Electrochemical Synthesis of Polypyrrole, Reduced Graphene Oxide, and Gold Nanoparticles Composite and Its Application to Hydrogen Peroxide Biosensor
Abstract
:1. Introduction
2. Results
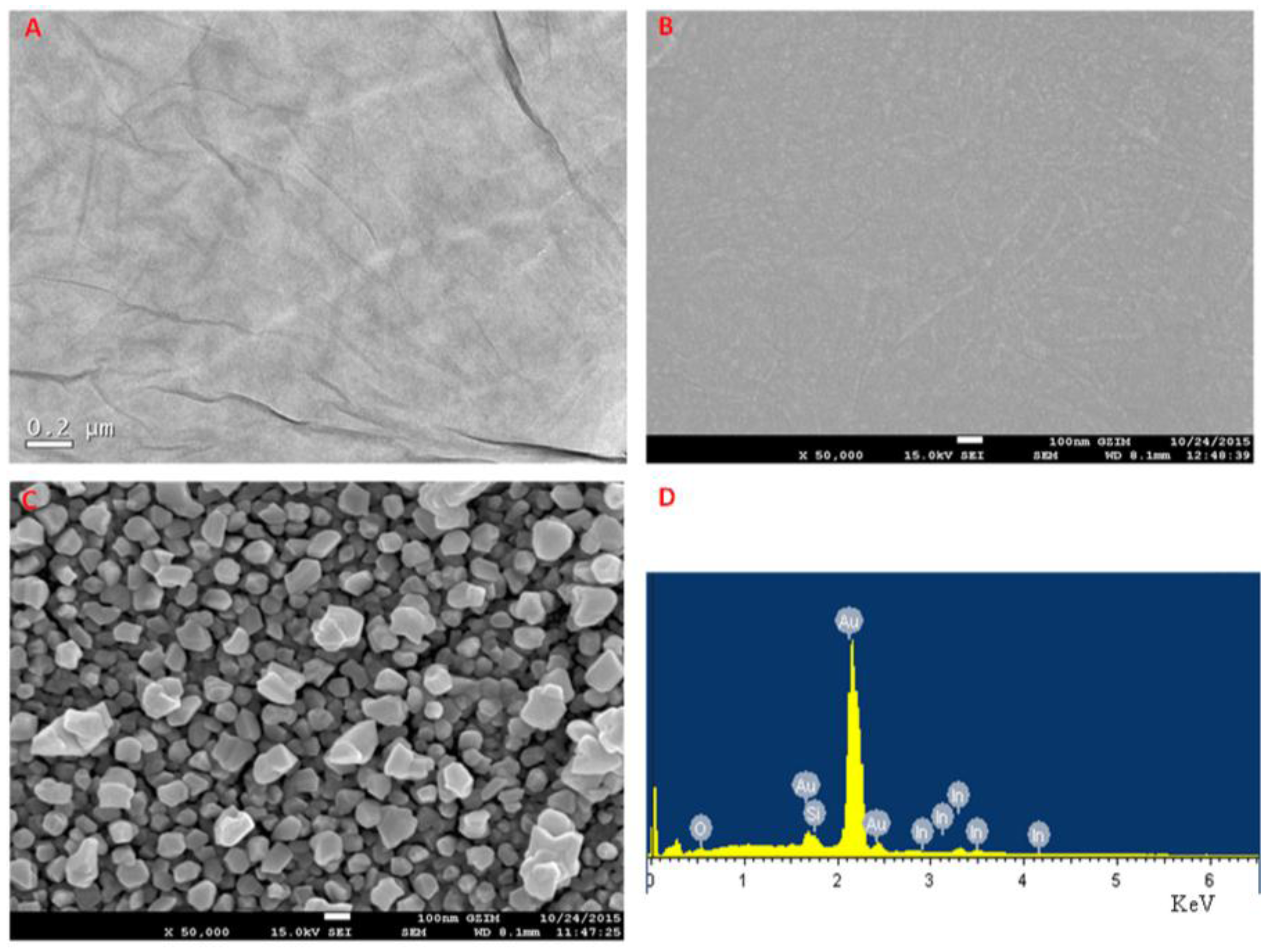
2.1. Preparation and Morphology Characterization
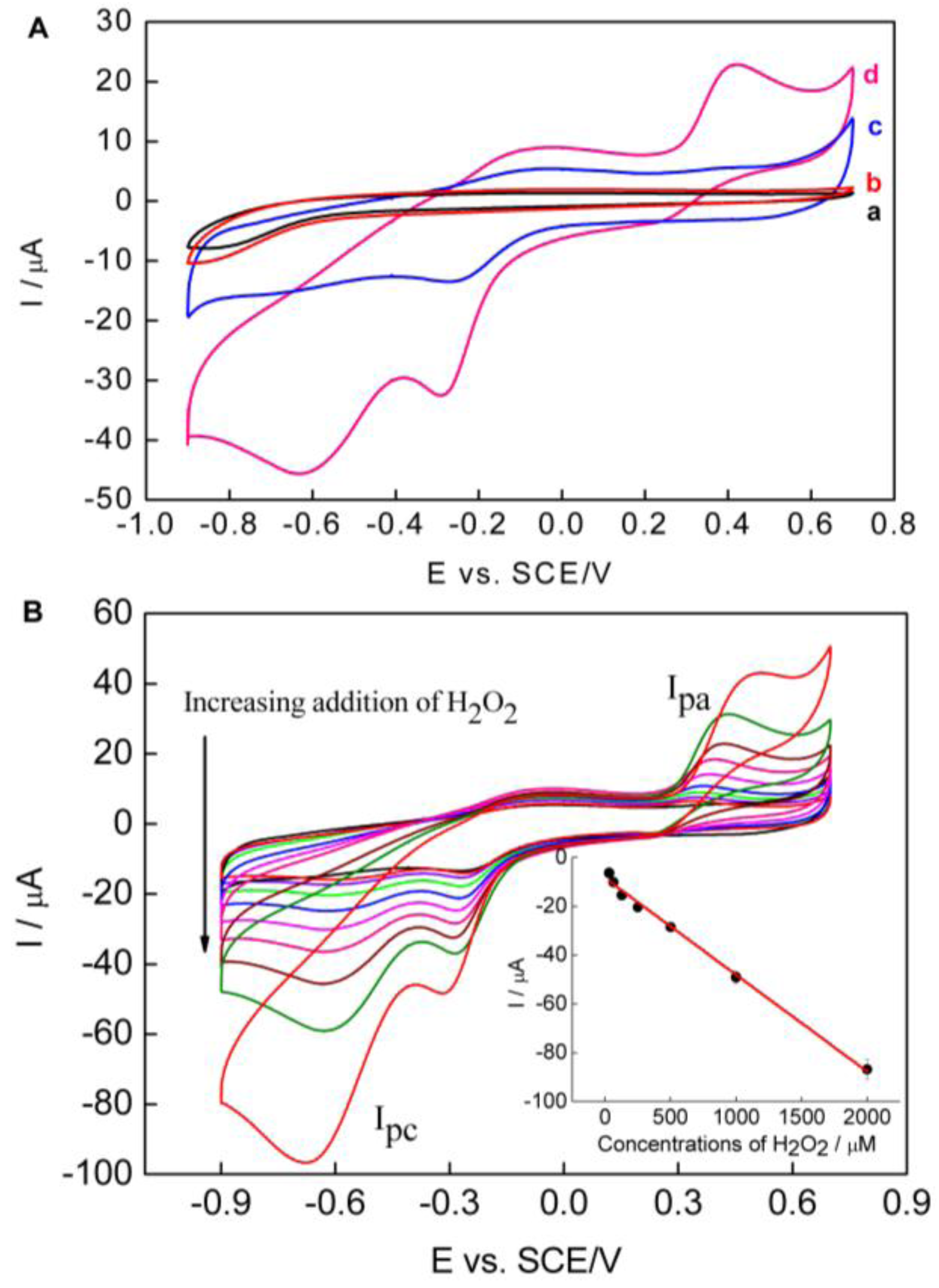
2.2. Electrochemical Behaviors of the PPy–RGO–NanoAu
2.3. Determination of Hydrogen Peroxide Using the PPy–RGO–NanoAu/GCE
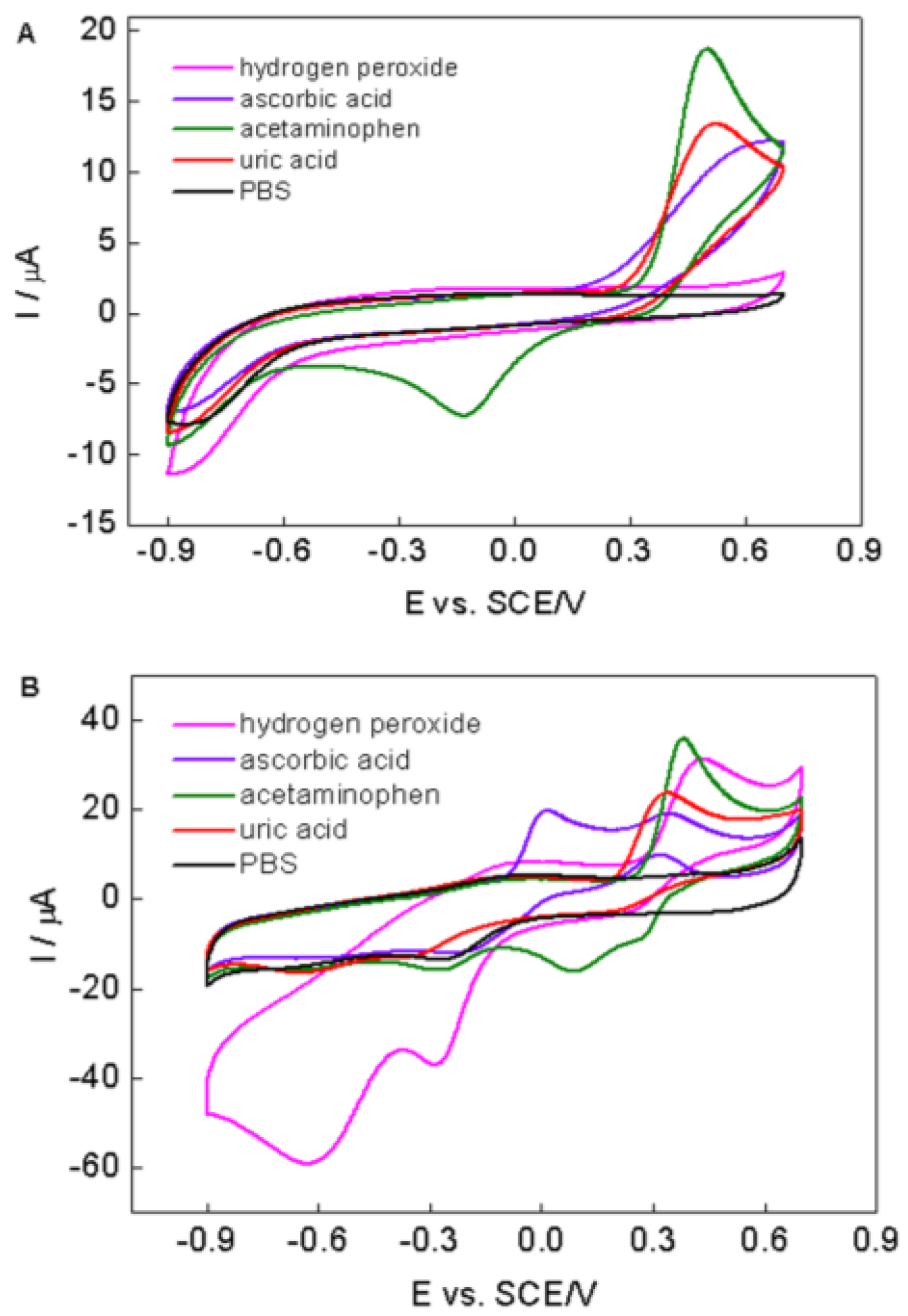
2.4. Stability and Selectivity of the PPy–RGO–NanoAu/GCE
3. Materials and Methods
3.1. Reagents
3.2. Apparatus and Measurements
3.3. Preparation of PPy–RGO–NanoAu/GCE
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hrapovic, S.; Liu, Y.; Male, K.; Luong, J. Electrochemical biosensing platforms using platinum nanoparticles and carbon nanotubes. Anal. Chem. 2004, 76, 1083–1088. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Hou, S.; Yin, F.; Zhao, Z.; Wang, Y.; Wang, X.; Chen, Q. Amperometric glucose biosensor based on multilayer films via layer-by-layer self-assembly of multi-wall carbon nanotubes, gold nanoparticles and glucose oxidase on the Pt electrode. Biosens. Bioelectron. 2007, 22, 2854–2860. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yang, M.; Qu, F.; Shen, G.; Yu, R. Enzyme-functionalized gold nanowires for the fabrication of biosensors. Bioelectrochemistry 2007, 71, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, X.; Guo, Z.; Hu, Z.; Xue, Z.; Lu, X. Horseradish peroxidase supported on porous graphene as a novel sensing platform for detection of hydrogen peroxide in living cells sensitively. Biosens. Bioelectron. 2017, 87, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, Y.; Du, X.; Chen, Y.; Dong, W.; Han, B.; Chen, Q. Facile fabrication of Pt-Ag bimetallic nanoparticles decorated reduced graphene oxide for highly sensitive non-enzymatic hydrogen peroxide sensing. Talanta 2016, 159, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Haghighi, B.; Tabrizi, M. Direct electron transfer from glucose oxidase immobilized on an overoxidized