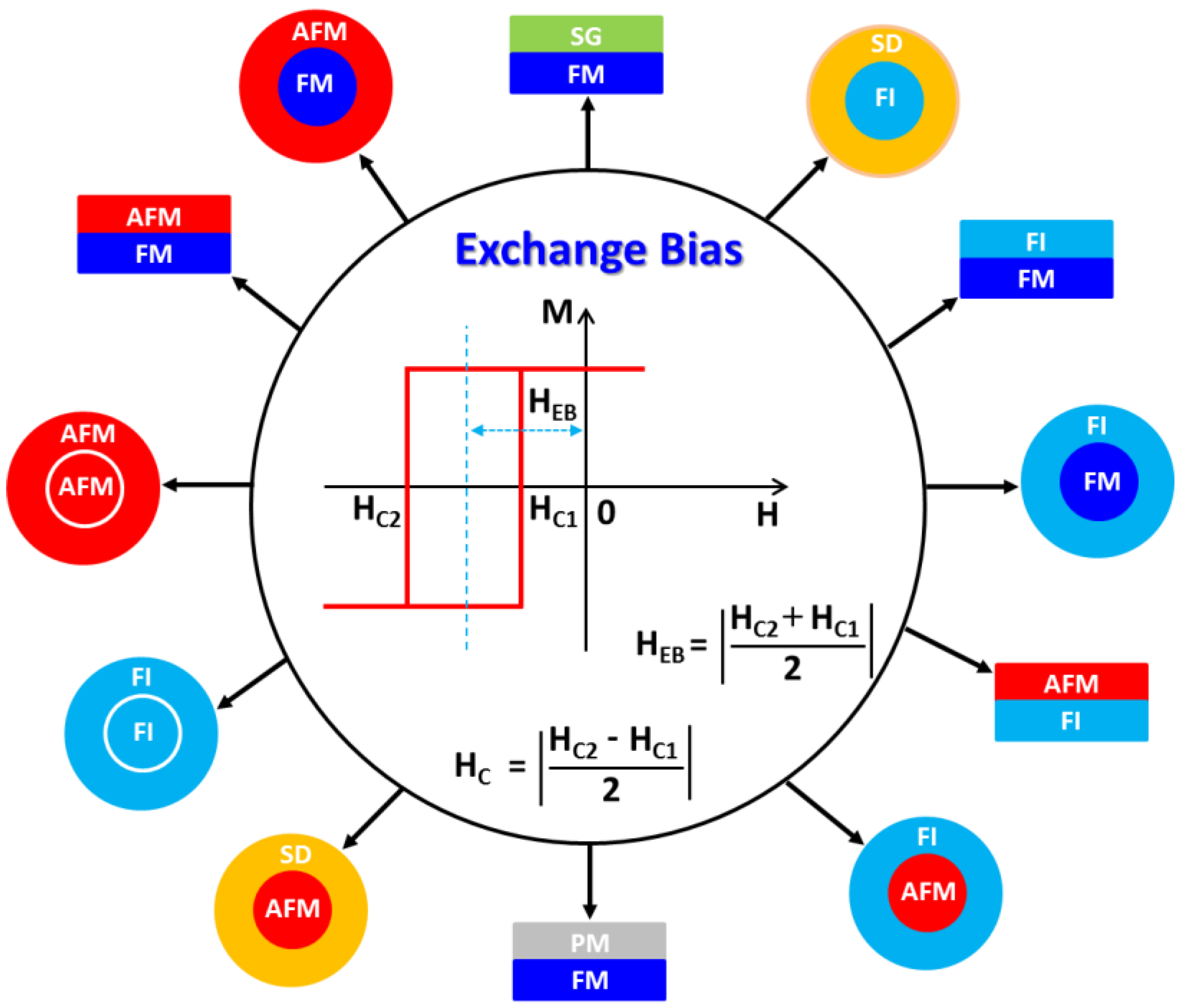
Exchange Bias Effects in Iron Oxide-Based Nanoparticle Systems
Abstract
:1. Introduction
2. Fundamental Aspects of Magnetite (Fe3O4) and Maghemite (γ-Fe2O3)
2.1. Crystal Structure and Electronic Configuration
2.2. Size-Dependent Magnetic Properties
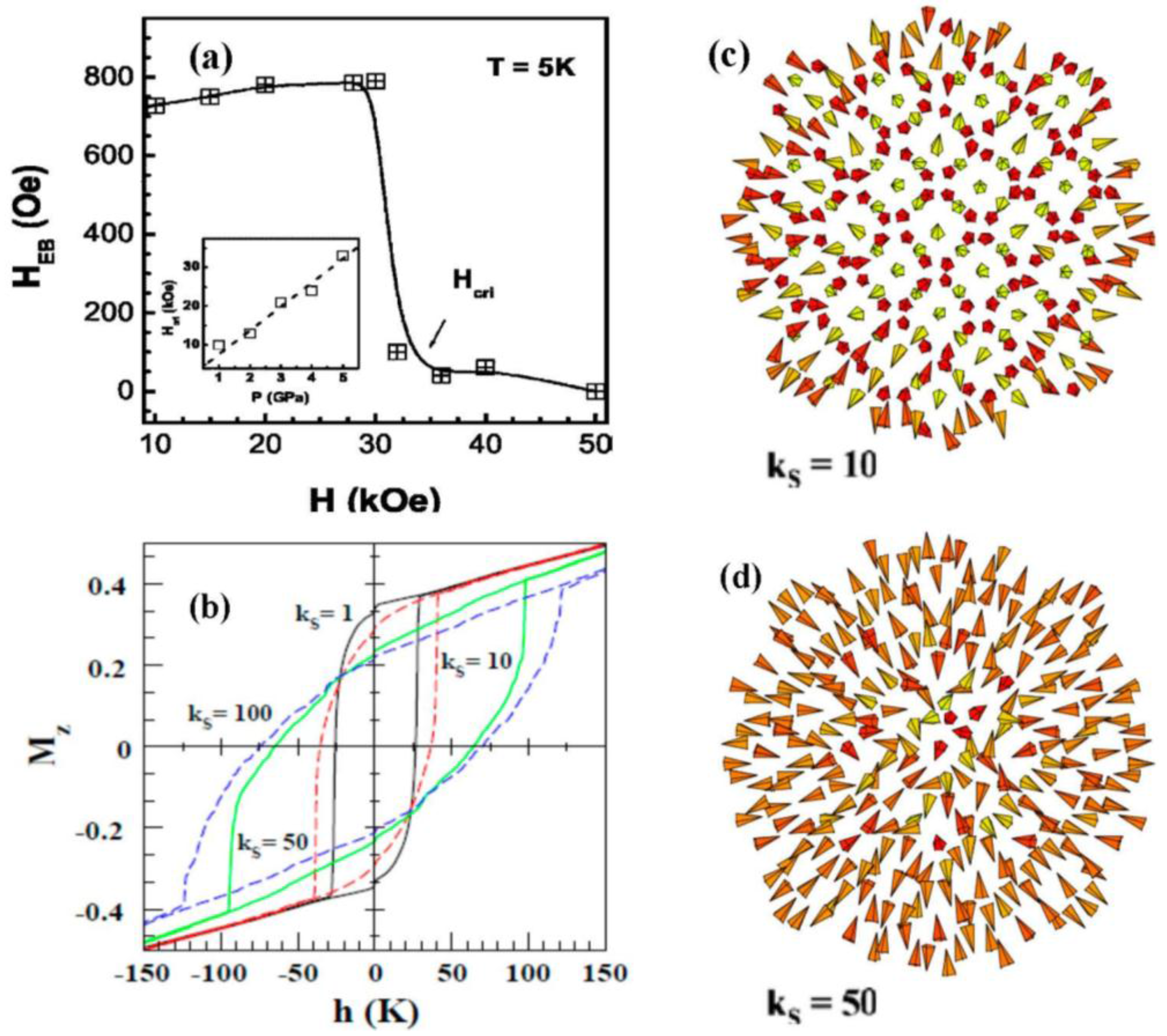
3. Exchange Bias Effect in Single-Component Solid Nanoparticles
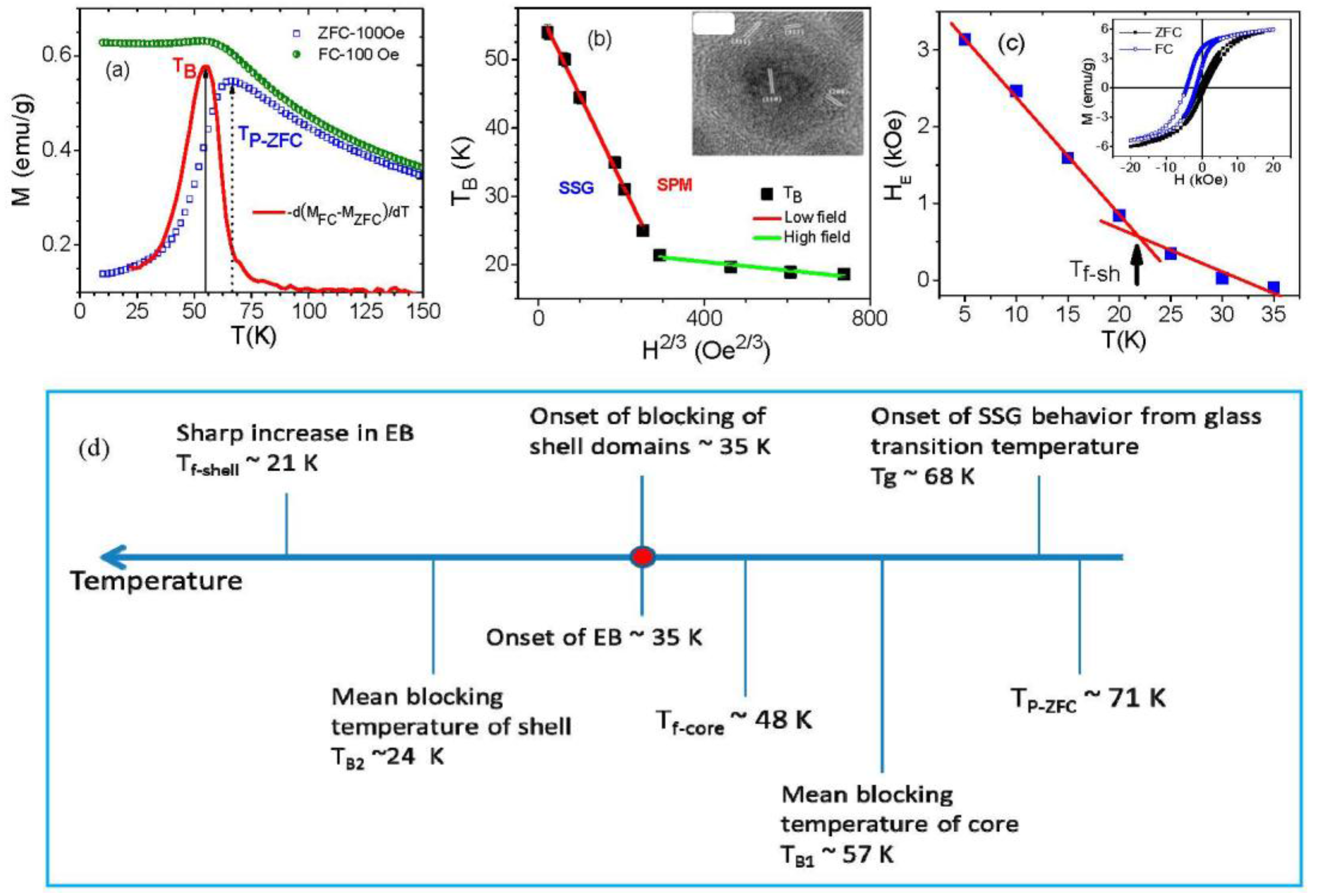
4. Exchange Bias Effect in Core/Shell Nanoparticles
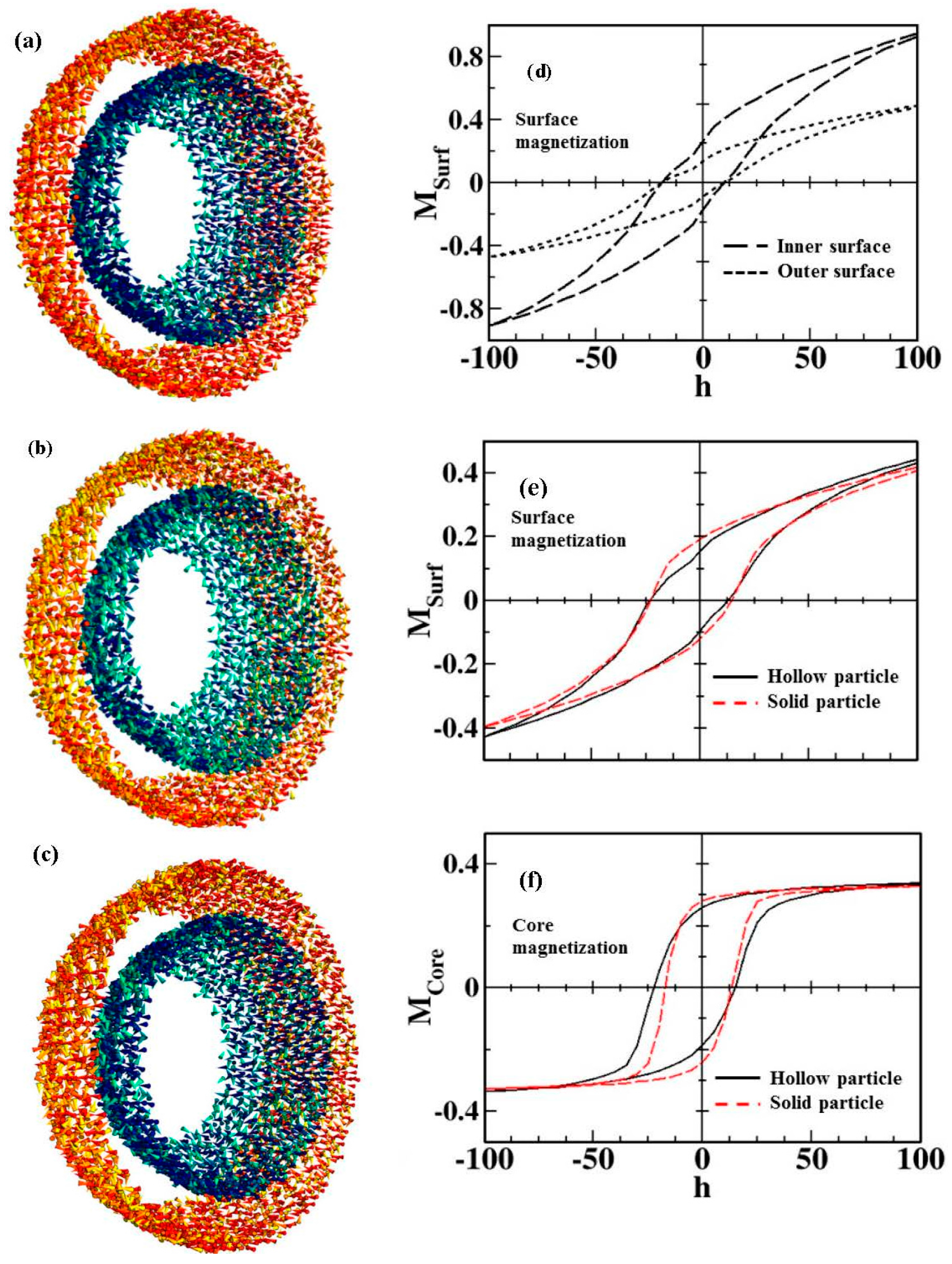
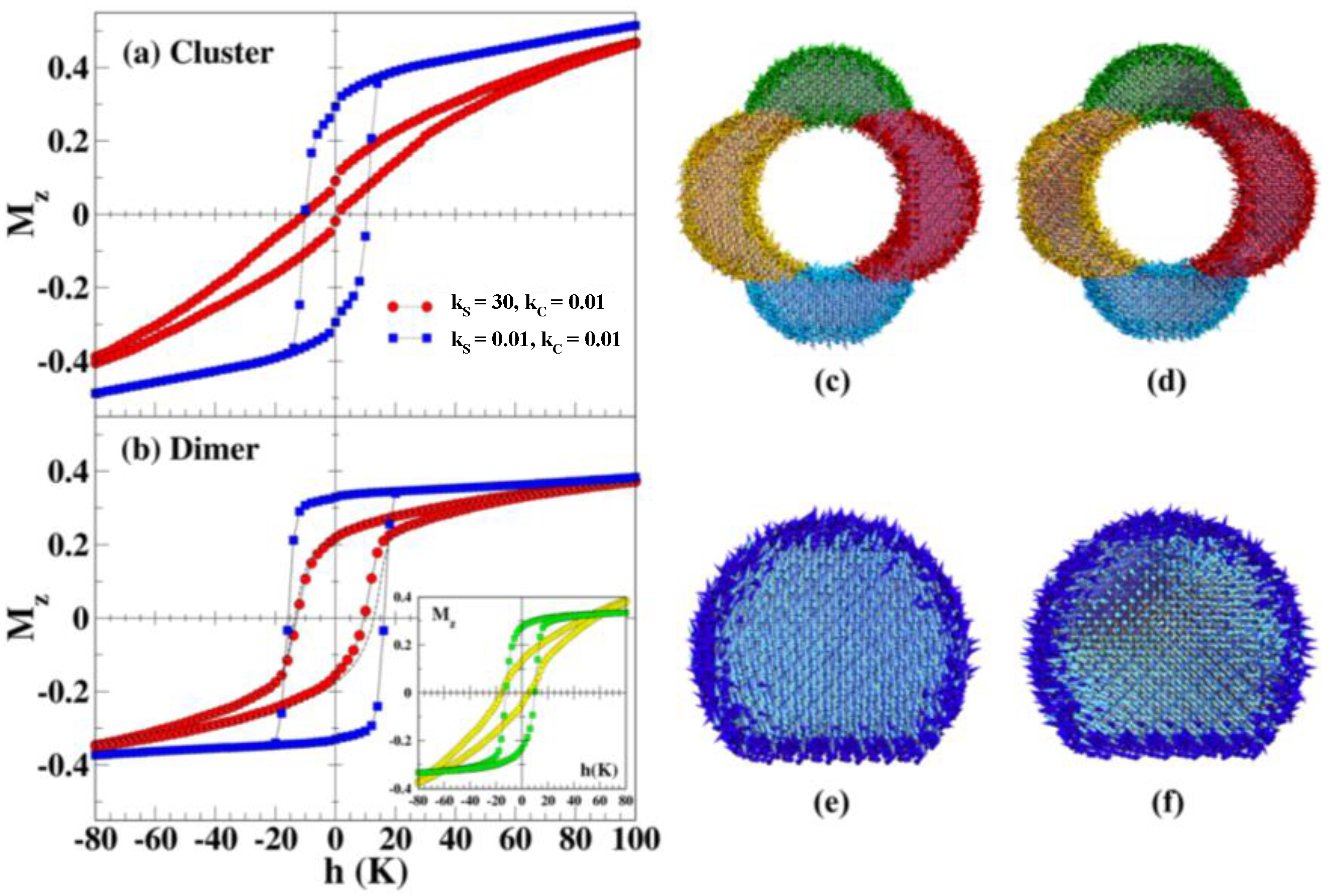
5. Exchange Bias Effect in Hollow Nanoparticles
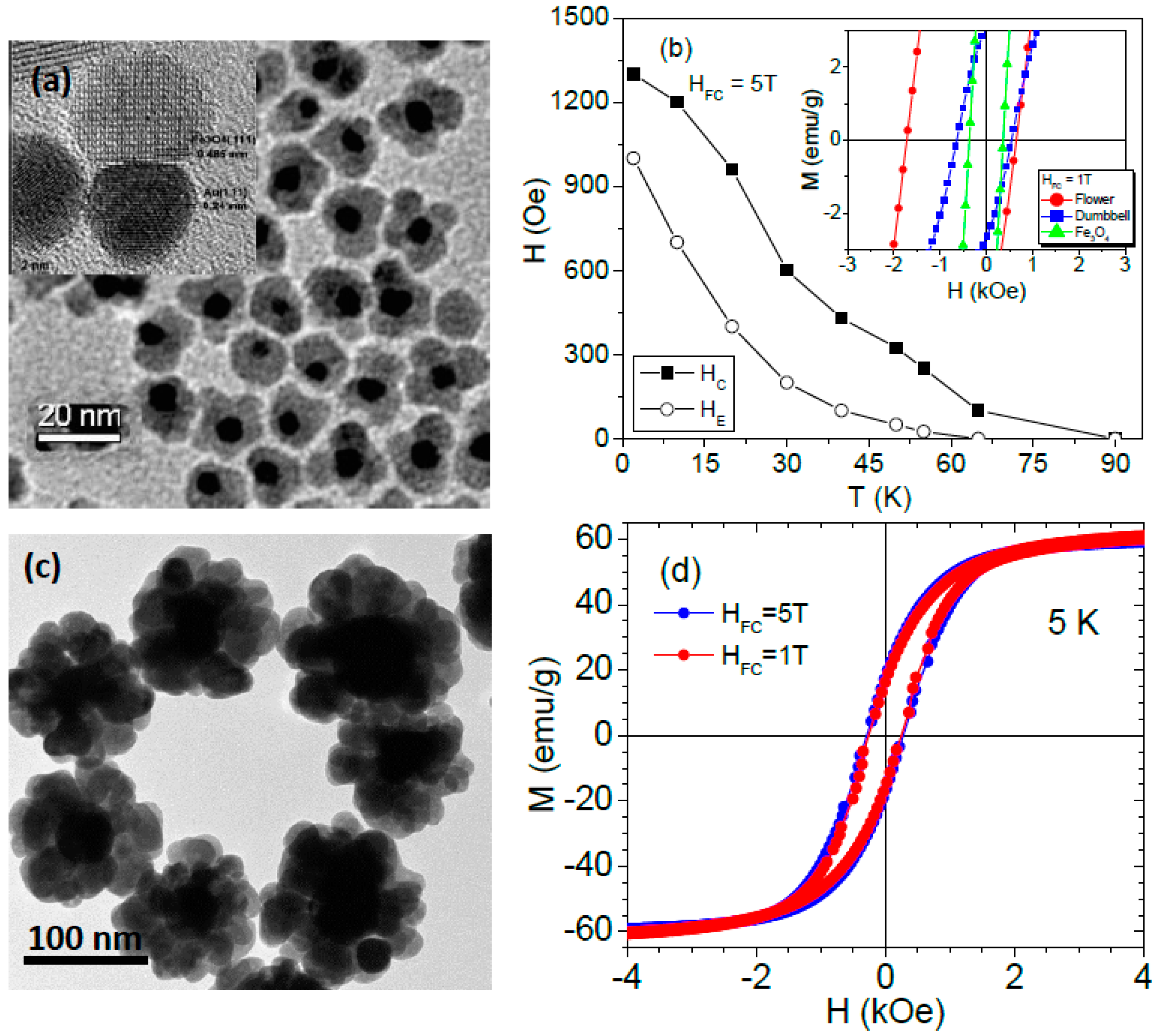
6. Exchange Bias Effect in Hybrid Composite Nanoparticles
