.; Kocha, S.S.; Pivovar, B.S. Oxidation of Platinum Nickel Nanowires to Improve Durability of Oxygen-Reducing Electrocatalysts. J. Electrochem. Soc. 2016, 163, F296–F301. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, B.; Cao, D.; Ye, K.; Xu, Y.; Yin, J.; Cheng, K.; Wang, G. N2H4 electrooxidation at negative potential on novel wearable nano-Ni-MWNTs-textile electrode. Mater. Sci. Eng. B 2014, 188, 48–53. [Google Scholar] [CrossRef]
- Guo, F.; Ye, K.; Du, M.; Cheng, K.; Gao, Y.; Wang, G.; Cao, D. Nickel nanowire arrays electrode as an efficient catalyst for urea peroxide electro-oxidation in alkaline media. Electrochim. Acta 2016, 190, 150–158. [Google Scholar] [CrossRef]
- Muench, F.; Oezaslan, M.; Rauber, M.; Kaserer, S.; Fuchs, A.; Mankel, E.; Brötz, J.; Strasser, P.; Roth, C.; Ensinger, W. Electroless synthesis of nanostructured nickel and nickel-boron tubes and their performance as unsupported ethanol electrooxidation catalysts. J. Power Sources 2013, 222, 243–252. [Google Scholar] [CrossRef]
- Pradhan, B.K.; Kyotani, T.; Tomita, A. Nickel nanowires of 4 nm diameter in the cavity of carbon nanotubes. Chem. Commun. 1999, 1317–1318. [Google Scholar] [CrossRef]
- Tang, S.; Vongehr, S.; Ren, H.; Meng, X. Diameter-controlled synthesis of polycrystalline nickel nanowires and their size dependent magnetic properties. CrystEngComm 2012, 14, 7209–7216. [Google Scholar] [CrossRef]
- Sun, L.; Chen, Q.; Tang, Y.; Xiong, Y. Formation of one-dimensional nickel wires by chemical reduction of nickel ions under magnetic fields. Chem. Commun. 2007, 2844–2846. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Wen, W. Synthesis of Nickel Nanowires with Tunable Characteristics. Nanomaterials 2016, 6, 19. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, R.; Liu, X.; Lin, D.; Pan, W. Electrospinning of Fe, Co, and Ni Nanofibers: Synthesis, Assembly, and Magnetic Properties. Chem. Mater. 2007, 19, 3506–3511. [Google Scholar] [CrossRef]
- Ji, Y.; Zhang, X.; Zhu, Y.; Li, B.; Wang, Y.; Zhang, J.; Feng, Y. Nickel nanofibers synthesized by the electrospinning method. Mater. Res. Bull. 2013, 48, 2426–2429. [Google Scholar] [CrossRef]
- Barakat, N.A.M.; Kim, B.; Kim, H.Y. Production of Smooth and Pure Nickel Metal Nanofibers by the Electrospinning Technique: Nanofibers Possess Splendid Magnetic Properties. J. Phys. Chem. C 2009, 113, 531–536. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; He, G.; Lin, Y.; Wei, J.; Zheng, J.; Zheng, G.; Sun, D. Electrospun nickel oxide nanofibers for gas sensor application. In Proceedings of the 8th Annual IEEE International Conference on Nano/Micro Engineered and Molecular Systems, IEEE NEMS 2013, Suzhou, China, 7–10 April 2013; pp. 377–380.
- Macdonald, T.; Xu, J.; Elmas, S.; Mange, Y.; Skinner, W.; Xu, H.; Nann, T. NiO Nanofibers as a Candidate for a Nanophotocathode. Nanomaterials 2014, 4, 256–266. [Google Scholar] [CrossRef] [Green Version]
- Cavaliere, S.; Subianto, S.; Savych, I.; Jones, D.J.; Rozière, J. Electrospinning: Designed architectures for energy conversion and storage devices. Energy Environ. Sci. 2011, 4, 4761–4785. [Google Scholar] [CrossRef]
- Khalil, A.; Hashaikeh, R. Electrospinning of nickel oxide nanofibers: Process parameters and morphology control. Mater. Charact. 2014, 95, 65–71. [Google Scholar] [CrossRef]
- Reneker, D.H.; Yarin, A.L. Electrospinning jets and polymer nanofibers. Polymer (Guildf.) 2008, 49, 2387–2425. [Google Scholar] [CrossRef]
- Koombhongse, S.; Liu, W.; Reneker, D.H. Flat polymer ribbons and other shapes by electrospinning. J. Polym. Sci. Part B 2001, 39, 2598–2606. [Google Scholar] [CrossRef]
- Bandrowski, J.; Bickling, C.R.; Yang, K.H.; Hougen, O.A. Kinetics of the reduction of nickel oxide by hydrogen. Chem. Eng. Sci. 1962, 17, 379–390. [Google Scholar] [CrossRef]
- Janković, B.; Adnađević, B.; Mentus, S. The kinetic study of temperature-programmed reduction of nickel oxide in hydrogen atmosphere. Chem. Eng. Sci. 2008, 63, 567–575. [Google Scholar] [CrossRef]