polypyrrole film decorated with Au nanoparticles. Colloids Surf. B 2013, 103, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yu, C.; Gao, R.; Xia, C.; Yuan, G.; Li, Y.; Zhao, Y.; Chen, Q.; He, J. A novel DNA biosensor integrated with polypyrrole/streptavidin and Au-PAMAM-CP bionanocomposite probes to detect the rs4839469 locus of the vangl1 gene for dysontogenesis prediction. Biosens. Bioelectron. 2016, 80, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Li, C.M. Nanocomposites from fabrications to electrochemical bioapplications. Electroanalysis 2008, 20, 648–662. [Google Scholar] [CrossRef]
- Dutta, D.; Chandra, S.; Swain, A.; Bahadur, D. SnO2 Quantum dots-reduced graphene oxide composite for enzyme-Free ultrasensitive electrochemical detection of urea. Anal. Chem. 2014, 86, 5914–5921. [Google Scholar] [CrossRef] [PubMed]
- Xue, K.; Zhou, S.; Shi, H.; Feng, X.; Xin, H.; Song, W. A novel amperometric glucose biosensor based on ternary goldnanoparticles/polypyrrole/reduced graphene oxide nanocomposite. Sens. Actuators B 2014, 203, 412–416. [Google Scholar] [CrossRef]
- Wang, F.; Zhu, L.; Zhang, J. Electrochemical sensor for levofloxacin based on molecularly imprinted polypyrrole-graphene-gold nanoparticles modified electrode. Sens. Actuators B 2014, 192, 642–647. [Google Scholar] [CrossRef]
- Yang, Y.; Asiri, A.; Du, D.; Lin, Y. Acetylcholinesterase biosensor based on a gold nanoparticle-polypyrrole-reduced grapheme oxide nanocomposite modified electrode for the amperometric detection of organophosphorus pesticides. Analyst 2014, 139, 3055–3060. [Google Scholar] [CrossRef] [PubMed]
- Tuyen, D.; Quan, D.; Binh, N.; Nguyen, V.; Van Chuc, N.; Dai, L.T.; Trong, H.L.; Le, H.N.; Hung, V.P.; Thai, L.N.; et al. A highly sensitive electrode modified with graphene, gold nanoparticles, and molecularly imprinted over-oxidized polypyrrole for electrochemical determination of dopamine. J. Mol. Liq. 2014, 198, 307–312. [Google Scholar]