7. Prospective Applications of Exchange-Coupled Nanoparticles
8. Concluding Remarks and Outlook
- (i)
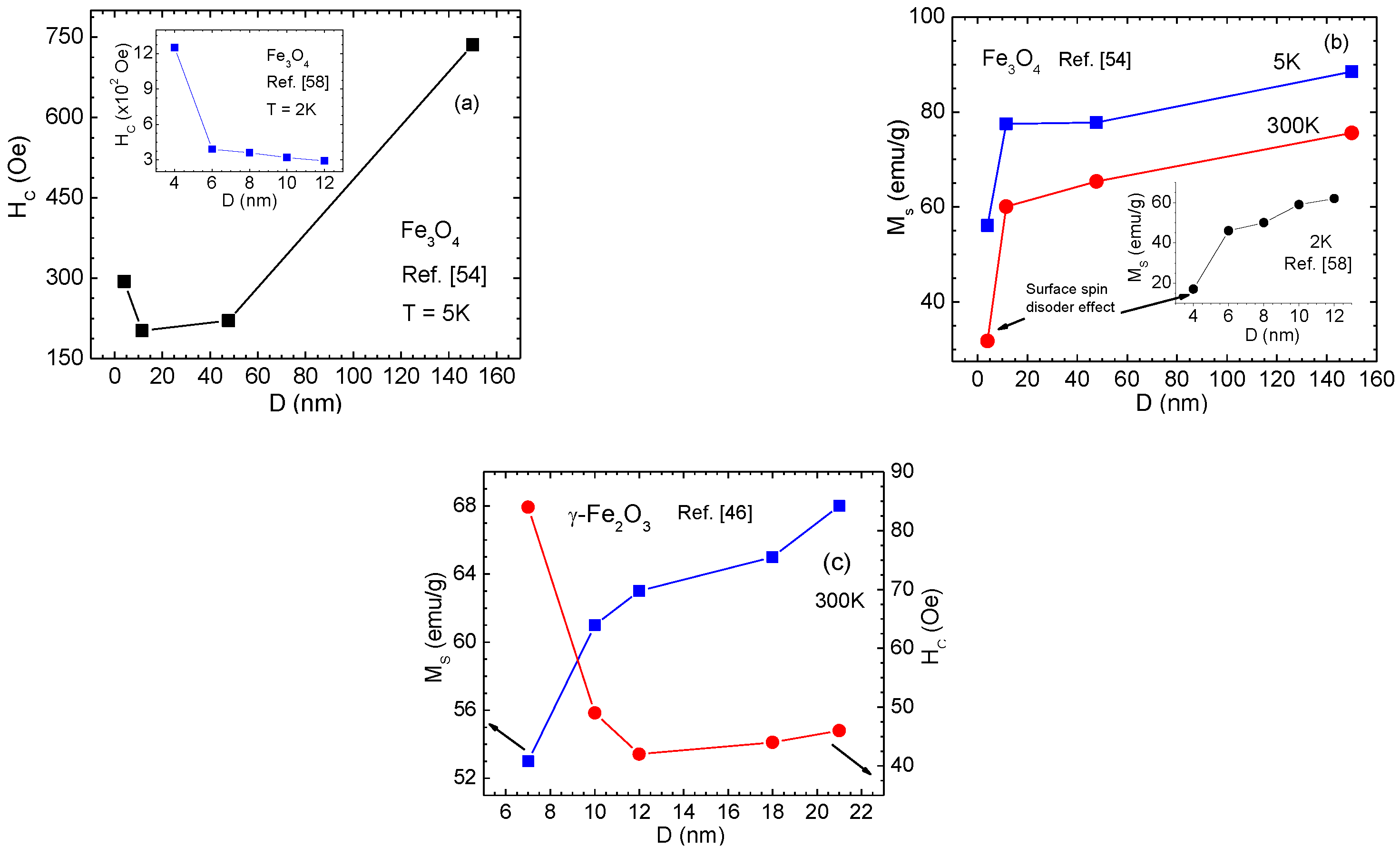
- In the case of solid, spherical, single-component MNPs, size reduction to the nanoscale (below 20 nm) has a greater influence on the magnetic ordering of surface spins, namely, a higher degree of disordering of surface spins of γ-Fe2O3 MNPs as compared to Fe3O4 MNPs of similar size. As a result, the γ-Fe2O3 MNPs possess a larger drop in MS and a greater EB effect.
- (ii)
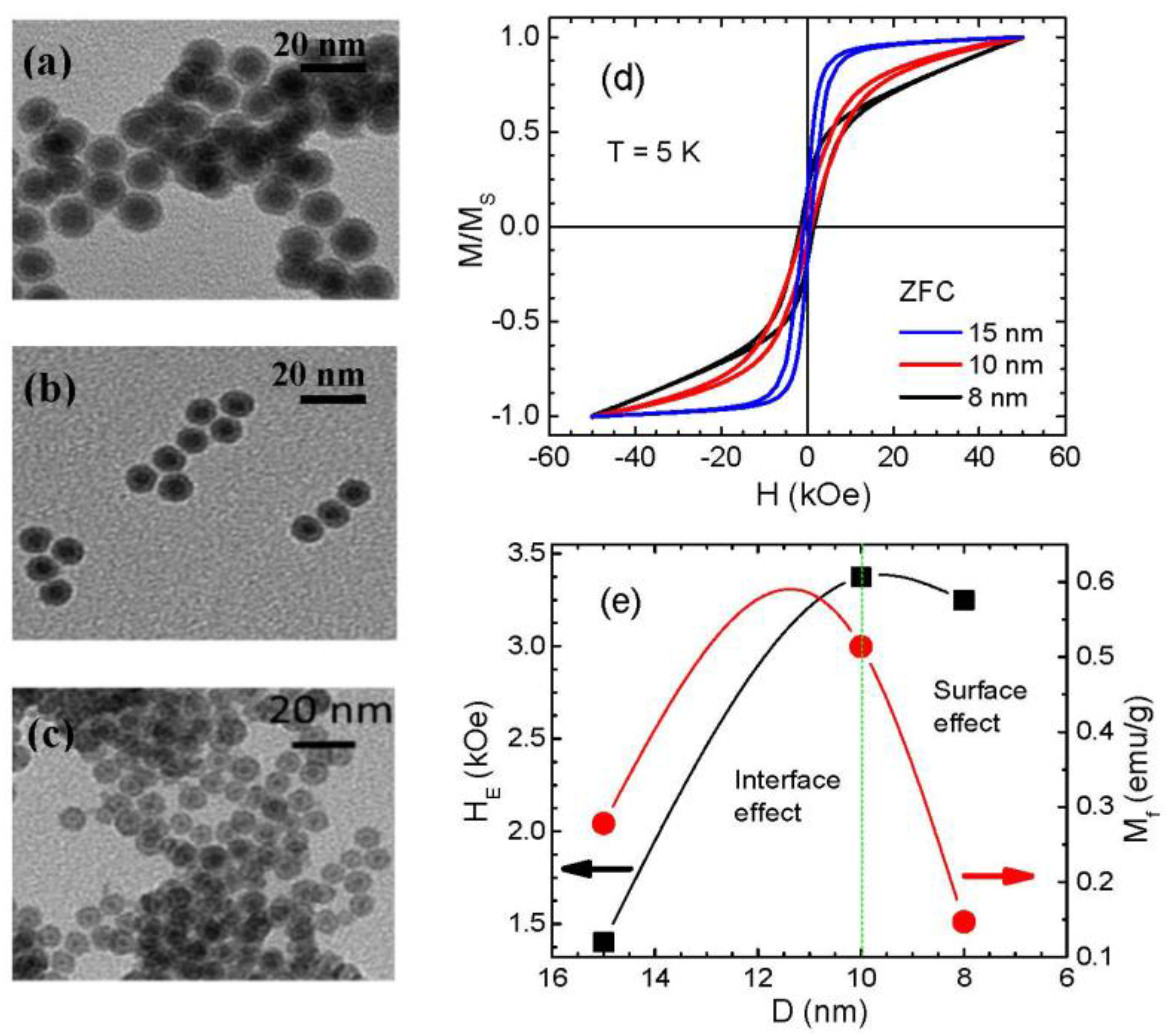
- In the case of core/shell MNPs, the interface spins play a more dominant role in triggering the EB effect in Fe3O4-based core/shell MNP systems such as Fe/Fe3O4 and FeO/Fe3O4 MNPs. The surface spins in the Fe3O4 shell layer have a much lower contribution to the EB than the interface spins. This is evident from the fact that the EB field is drastically reduced when the Fe core is removed from the core/shell structure. On the other hand, for the case of γ-Fe2O3-based core/shell nanoparticle systems like Fe/γ-Fe2O3 MNPs, there exists a critical particle size (do ~ 10 nm for Fe/γ-Fe2O3 MNPs of ~2 nm shell thickness), above which the interface spins contribute primarily to the EB, but below which the surface spin effect is dominant. Different values of do may be obtained when shell thickness is varied.