- Richardson, J.T.; Scates, R.; Twigg, M.V. X-ray diffraction study of nickel oxide reduction by hydrogen. Appl. Catal. A 2003, 246, 137–150. [Google Scholar] [CrossRef]
- Manukyan, K.V.; Avetisyan, A.G.; Shuck, C.E.; Chatilyan, H.A.; Rouvimov, S.; Kharatyan, S.L.; Mukasyan, A.S. Nickel Oxide Reduction by Hydrogen: Kinetics and Structural Transformations. J. Phys. Chem. C 2015, 119, 16131–16138. [Google Scholar] [CrossRef]
- Afanasov, I.M.; Lebedev, O.I.; Kolozhvary, B.A.; Smirnov, A.V.; van Tendeloo, G. Nickel/Carbon composite materials based on expanded graphite. New Carbon Mater. 2011, 26, 335–340. [Google Scholar] [CrossRef]
- Li, J.; Liu, E.H.; Li, W.; Meng, X.Y.; Tan, S.T. Nickel/carbon nanofibers composite electrodes as supercapacitors prepared by electrospinning. J. Alloys Compd. 2009, 478, 371–374. [Google Scholar] [CrossRef]
- Balogun, M.-S.; Zeng, Y.; Qiu, W.; Luo, Y.; Onasanya, A.; Olaniyi, T.K.; Tong, Y. Three-dimensional nickel nitride (Ni 3 N) nanosheets: Free standing and flexible electrodes for lithium ion batteries and supercapacitors. J. Mater. Chem. A 2016, 4, 9844–9849. [Google Scholar] [CrossRef]
- Soo Kang, J.; Park, M.-A.; Kim, J.J.-Y.; Ha Park, S.; Young Chung, D.; Yu, S.-H.; Kim, J.J.-Y.; Park, J.; Choi, J.-W.; Jae Lee, K.; et al. Reactively sputtered nickel nitride as electrocatalytic counter electrode for dye- and quantum dot-sensitized solar cells. Sci. Rep. 2015, 5, 10450. [Google Scholar] [CrossRef] [PubMed]
- Baiker, A.; Maciejewski, M. Formation and thermal stability of copper and nickel nitrides. J. Chem. Soc. Faraday Trans. 1984, 80, 2331–2341. [Google Scholar] [CrossRef]
- Debe, M.K. Electrocatalyst approaches and challenges for automotive fuel cells. Nature 2012, 486, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Ercolano, G.; Cavaliere, S.; Jones, D.; Rozière, J. Electrospun Ni nanofibres as Pt supports for PEMFC electrodes. ECS Trans. 2015, 69, 1237–1242. [Google Scholar] [CrossRef]

| Carrier Polymer (wt %) | Nickel Precursor (wt %) | Solvent | Dynamic Viscosity (Pa·s) | Applied Voltage (kV) | Flow Rate (mL/h) | Target Distance (cm) |
|---|---|---|---|---|---|---|
| PVA (12) | Ni acetate (12) | Water | 0.460 | 15 | 0.10 | 12 |
| PVP (9-10-11) | Ni acetate (9-10-11) | Ethanol | 0.086/0.092/0.126 | 10 | 1.00 | 12 |
| PVP (10) | Ni acetate (10) | Ethanol:DMF 7:3 vol | 0.064 | 10 | 1.00 | 12 |
| PVP (11) | Ni nitrate (11) | Ethanol:water 1:1 wt | 0.130 | 14 | 0.20 | 10 |
| PVP (9) | Ni nitrate (9) | Ethanol:DMF 1:1 wt | 0.071 | 20 | 0.25 | 12 |
| Procedure | Starting Material | Ramp (°C/min) | Dwell Time (min) | Dwell Temperature (°C) | Gas | Gas Flow (cc/h) | Obtained Phase |
|---|---|---|---|---|---|---|---|
| Calcination | Polymer + nickel salt | 1 | 60 | (I) 150 | air | -- | NiO |
| (II) 250 | |||||||
| (III) 600 | |||||||
| Reduction | NiO | 5 | 30 | 250 | H2 | 50 | Ni |
| High temperature decomposition | Polymer + nickel salt | 1 | 300 | 700 | N2 | 50 | Ni (C matrix) |
| Direct conversion into nitride | NiO | 5 | 360 | (I) 600 | NH3 | 50 | Ni3N |
| (II) 250 | |||||||
| Conversion into nitride | Ni | 5 | 0-60 | 250 | NH3 | 50 | Ni3N |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ercolano, G.; Farina, F.; Cavaliere, S.; Jones, D.J.; Rozière, J. Nickel Based Electrospun Materials with Tuned Morphology and Composition. Nanomaterials 2016, 6, 236. https://doi.org/10.3390/nano6120236
Ercolano G, Farina F, Cavaliere S, Jones DJ, Rozière J. Nickel Based Electrospun Materials with Tuned Morphology and Composition. Nanomaterials. 2016; 6(12):236. https://doi.org/10.3390/nano6120236
Chicago/Turabian StyleErcolano, Giorgio, Filippo Farina, Sara Cavaliere, Deborah J. Jones, and Jacques Rozière. 2016. "Nickel Based Electrospun Materials with Tuned Morphology and Composition" Nanomaterials 6, no. 12: 236. https://doi.org/10.3390/nano6120236