- Tiwari, I.; Gupta, M.; Pandey, C.; Mishra, V. Gold nanoparticle decorated graphene sheet polypyrrole based nanocomposite: Its synthesis, characterization and genosensing application. Dalton Trans. 2015, 44, 15557–15566. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, X. Electrochemical co-reduction synthesis of graphene/nano-gold composites and its application to electrochemical glucose biosensor. Electrochim. Acta 2013, 112, 774–782. [Google Scholar] [CrossRef]
- Guo, H.; Wang, X.; Qian, Q.; Wang, F.; Xia, X. A Green approach to the synthesis of graphene nanosheets. ACS Nano 2009, 3, 2653–2659. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Wang, Y.; Zhai, Y.; Zhai, J.; Ren, W.; Wang, F.; Dong, S. Controlled synthesis of large-area and patterned electrochemically reduced graphene oxide Films. Chem. Eur. J. 2009, 15, 6116–6120. [Google Scholar] [CrossRef] [PubMed]
- Camacho, C.; Matías, J.; Cao, R.; Matos, M.; Chico, B.; Herna’ndez, J.; Longo, M.; Sanroma’n, M.; Villalonga, R. Hydrogen peroxide biosensor with a supramolecular layer-by-layer design. Langmuir 2008, 24, 7654–7657. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Yang, X.; Wu, T.; Li, Y.; Li, M.; Tan, Q.; Wang, X.; Tang, B. Rational design of an α-ketoamide-based near-infrared fluorescent probe specific for hydrogen peroxide in living systems. Anal. Chem. 2016, 88, 8019–8025. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Hou, S.; Yin, F.; Li, J.; Zhao, Z.; Huang, J.; Chen, Q. Amperometric glucose biosensor based on layer-by-layer assembly of multilayer films composed of chitosan, gold nanoparticles and glucose oxidase modefied Pt electrode. Biosens. Bioelectron. 2007, 22, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Sampson, N.; Verielink, A. Cholesterol oxidases: A study of nature’s approach to protein design. Acc. Chem. Res. 2003, 36, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Ou, Z.; Chen, Q.; Wu, B. Amperometric acetylcholine biosensor based on self-assembly of gold nanoparticles and acetylcholinesterase on the sol-gel/multi-walled carbon nanotubes/choline oxidase composite-modified platinum electrode. Biosens. Bioelectron. 2012, 33, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Wang, H.; Schultz, Z.; Camden, P. Sensing glucose in urine and serum and hydrogen peroxide in living cells by use of a novel boronate nanoprobe based on surface-enhanced raman spectroscopy. Anal. Chem. 2016, 88, 7191–7197. [Google Scholar] [CrossRef] [PubMed]