- (iii)
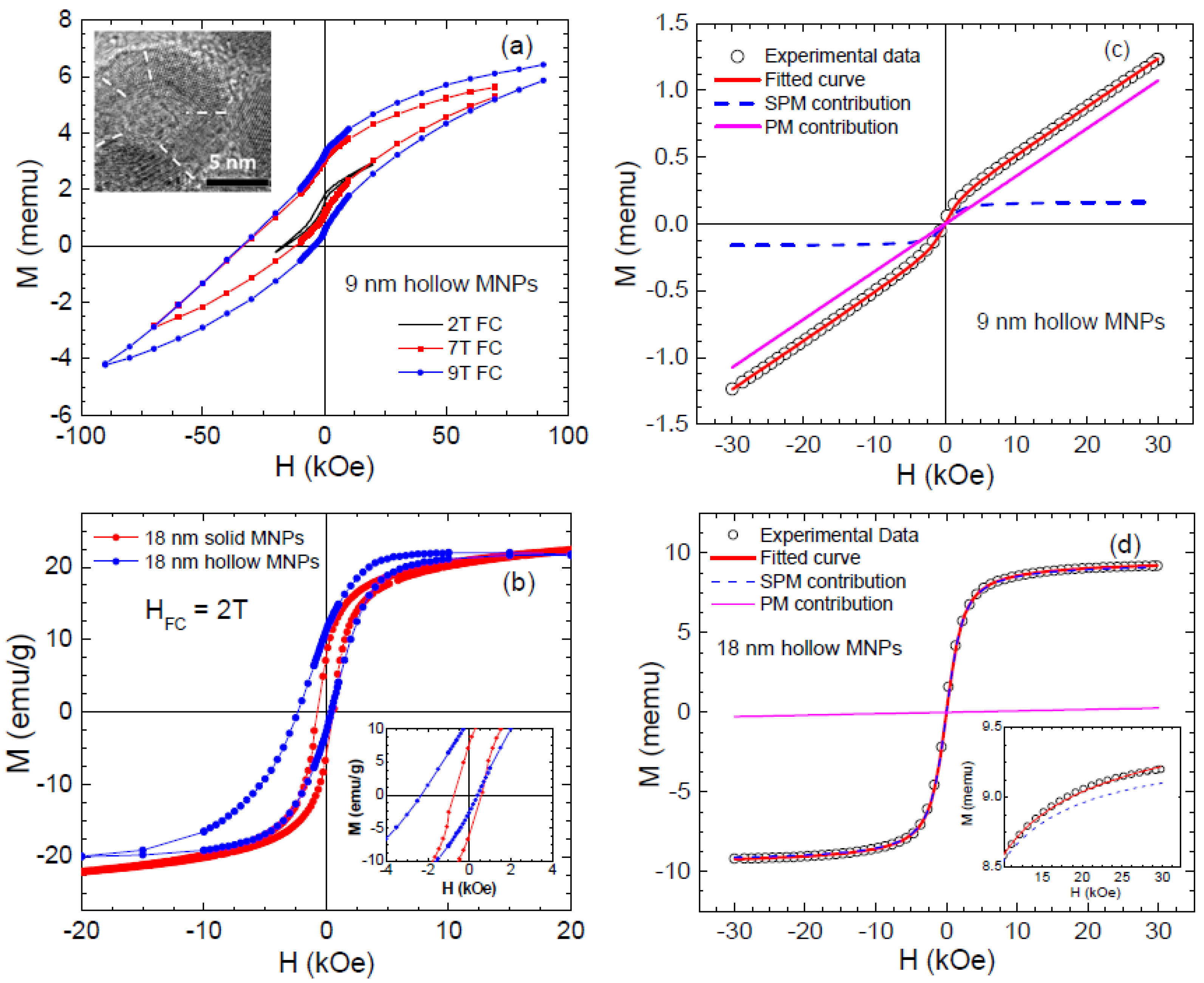
- In the case of hollow MNPs, both the inner and outer surface spins play important roles in determining the magnetic behavior and EB. The magnetic behavior is not identical at the inner and outer surfaces, with spins at the outer surface layer exhibiting a higher degree of frustration and thus contributing more dominantly to the EB. It is therefore possible to optimize EB by tuning the number of surface spins through varying the thickness of the shell layer and/or the outer diameter of the MNPs. The surface contributions to the coercivity and EB are almost identical for both solid and hollow morphologies, but the core magnetization contributions are different.
- (iv)
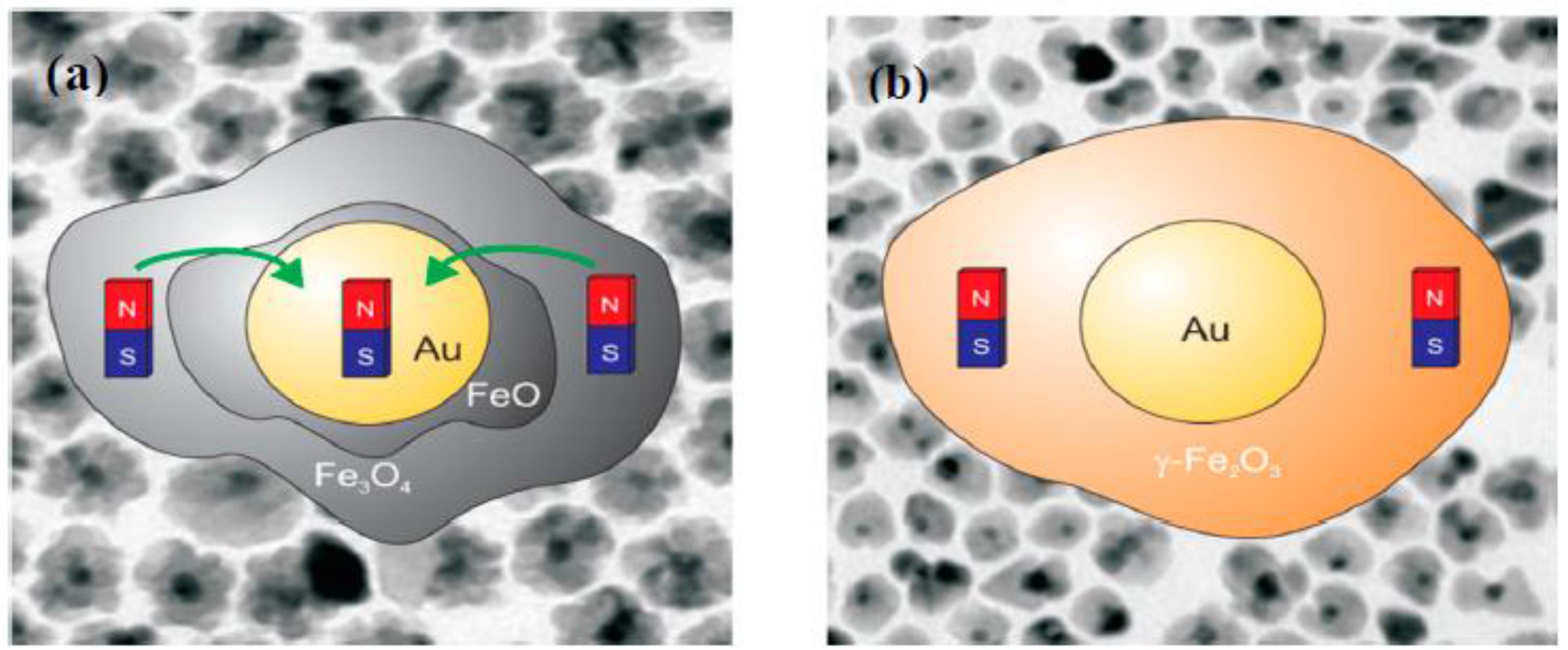
- In the case of hybrid composite nanoparticles, coating iron oxide MNPs with other non-magnetic metals can result in different consequences, depending upon the electronic states of the metals used and sample synthesis conditions. While the unexpected formation of an intermediate phase during sample synthesis, such as CuO in the case of γ-Fe2O3/Cu MNPs [92] and FeO in the case of Au/Fe3O4 MNPs [94], complicates the magnetic picture of the systems, it opens up a new pathway for tailoring the anisotropic magnetic properties of these nanostructures if this intermediate phase can be controllably engineered.