- Thanh, T.; Balamurugan, J.; Lee, S.; Kim, N.; Lee, J. Novel porous gold-palladium nanoalloy network-supported graphene as an advanced catalyst for non-enzymatic hydrogen peroxide sensing. Biosens. Bioelectron. 2016, 85, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.G.; Voinov, M.A.; Schmidt, A.C.; Smirnova, T.I.; Sombers, L.A. The hydroxyl radical is a critical intermediate in the voltammetric detection of hydrogen peroxide. J. Am. Chem. Soc. 2016, 138, 2516–2519. [Google Scholar] [CrossRef] [PubMed]
- Molina, J.; Bonastre, J.; Fernández, J.; del Río, A.I.; Cases, F. Electrochemical synthesis of polypyrrole doped with graphene oxide and its electrochemical characterization as membrane material. Synth. Met. 2016, 220, 300–310. [Google Scholar] [CrossRef]
- Phatthanakittiphong, H.; Seo, G. Characteristic evaluation of graphene oxide for bisphenol a adsorption in aqueous solution. Nanomaterials 2016, 6, 128. [Google Scholar] [CrossRef]
- Sadki, S.; Schottland, P.; Brodie, N.; Sabourauda, G. The mechanisms of pyrrole electropolymerization. Chem. Soc. Rev. 2000, 29, 283–293. [Google Scholar]
- Lim, Y.S.; Tan, Y.P.; Lim, H.N.; Tan, W.T.; Mahnaz, M.A.; Talib, Z.A.; Huang, N.M.; Kassim, A.; Yarmo, M.A. Polypyrrole/graphene composite films synthesized via potentiostatic deposition. J. Appl. Polym. Sci. 2013, 128, 224–229. [Google Scholar] [CrossRef] [Green Version]
- Nia, P.; Meng, W.; Lorestani, F.; Mahmoudian, M.; Alias, Y. Electrodeposition of copper oxide/polypyrrole/reduced graphene oxide as a nonenzymatic glucose biosensor. Sens. Actuators B 2015, 209, 100–108. [Google Scholar]
- Shao, Y.; Wang, J.; Engelhard, M.; Wang, C.; Lin, Y. Facile and controllable electrochemical reduction of graphene oxide and its applications. J. Mater. Chem. 2010, 20, 743–748. [Google Scholar] [CrossRef]
- Astratine, L.; Magner, E.; Cassidy, J.; Betts, A. Electrodeposition and characterisation of copolymers based on pyrrole and 3,4-ethylenedioxythiophene in BMIMBF4 usinga microcell configuration. Electrochim. Acta 2014, 115, 440–448. [Google Scholar] [CrossRef]
- Shrestha, B.; Ahmad, R.; Mousa, H.; Kim, I.; Neupane, M.; Park, C.; Kim, C. High-performance glucose biosensor based on chitosan-glucose oxidase immobilized polypyrrole/Nafion/functionalized multi-walled carbon nanotubes bio-nanohybrid film. J. Colloid Interface Sci. 2016, 482, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, K.; Luo, S.; Tang, Y.; Chen, L. Direct electrodeposition of graphene enabling the one-step synthesis of graphene-metal nanocomposite Films. Small 2011, 7, 1203–1206. [Google Scholar] [CrossRef] [PubMed]
- Hezard, T.; Fajerwerg, K.; Evrard, D.; Collière, V.; Behra, P.; Gros, P. Gold nanoparticles electrodeposited on glassy carbon using cyclic voltammetry: Application to Hg (II) trace analysis. J. Electroanal. Chem. 2012, 664, 46–52. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Zhou, X.; Zhang, J.; Boey, F.; Zhang, H. Direct electrochemical reduction of single-layer graphene oxide and subsequent functionalization with glucose oxidase. J. Phys. Chem. C 2009, 113, 14071–14075. [Google Scholar] [CrossRef]
- Chang, H.; Chang, C.; Tsai, Y.; Liao, C. Electrochemically synthesized graphene/polypyrrole composites and their use in supercapacitor. Carbon 2012, 50, 2331–2336. [Google Scholar] [CrossRef]
- Chen, L.; Tang, Y.; Wang, K.; Liu, C.; Luo, S. Direct electrodeposition of reduced graphene oxide on glassy carbon electrode and its electrochemical application. Electrochem. Commun. 2011, 13, 133–137. [Google Scholar] [CrossRef]
- Sakai, N.; Fujiwara, Y.; Arai, M.; Yu, K.; Tatsum, T. Electrodeposition of gold nanoparticles on ITO: Control of morphology and plasmon resonance based absorption and scattering. J. Electroanal. Chem. 2009, 628, 7–15. [Google Scholar] [CrossRef]