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B
Appendix B.1. Synthesis of Core/Shell Fe/γ-Fe2O3 and Hollow γ-Fe2O3

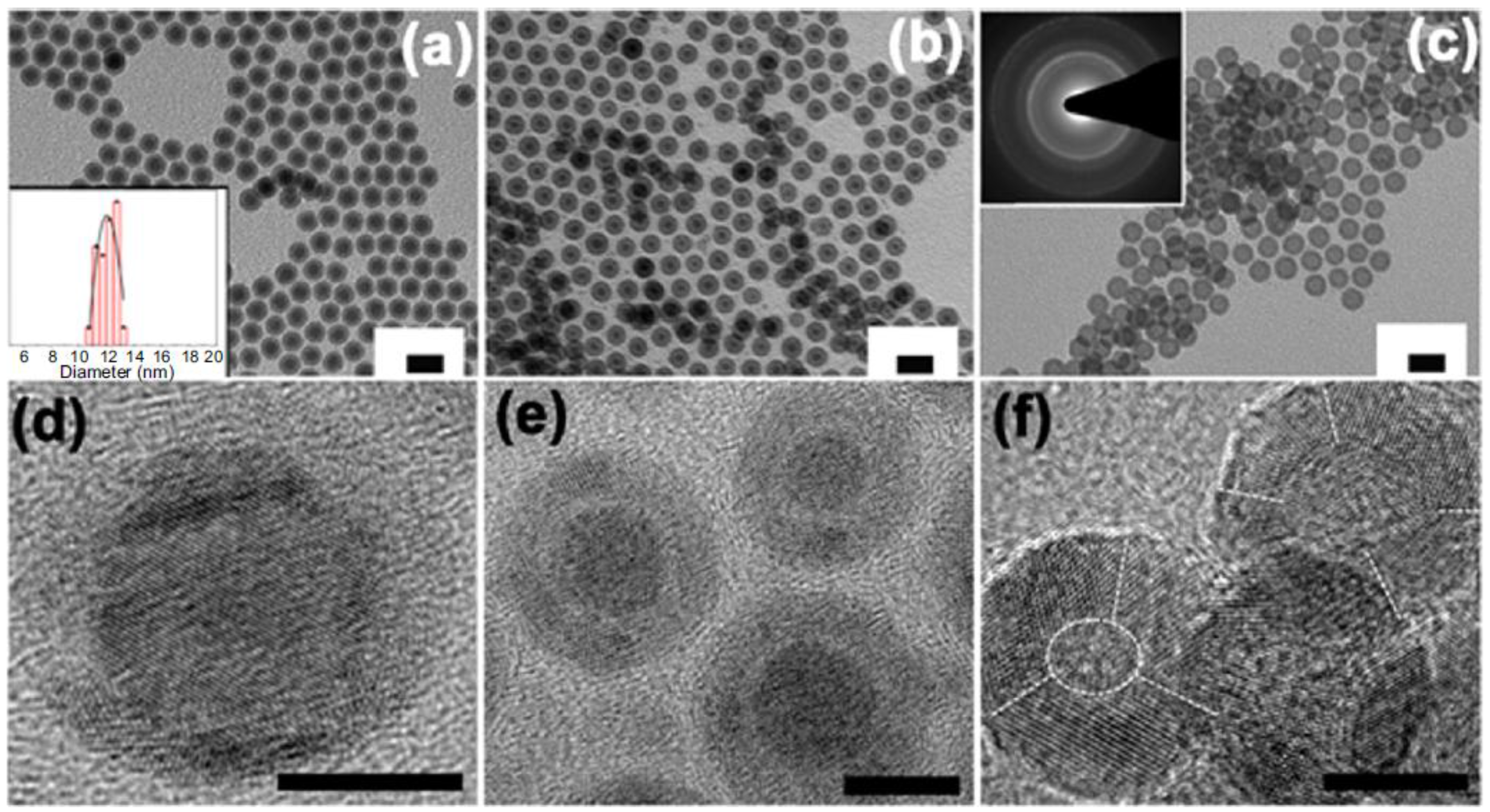
Appendix B.2. Synthesis of Ag/Fe3O4 Composite Nanoflowers
References
- Meiklejohn, W.H.; Bean, C.P. New Magnetic Anisotropy. Phys. Rev. 1956, 102, 1413. [Google Scholar] [CrossRef]
- Nogués, J.; Schuller, I.K. Exchange bias. J. Magn. Magn. Mater. 1999, 192, 203–232. [Google Scholar] [CrossRef]
- Skumryev, V.; Stoyanov, S.; Zhang, Y.; Hadjipanayis, G.; Givord, D.; Nogues, J. Beating the superparamagnetic limit with exchange bias. Nature 2003, 423, 850–853. [Google Scholar] [CrossRef] [PubMed]
- Nogués, J.; Sort, J.; Langlais, V.; Skumryev, V.; Suriñach, S.; Muñoz, J.S.; Baró, M.D. Exchange bias in nanostructures. Phys. Rep. 2005, 422, 65–117. [Google Scholar] [CrossRef]
- Iglesias, O.; Labarta, A.; Batlle, X. Exchange bias phenomenology and models of core/shell nanoparticles. J. Nanosci. Nanotechnol. 2008, 8, 2761–2780. [Google Scholar] [CrossRef] [PubMed]
- O’Grady, K.; Fernandez-Outon, L.E.; Vallejo-Fernandez, G. A new paradigm for exchange bias in polycrystalline thin films. J. Magn. Magn. Mater. 2010, 322, 883–899. [Google Scholar] [CrossRef]
- Giri, S.; Patra, M.; Majumdar, S. Exchange bias effect in alloys and compounds. J. Phys. Condens. Matter 2011, 23, 073201. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.K.; Yusuf, S.M. Two interface effects: Exchange bias and magnetic proximity. Phys. Rep. 2014, 535, 61–99. [Google Scholar] [CrossRef]
- Berkowitz, A.E.; Takano, K. Exchange anisotropy. J. Magn. Magn. Mater. 1999, 200, 552. [Google Scholar] [CrossRef]
- Stamps, R.L. Mechanisms for Exchange Bias. J. Phys. D 2000, 33, R247–R268. [Google Scholar] [CrossRef]
- Berkowitz, A.E.; Hong, J.I.; McCall, S.K.; Shipton, E.; Chan, K.T.; Leo, T.; Smith, D.J. Refining the exchange anisotropy paradigm: Magnetic and microstructural heterogeneity at the Permalloy-CoO interface. Phys. Rev. B 2010, 81, 134404. [Google Scholar] [CrossRef]
- Ali, M.; Adie, P.; Marrows, C.H.; Greig, D.; Hickey, B.J.; Stamps, R.L. Exchange bias using a spin glass. Nat. Mater. 2007, 6, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Cain, W.C.; Kryder, M.H. Investigation of the exchange mechanism in NiFe-TbCo bilayers. J. Appl. Phys. 1990, 67, 5722–5724. [Google Scholar] [CrossRef]
- Binek, C.; Polisetty, S.; He, X.; Berger, A. Exchange bias training effect in coupled all ferromagnetic bilayer structures. Phys. Rev. Lett. 2006, 96, 067201. [Google Scholar] [CrossRef] [PubMed]
- Van der Zaag, P.J.; Wolf, R.M.; Ball, A.R.; Bordel, C.; Feiner, L.F.; Jungblut, R. A study of the magnitude of exchange biasing in [111] Fe3O4/CoO bilayers. J. Magn. Magn. Mater. 1995, 148, 346–348. [Google Scholar] [CrossRef]
- Kishimoto, M.; Sueyoshi, T.; Hirata, J.; Amemiya, M.; Hayama, F. Coercivity of γ-Fe2O3 particles growing iron-cobalt ferrite. J. Appl. Phys. 1979, 50, 450–452. [Google Scholar] [CrossRef]
- Zheng, R.K.; Liu, H.; Zhang, X.X.; Roy, V.A.L.; Djurisic, A.B. Exchange bias and the origin of magnetism in Mn-doped ZnO tetrapods. Appl. Phys. Lett. 2004, 85, 2589–2591. [Google Scholar] [CrossRef] [Green Version]
- Kodama, R.H.; Berkowitz, A.E.; McNiff, E.J., Jr.; Foner, S. Surface Spin Disorder in NiFe2O4 Nanoparticles. Phys. Rev. Lett. 1996, 77, 394–397. [Google Scholar] [CrossRef] [PubMed]
- Martínez, B.; Obradors, X.; Balcells, L.; Rouanet, A.; Monty, C. Low temperature surface spin-glass transition in γ-Fe2O3 nanoparticles. Phys. Rev. Lett. 1998, 80, 181–184. [Google Scholar] [CrossRef]
- Peddis, D.; Cannas, C.; Piccaluga, G.; Agostinelli, E.; Fiorani, D. Spin-glass-like freezing and enhanced magnetization in ultra-small CoFe2O4 nanoparticles. Nanotechnology 2010, 21, 125705. [Google Scholar] [CrossRef] [PubMed]
- Pankhurst, Q.A.; Connolly, J.; Jones, S.K.; Dobson, J. Applications of magnetic nanoparticles in biomedicine. J. Phys. D 2003, 167, R167–R181. [Google Scholar] [CrossRef]
- Frey, N.A.; Peng, S.; Cheng, K.; Sun, S. Magnetic nanoparticles: Synthesis, functionalization, and applications in bioimaging and magnetic energy storage. Chem. Soc. Rev. 2009, 38, 2532–2542. [Google Scholar] [CrossRef] [PubMed]
- Kolhatkar, A.G.; Jamison, A.C.; Litvinov, D.; Willson, R.C.; Lee, T.R. Tuning the magnetic properties of nanoparticles. Int. J. Mol. Sci. 2013, 14, 15977–16009. [Google Scholar] [CrossRef] [PubMed]
- Hadjipanayis, C.G.; Bonder, M.J.; Balakrishnan, S.; Wang, X.; Mao, H.; Hadjipanayis, G.C. Metallic Iron Nanoparticles for MRI Contrast Enhancement and Local Hyperthermia. Small 2008, 4, 1925–1929. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, H.; Hadjipanayis, C.G.; Chen, H.; Li, W.; Mao, H.; MacHaidze, R.; Tzitzios, V.; Hadjipanayis, G.C. Core/shell structured iron/iron-oxide nanoparticles as excellent MRI contrast enhancement agents. J. Magn. Magn. Mater. 2013, 331, 17–20. [Google Scholar] [CrossRef]
- Zhou, Z.; Sun, Y.; Shen, J.; Wei, J.; Yu, C.; Kong, B.; Liu, W.; Yang, H.; Yang, S.; Wang, W. Iron/iron oxide core/shell nanoparticles for magnetic targeting MRI and near-infrared photothermal therapy. Biomaterials 2014, 35, 7470–7478. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.K.; Wen, G.H.; Fung, K.K.; Zhang, X.X. Training effect of exchange bias in γ-Fe2O3 coated Fe nanoparticles. Phys. Rev. B 2004, 69, 214431. [Google Scholar] [CrossRef]
- Chandra, S.; Khurshid, H.; Li, W.; Hadjipanayis, G.C.; Phan, M.H.; Srikanth, H. Spin dynamics and criteria for onset of exchange bias in superspin glass Fe/γ-Fe2O3 core-shell nanoparticles. Phys. Rev. B 2012, 86, 014426. [Google Scholar] [CrossRef]
- Khurshid, H.; Phan, M.-H.; Mukherjee, P.; Srikanth, H. Tuning exchange bias in Fe/γ-Fe2O3 core-shell nanoparticles: Impacts of interface and surface spins. Appl. Phys. Lett. 2014, 104, 072407. [Google Scholar] [CrossRef]
- Ong, Q.K.; Wei, A.; Lin, X.-M. Exchange bias in Fe/Fe3O4 core-shell magnetic nanoparticles mediated by frozen interfacial spins. Phys. Rev. B 2009, 80, 134418. [Google Scholar] [CrossRef]
- Ong, Q.K.; Lin, X.-M.; Wei, A. Role of Frozen Spins in the Exchange Anisotropy of Core-Shell Fe@Fe3O4 Nanoparticles. J. Phys. Chem. C 2011, 115, 2665–2672. [Google Scholar] [CrossRef] [PubMed]
- Kavich, D.W.; Dickerson, J.H.; Mahajan, S.V.; Hasan, S.A.; Park, J.H. Exchange bias of singly inverted FeO/Fe3O4 core-shell nanocrystals. Phys. Rev. B 2008, 78, 174414. [Google Scholar] [CrossRef]
- Sun, X.; Huls, N.F.; Sigdel, A.; Sun, S. Tuning exchange bias in core/shell FeO/Fe3O4 nanoparticles. Nano Lett. 2012, 12, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, H.; Li, W.; Chandra, S.; Phan, M.-H.; Hadjipanayis, G.C.; Mukherjee, P.; Srikanth, H. Mechanism and controlled growth of shape and size variant core/shell FeO/Fe3O4 nanoparticles. Nanoscale 2013, 5, 7942–7952. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.; Angappane, S.; Park, J.; An, K.; Hyeon, T.; Park, J.-G. Exchange bias behavior of monodisperse Fe3O4/γ-Fe2O3 core/shell nanoparticles. Curr. Appl. Phys. 2012, 12, 808–811. [Google Scholar] [CrossRef]