- Wu, B.; Hou, S.; Miao, Z.; Zhang, C.; Ji, Y. Layer-by-layer self-assembling gold nanorods and glucose oxidase onto carbon nanotubes functionalized sol-gel matrix for an amperometric glucose biosensor. Nanomaterials 2015, 5, 1544–1555. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods-Fundamentals and Applications; John Wiley and Sons: New York, NY, USA, 2000. [Google Scholar]
- Karuppiah, C.; Palanisamy, S.; Chen, S.M.; Veeramani, V.; Periakaruppan, P. A novel enzymatic glucose biosensor and sensitive non-enzymatichydrogen peroxide sensor based on graphene and cobalt oxidenanoparticles composite modified glassy carbon electrode. Sens. Actuators B 2014, 196, 450–456. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Zhong, Y.; Li, S. Enzyme-free hydrogen peroxide sensor based on Au@Ag@Ccore-double shell nanocomposites. Appl. Surf. Sci. 2015, 347, 428–434. [Google Scholar] [CrossRef]
- Yang, Q.; Atanasov, P.; Wilkins, E. Development of needle-type glucose sensor with high selectivity. Sens. Actuators B 1998, 46, 249–256. [Google Scholar] [CrossRef]
- Wang, J.; Musameh, M.; Lin, Y. Solubilization of carbon nanotubes by nafion toward the preparation of amperometric biosensors. J. Am. Chem. Soc. 2003, 125, 2408–2409. [Google Scholar] [CrossRef] [PubMed]



© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, B.; Zhao, N.; Hou, S.; Zhang, C. Electrochemical Synthesis of Polypyrrole, Reduced Graphene Oxide, and Gold Nanoparticles Composite and Its Application to Hydrogen Peroxide Biosensor. Nanomaterials 2016, 6, 220. https://doi.org/10.3390/nano6110220
Wu B, Zhao N, Hou S, Zhang C. Electrochemical Synthesis of Polypyrrole, Reduced Graphene Oxide, and Gold Nanoparticles Composite and Its Application to Hydrogen Peroxide Biosensor. Nanomaterials. 2016; 6(11):220. https://doi.org/10.3390/nano6110220
Chicago/Turabian StyleWu, Baoyan, Na Zhao, Shihua Hou, and Cong Zhang. 2016. "Electrochemical Synthesis of Polypyrrole, Reduced Graphene Oxide, and Gold Nanoparticles Composite and Its Application to Hydrogen Peroxide Biosensor" Nanomaterials 6, no. 11: 220. https://doi.org/10.3390/nano6110220