- Panagiotopoulos, I.; Basina, G.; Alexandrakis, V.; Devlin, E.; Hadjipanayis, G.; Colak, L.; Niarchos, D.; Tzitzios, V. Synthesis and Exchange Bias in γ-Fe2O3 /CoO and Reverse CoO/γ-Fe2O3 Binary Nanoparticles. J. Phys. Chem. C 2009, 113, 14609–14614. [Google Scholar] [CrossRef]
- Chandra, S.; Huls, N.A.F.; Phan, M.H.; Srinath, S.; Garcia, M.A.; Lee, Y.; Wang, C.; Sun, S.; Iglesias, Ò.; Srikanth, H. Exchange bias effect in Au-Fe3O4 nanocomposites. Nanotechnology 2014, 25, 055702. [Google Scholar] [CrossRef] [PubMed]
- Feygenson, M.; Bauer, J.C.; Gai, Z.; Marques, C.; Aronson, M.C.; Teng, X.; Su, D.; Stanic, V.; Urban, V.S.; Beyer, K.A.; et al. Exchange bias effect in Au-Fe3O4 dumbbell nanoparticles induced by the charge transfer from gold. Phys. Rev. B 2015, 92, 054416. [Google Scholar] [CrossRef]
- Dimitriadis, V.; Kechrakos, D.; Chubykalo-Fesenko, O.; Tsiantos, V. Shape-dependent exchange bias effect in magnetic nanoparticles with core-shell morphology. Phys. Rev. B 2015, 92, 064420. [Google Scholar] [CrossRef]
- Cabot, A.; Alivisatos, A.P.; Puntes, V.F.; Balcells, L.; Iglesias, Ò.; Labarta, A. Magnetic domains and surface effects in hollow maghemite nanoparticles. Phys. Rev. B 2009, 79, 094419. [Google Scholar] [CrossRef]
- Khurshid, H.; Li, W.; Phan, M.-H.; Mukherjee, P.; Hadjipanayis, G.C.; Srikanth, H. Surface spin disorder and exchange-bias in hollow maghemite nanoparticles. Appl. Phys. Lett. 2012, 101, 022403. [Google Scholar] [CrossRef]
- Khurshid, H.; Alonso, J.; Nemati, Z.; Phan, M.H.; Mukherjee, P.; Fdez-Gubieda, M.L.; Barandiarán, J.M.; Srikanth, H. Anisotropy effects in magnetic hyperthermia: A comparison between spherical and cubic exchange-coupled FeO/Fe3O4 nanoparticles. J. Appl. Phys. 2015, 117, 17A337. [Google Scholar] [CrossRef]
- Nemati, Z.; Khurshid, H.; Alonso, J.; Phan, M.H.; Mukherjee, P.; Srikanth, H. From core/shell to hollow Fe/γ-Fe2O3 nanoparticles: Evolution of the magnetic behavior. Nanotechnology 2015, 26, 405705. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, H.; Lampen-Kelley, P.; Iglesias, Ò.; Alonso, J.; Phan, M.-H.; Sun, C.-J.; Saboungi, M.-L.; Srikanth, H. Spin-glass-like freezing of inner and outer surface layers in hollow γ-Fe2O3 nanoparticles. Sci. Rep. 2015, 5, 15054. [Google Scholar] [CrossRef] [PubMed]
- Cornell, R.M.; Schwertmann, U. The Iron Oxydes: Structure, Properties, Reactions, Occurences and Uses; Wiley-Vch: Weinheim, Germany, 2003. [Google Scholar]
- Demortière, A.; Panissod, P.; Pichon, B.P.; Pourroy, G.; Guillon, D.; Donnio, B.; Bégin-Colin, S. Size-dependent properties of magnetic iron oxide nanocrystals. Nanoscale 2011, 3, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.P.; Verdangure, S.V.; Montero, M.I.; Serna, C.J.; Roig, A.; Casas, L.; Martinez, B.; Sandiumenge, F. Surface and Internal Spin Canting in. Chem. Mater. 1999, 11, 3058–3064. [Google Scholar] [CrossRef]
- Lak, A. Synthesis and Characterization of Magnetic Iron Oxide Nanoparticles. Ph.D. Thesis, Institut für Elektrische Messtechnik und Grundlagen der Elektrotechnik, Braunschweig, Germany, 2013. [Google Scholar]
- Greaves, C. A powder neutron diffraction investigation of vacancy ordering and covalence in γ-Fe2O3. J. Solid State Chem. 1983, 49, 325–333. [Google Scholar] [CrossRef]
- Grau-Crespo, R.; Al-Baitai, A.Y.; Saadoune, I.; De Leeuw, N.H. Vacancy ordering and electronic structure of γ-Fe2O3 (maghemite): A theoretical investigation. J. Phys. Condens. Matter 2010, 22, 255401. [Google Scholar] [CrossRef] [PubMed]
- Cullity, B.D.; Graham, C.D. Introduction to Magnetic Materials, 2nd ed.; Wiley-IEEE Press: Hoboken, NJ, USA, 2009. [Google Scholar]
- Verwey, E.J.W. Electronic Conduction of Magnetite (Fe3O4) and its Transition Point at Low Temperatures. Nature 1939, 144, 327–328. [Google Scholar] [CrossRef]
- Walz, F. The Verwey transition—A topical review. J. Phys. Condens. Matter 2002, 14, R285–R340. [Google Scholar] [CrossRef]
- Goya, G.F.; Berquó, T.S.; Fonseca, F.C.; Morales, M.P. Static and dynamic magnetic properties of spherical magnetite nanoparticles. J. Appl. Phys. 2003, 94, 3520–3528. [Google Scholar] [CrossRef]
- Majetich, S.A.; Sachan, M. Magnetostatic interactions in magnetic nanoparticle assemblies: Energy, time and length scales. J. Phys. D 2006, 39, R407–R422. [Google Scholar] [CrossRef]
- Bedanta, S.; Kleemann, W. Supermagnetism. J. Phys. D 2009, 42, 013001. [Google Scholar] [CrossRef]
- Krishnan, K.M. Biomedical nanomagnetics: A spin through possibilities in imaging, diagnostics, and therapy. IEEE Trans. Magn. 2010, 46, 2523–2558. [Google Scholar] [CrossRef] [PubMed]
- Dutta, P.; Pal, S.; Seehra, M.S.; Shah, N.; Huffman, G.P. Size dependence of magnetic parameters and surface disorder in magnetite nanoparticles. J. Appl. Phys. 2009, 105, 07B501. [Google Scholar] [CrossRef]
- Martínez-Boubeta, C.; Simeonidis, K.; Angelakeris, M.; Pazos-Pérez, N.; Giersig, M.; Delimitis, A.; Nalbandian, L.; Alexandrakis, V.; Niarchos, D. Critical radius for exchange bias in naturally oxidized Fe nanoparticles. Phys. Rev. B Condens. Matter Mater. Phys. 2006, 74, 054430. [Google Scholar] [CrossRef]
- Frison, R.; Cernuto, G.; Cervellino, A.; Zaharko, O.; Colonna, G.M.; Guagliardi, A.; Masciocchi, N. Magnetite-maghemite nanoparticles in the 5–15 nm range: Correlating the core-shell composition and the surface structure to the magnetic properties. A total scattering study. Chem. Mater. 2013, 25, 4820–4827. [Google Scholar] [CrossRef]
- Mazo-Zuluaga, J.; Restrepo, J.; Muñoz, F.; Mejía-López, J. Surface anisotropy, hysteretic, and magnetic properties of magnetite nanoparticles: A simulation study. J. Appl. Phys. 2009, 105, 123907. [Google Scholar] [CrossRef]
- Krycka, K.L.; Booth, R.A.; Hogg, C.R.; Ijiri, Y.; Borchers, J.A.; Chen, W.C.; Watson, S.M.; Laver, M.; Gentile, T.R.; Dedon, L.R.; et al. Core-shell magnetic morphology of structurally uniform magnetite nanoparticles. Phys. Rev. Lett. 2010, 104, 207723. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhu, T.; Zhao, K.; Wang, W.N.; Wang, C.S.; Wang, Y.J.; Zhan, W.S. Surface spin glass and exchange bias in Fe3O4 nanoparticles compacted under high pressure. Phys. Rev. B 2004, 70, 092409. [Google Scholar] [CrossRef]
- De Montferrand, C.; Lalatonne, Y.; Bonnin, D.; Lièvre, N.; Lecouvey, M.; Monod, P.; Russier, V.; Motte, L. Size-dependent nonlinear weak-field magnetic behavior of maghemite nanoparticles. Small 2012, 8, 1945–1956. [Google Scholar] [CrossRef] [PubMed]
- Shendruk, T.N.; Desautels, R.D.; Southern, B.W.; van Lierop, J. The effect of surface spin disorder on the magnetism of gamma-Fe2O3 nanoparticle dispersions. Nanotechnology 2007, 18, 455704. [Google Scholar] [CrossRef]
- Itoh, H.; Sugimoto, T. Systematic control of size, shape, structure, and magnetic properties of uniform magnetite and maghemite particles. J. Colloid Interface Sci. 2003, 265, 283–295. [Google Scholar] [CrossRef]
- Mukadam, M.D.; Yusuf, S.M.; Sharma, P.; Kulshreshtha, S.K. Particle size-dependent magnetic properties of γ-Fe2O3 nanoparticles. J. Magn. Magn. Mater. 2004, 272, 1401–1403. [Google Scholar] [CrossRef]
- Fiorani, D.; Testa, A.M.; Lucari, F.; D’Orazio, F.; Romero, H. Magnetic properties of maghemite nanoparticle systems: Surface anisotropy and interparticle interaction effects. Phys. B 2002, 320, 122–126. [Google Scholar] [CrossRef]
- Iglesias, Ò.; Labarta, A. Role of surface disorder on the magnetic properties and hysteresis of nanoparticles. Phys. B 2004, 343, 286–292. [Google Scholar] [CrossRef]
- Iglesias, Ò.; Labarta, A. Influence of surface anisotropy on the hysteresis of magnetic nanoparticles. J. Magn. Magn. Mater. 2005, 290–291, 738–741. [Google Scholar] [CrossRef]
- Iglesias, Ò.; Labarta, A. Influence of surface anisotropy on the magnetization reversal of nanoparticles. Phys. Status Solidi 2004, 1, 3481–3484. [Google Scholar] [CrossRef]
- Iglesias, Ò.; Labarta, A. Finite-size and surface effects in maghemite nanoparticles: Monte Carlo simulations. Phys. Rev. B 2001, 63, 184416. [Google Scholar] [CrossRef]
- Cabreira-Gomes, R.; Silva, F.G.; Aquino, R.; Bonville, P.; Tourinho, F.A.; Perzynski, R.; Depeyrot, J. Exchange bias of MnFe2O4@γFe2O3 and Co Fe2O4@γFe2O3 core/shell nanoparticles. J. Magn. Magn. Mater. 2014, 368, 409–414. [Google Scholar] [CrossRef]
- Kaur, M.; McCloy, J.S.; Qiang, Y. Exchange bias in core-shell iron-iron oxide nanoclusters. J. Appl. Phys. 2013, 113, 17D715. [Google Scholar] [CrossRef]
- Kaur, M.; McCloy, J.S.; Jiang, W.; Yao, Q.; Qiang, Y. Size dependence of inter- and intra-cluster interactions in core-shell iron-iron oxide nanoclusters. J. Phys. Chem. C 2012, 116, 12875–12885. [Google Scholar] [CrossRef]
- Bodnarchuk, M.I.; Kovalenko, M.V.; Groiss, H.; Resel, R.; Reissner, M.; Hesser, G.; Lechner, R.T.; Steiner, W.; Schöffler, F.; Heiss, W. Exchange-coupled bimagnetic wüstite/metal ferrite core/shell nanocrystals: Size, shape, and compositional control. Small 2009, 5, 2247–2252. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Li, J.; Wang, Z.L.; Liu, J.P.; Sun, S. Bimagnetic Core/Shell FePt/Fe3O4 Nanoparticles. Nano Lett. 2004, 4, 187–190. [Google Scholar] [CrossRef]
- Tracy, J.B.; Weiss, D.N.; Dinega, D.P.; Bawendi, M.G. Exchange biasing and magnetic properties of partially and fully oxidized colloidal cobalt nanoparticles. Phys. Rev. B 2005, 72, 064404. [Google Scholar] [CrossRef]
- Feygenson, M.; Yiu, Y.; Kou, A.; Kim, K.S.; Aronson, M.C. Controlling the exchange bias field in Co core/CoO shell nanoparticles. Phys. Rev. B 2010, 81, 195445. [Google Scholar] [CrossRef]
- Nguyen, H.M.; Hsiao, P.-Y.; Phan, M.-H. Monte Carlo study of a bilayer model for exchange bias using a magnetic glass exhibiting random magnetic anisotropy. J. Appl. Phys. 2010, 107, 09D706. [Google Scholar] [CrossRef]
- Iglesias, Ò.; Batlle, X.; Labarta, A. Microscopic origin of exchange bias in core/shell nanoparticles. Phys. Rev. B 2005, 72, 212401. [Google Scholar] [CrossRef]
- Evans, R.F.L.; Bate, D.; Chantrell, R.W.; Yanes, R.; Chubykalo-Fesenko, O. Influence of interfacial roughness on exchange bias in core-shell nanoparticles. Phys. Rev. B 2011, 84, 092404. [Google Scholar] [CrossRef]
- Margaris, G.; Trohidou, K.N.; Nogués, J. Mesoscopic model for the simulation of large arrays of Bi-magnetic core/shell nanoparticles. Adv. Mater. 2012, 24, 4331–4336. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.-P.P.; Guo, Y.-G.G.; Zhang, H.-M.M.; Hu, J.-S.S.; Wan, L.-J.J.; Bai, C.-L.L. Controllable AuPt bimetallic hollow nanostructures. Chem. Commun. 2004, 10, 1496–1497. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Im, S.S.; Lee, C.W.; Yu, J.H.; Choa, Y.H.; Oh, S.T. Hollow nanoparticles of beta-iron oxide synthesized by chemical vapor condensation. J. Nanopart. Res. 2004, 6, 627–631. [Google Scholar] [CrossRef]
- Ma, Y.; Huo, K.; Wu, Q.; Lu, Y.; Hu, Y.; Hu, Z.; Chen, Y. Self-templated synthesis of polycrystalline hollow aluminium nitride nanospheres. J. Mater. Chem. 2006, 16, 2834. [Google Scholar] [CrossRef]
- Cheng, K.; Peng, S.; Xu, C.; Sun, S. Porous hollow Fe3O4 nanoparticles for targeted delivery and controlled release of cisplatin. J. Am. Chem. Soc. 2009, 131, 10637–10644. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.J.; Gösele, U.; Zacharias, M. Formation of Nanotubes and Hollow Nanoparticles Based on Kirkendall and Diffusion Processes: A Review. Small 2007, 3, 1660–1671. [Google Scholar] [CrossRef] [PubMed]
- Jaffari, G.H.; Ceylan, A.; Ni, C.; Shah, S.I. Enhancement of surface spin disorder in hollow NiFe2O4 nanoparticles. J. Appl. Phys. 2010, 107, 013910. [Google Scholar] [CrossRef]
- Jaffari, G.H.; Ceylan, A.; Bui, H.P.; Beebe, T.P.; Ozcan, S.; Shah, S.I. Non-equilibrium cation distribution and enhanced spin disorder in hollow CoFe2O4 nanoparticles. J. Phys. Condens. Matter 2012, 24, 336004. [Google Scholar] [CrossRef] [PubMed]
- Desautels, R.D.; Skoropata, E.; Chen, Y.-Y.; Ouyang, H.; Freeland, J.W.; van Lierop, J. Tuning the surface magnetism of γ-Fe2O3 nanoparticles with a Cu shell. Appl. Phys. Lett. 2011, 99, 262501. [Google Scholar] [CrossRef]
- Desautels, R.D.; Skoropata, E.; Chen, Y.-Y.; Ouyang, H.; Freeland, J.W.; van Lierop, J. Increased surface spin stability in γ-Fe2O3 nanoparticles with a Cu shell. J. Phys. Condens. Matter 2012, 24, 146001. [Google Scholar] [CrossRef] [PubMed]
- Shevchenko, E.V.; Bodnarchuk, M.I.; Kovalenko, M.V.; Talapin, D.V.; Smith, R.K.; Aloni, S.; Heiss, W.; Alivisatos, A.P. Gold/Iron Oxide Core/Hollow-Shell Nanoparticles. Adv. Mater. 2008, 20, 4323–4329. [Google Scholar] [CrossRef]
- Pineider, F.; de Julián Fernández, C.; Videtta, V.; Carlino, E.; al Hourani, A.; Wilhelm, F.; Rogalev, A.; Cozzoli, P.D.; Ghigna, P.; Sangregorio, C. Spin-Polarization Transfer in Colloidal Magnetic-Plasmonic Au/Iron Oxide Hetero-nanocrystals. ACS Nano 2013, 7, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Velasco, V.; Muñoz, L.; Mazarío, E.; Menéndez, N.; Herrasti, P.; Hernando, A.; Crespo, P. Chemically synthesized Au–Fe3O4 nanostructures with controlled optical and magnetic properties. J. Phys. D 2015, 48, 035502. [Google Scholar] [CrossRef]
- Yu, H.; Chen, M.; Rice, P.M.; Wang, S.X.; White, R.L.; Sun, S. Dumbbell-like bifunctional Au-Fe3O4 nanoparticles. Nano Lett. 2005, 5, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Encina, E.R.; Coronado, E.A. Size Optimization of Iron Oxide@Noble Metal Core–Shell Nanohybrids for Photothermal Applications. J. Phys. Chem. C. 2016, 120, 5630–5639. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, F.; Aronova, M.; Zhu, L.; Lin, X.; Quan, Q.; Liu, G.; Zhang, G.; Choi, K.Y.; Kim, K.; et al. Manipulating the power of an additional phase: A flower-like au-Fe 3O4 optical nanosensor for imaging protease expressions in vivo. ACS Nano 2011, 5, 3043–3051. [Google Scholar] [CrossRef] [PubMed]
- Bødker, F.; Mørup, S.; Linderoth, S. Surface effects in metallic iron nanoparticles. Phys. Rev. Lett. 1994, 72, 282–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernán, O.S.; Vázquez de Parga, A.L.; Gallego, J.M.; Miranda, R. Self-surfactant effect on Fe/Au(100): Place exchange plus Au self-diffusion. Surf. Sci. 1998, 415, 106–121. [Google Scholar] [CrossRef]
- Wang, C.; Wei, Y.; Jiang, H.; Sun, S. Tug-of-war in nanoparticles: Competitive growth of Au on Au-Fe3O4 nanoparticles. Nano Lett. 2009, 9, 4544–4547. [Google Scholar] [CrossRef] [PubMed]
- Uhl, M.; Siberchicot, B. A first-principles study of exchange integrals in magnetite. J. Phys. Condens. Matter 1999, 7, 4227. [Google Scholar] [CrossRef]
- Swiatkowska-Warkocka, Z.; Kawaguchi, K.; Wang, H.; Katou, Y.; Koshizaki, N. Controlling exchange bias in Fe3O4/FeO composite particles prepared by pulsed laser irradiation. Nanoscale Res. Lett. 2011, 6, 226. [Google Scholar] [CrossRef] [PubMed]
- Lak, A.; Kraken, M.; Ludwig, F.; Kornowski, A.; Eberbeck, D.; Sievers, S.; Litterst, F.J.; Weller, H.; Schilling, M. Size dependent structural and magnetic properties of FeO-Fe3O4 nanoparticles. Nanoscale 2013, 5, 12286–12295. [Google Scholar] [CrossRef] [PubMed]
- Wetterskog, E.; Tai, C.W.; Grins, J.; Bergstrom, L.; Salazar-Alvarez, G. Anomalous magnetic properties of nanoparticles arising from defect structures: Topotaxial oxidation of Fe1-xO|Fe3-δO4 core|shell nanocubes to single-phase particles. ACS Nano 2013, 7, 7132. [Google Scholar]
- Cao, X.; Wang, W.; Zhang, X.; Li, L.; Cheng, Y.; Liu, H.; Du, S.; Zheng, R. Magnetic properties of fluffy Fe@α-Fe2O3 core-shell nanowires. Nanoscale Res. Lett. 2013, 8, 423. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.P.; Fullerton, E.; Gutfleisch, O.; Sellmyer, D.J. Nanoscale Magnetic Materials and Applications; Springer: New York, NY, USA, 2009. [Google Scholar]
- López-Ortega, A.; Estrader, M.; Salazar-Alvarez, G.; Roca, A.G.; Nogués, J. Applications of exchange coupled bi-magnetic hard/soft and soft/hard magnetic core/shell nanoparticles. Phys. Rep. 2015, 553, 1–32. [Google Scholar] [CrossRef]
- Lottini, E.; López-Ortega, A.; Bertoni, G.; Turner, S.; Meledina, M.; Van Tendeloo, G.; de Julián Fernández, C.; Sangregorio, C. Strongly Exchange Coupled Core|Shell Nanoparticles with High Magnetic Anisotropy: A Strategy toward Rare-Earth-Free Permanent Magnets. Chem. Mater. 2016, 28, 4214–4222. [Google Scholar] [CrossRef]
- Lee, J.-H.; Jang, J.-T.; Choi, J.-S.; Moon, S.H.; Noh, S.-H.; Kim, J.-W.; Kim, J.-G.; Kim, I.-S.; Park, K.I.; Cheon, J. Exchange-coupled magnetic nanoparticles for efficient heat induction. Nat. Nanotechnol. 2011, 6, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Lak, A.; Niculaes, D.; Anyfantis, G.C.; Bertoni, G.; Barthel, M.J.; Marras, S.; Cassani, M.; Nitti, S.; Athanassiou, A.; Giannini, C.; et al. Facile transformation of FeO/Fe3O4 core-shell nanocubes to Fe3O4 via magnetic stimulation. Sci. Rep. 2016, 6, 33295. [Google Scholar] [CrossRef] [PubMed]
- Nemati, Z.; Alonso, J.; Khurshid, H.; Phan, M.H.; Srikanth, H. Core/shell iron/iron oxide nanoparticles: Are they promising for magnetic hyperthermia? RSC Adv. 2016, 6, 38697–38702. [Google Scholar] [CrossRef]
- Frey, N.A.; Phan, M.H.; Srikanth, H.; Srinath, S.; Wang, C.; Sun, S. Interparticle interactions in coupled Au–Fe3O4 nanoparticles. J. Appl. Phys. 2009, 105, 07B502. [Google Scholar] [CrossRef]
- Hai, H.T.; Yang, H.T.; Kura, H.; Hasegawa, D.; Ogata, Y.; Takahashi, M.; Ogawa, T. Size control and characterization of wustite (core)/spinel (shell) nanocubes obtained by decomposition of iron oleate complex. J. Colloid Interface Sci. 2010, 346, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Dai, Q.; Bowden, M.; Mh, E.; Wu, Y.; Tang, J.; Qiang, Y. Watermelon-like iron nanoparticles: Cr doping effect on magnetism and magnetization interaction reversal. Nanoscale 2013, 5, 7872–7881. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Rinaldi-Montes, N.; Alonso, J.; Amghouz, Z.; Gorria, P.; Blanco, J.A.; Phan, M.H.; Srikanth, H. Ag@Fe3O4 nanoflowers as dual agents for magnetic hyperthermia and photo-thermal therapy. ACS Appl. Mater. Interfaces 2016, 8, 25162–25169. [Google Scholar] [CrossRef] [PubMed]
- Landau, D.P.; Binder, K. A Guide to Monte Carlo Simulations in Statistical Physics; Cambridge University Press: New York, NY, USA, 2005. [Google Scholar]


| Material | Total Diameter, d (nm) | Types of Magnetic Coupling | Loop Shift, HE (Oe) | Blocking Temperature, TB (K) | Reference |
|---|---|---|---|---|---|
| Fe3O4 (solid) | 9 | FI | 0 | 36 | [37] |
| Fe3O4 (solid), under 5 GPa pressure | 20 | FI | 800 | 40 | [63] |
| Fe3O4 (hollow) | 16/4.5 | FI | 133 | -- | [31] |
| Fe/Fe3O4 (C/S) | 14/2.5 | FM/FI | 1190 | 115 | [31] |
| FeO/Fe3O4 (C/S) | 14/3.5 | AFM/FI | 471 | -- | [32] |
| FeO/Fe3O4 (C/S) | 35/4 | AFM/FI | 2250 | 270 | [33] |
| FeO/Fe3O4 (C/S) | 18/6 | AFM/FI | 1000 | 210 | [104] |
| FeO/Fe3O4 (C/S) | 24/5 | AFM/FI | 3700 | 260 | [104] |
| FeO/Fe3O4 (C/S) | 10/0.6 | AFM/FI | 1700 | 220 | [34] |
| FeO/Fe3O4 (C/S) | 20 | AFM/FI | 1550 | >275 | [105] |
| Fe3O4/FeO (C/S) | 6 | FI/AFM | 514 | -- | [103] |
| Fe3O4/γ-Fe2O3 (C/S) | 12 | FI/FI | 140 | 105 | [35] |
| Au/Fe3O4 (dumbbell) | 8/9 | FI | 260 | 65 | [37] |
| Au/Fe3O4 (flower) | 8/9 | FI | 500 | 90 | [37] |
| Au/FeO/Fe3O4 (C/S) | 10/14 | AFM/FI | 860 | 250 | [92] |
| γ-Fe2O3 (solid) | 4 | FI | 750 | 7 | [58] |
| γ-Fe2O3 (solid) | 7 | FI | 60 | 20 | [65] |
| γ-Fe2O3 (solid) | 10 | FI | 1500 | 42 | [19] |
| γ-Fe2O3 (hollow) | 19/4.5 | FI | 960 | 150 | [41] |
| γ-Fe2O3/CuO/Cu (C/S) | 7/0.5 | FI/AFM | 18 | 50 | [92] |
| Fe/γ-Fe2O3 (C/S) | 11 | FM/FI | 6300 | 110 | [27] |
| γ-Fe2O3/CoO (C/S) | 5 | FI/AFM | 1200 | -- | [36] |
| CoO/γ-Fe2O3 (C/S) | 5 | AFM/FI | 2000 | -- | [36] |
| MnFe2O4/γ-Fe2O3 (C/S) | 3.3/0.4 | FI/FI | 86 | -- | [73] |
| CoFe2O4/γ-Fe2O3 (C/S) | 3.1/0.5 | FI/FI | 125 | -- | [73] |
| Au/γ-Fe2O3 (C/S) | 13/3 | FI | 1200 | 65 | [93] |
| Fe/α-Fe2O3 (C/S) | 70/10 | FM/AFM | 78 | -- | [106] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phan, M.-H.; Alonso, J.; Khurshid, H.; Lampen-Kelley, P.; Chandra, S.; Stojak Repa, K.; Nemati, Z.; Das, R.; Iglesias, Ó.; Srikanth, H. Exchange Bias Effects in Iron Oxide-Based Nanoparticle Systems. Nanomaterials 2016, 6, 221. https://doi.org/10.3390/nano6110221
Phan M-H, Alonso J, Khurshid H, Lampen-Kelley P, Chandra S, Stojak Repa K, Nemati Z, Das R, Iglesias Ó, Srikanth H. Exchange Bias Effects in Iron Oxide-Based Nanoparticle Systems. Nanomaterials. 2016; 6(11):221. https://doi.org/10.3390/nano6110221
Chicago/Turabian StylePhan, Manh-Huong, Javier Alonso, Hafsa Khurshid, Paula Lampen-Kelley, Sayan Chandra, Kristen Stojak Repa, Zohreh Nemati, Raja Das, Óscar Iglesias, and Hariharan Srikanth. 2016. "Exchange Bias Effects in Iron Oxide-Based Nanoparticle Systems" Nanomaterials 6, no. 11: 221. https://doi.org/10.3390/nano6110221