Determination of Cd2+ and Pb2+ Based on Mesoporous Carbon Nitride/Self-Doped Polyaniline Nanofibers and Square Wave Anodic Stripping Voltammetry
Abstract
:1. Introduction
2. Results and Discussion
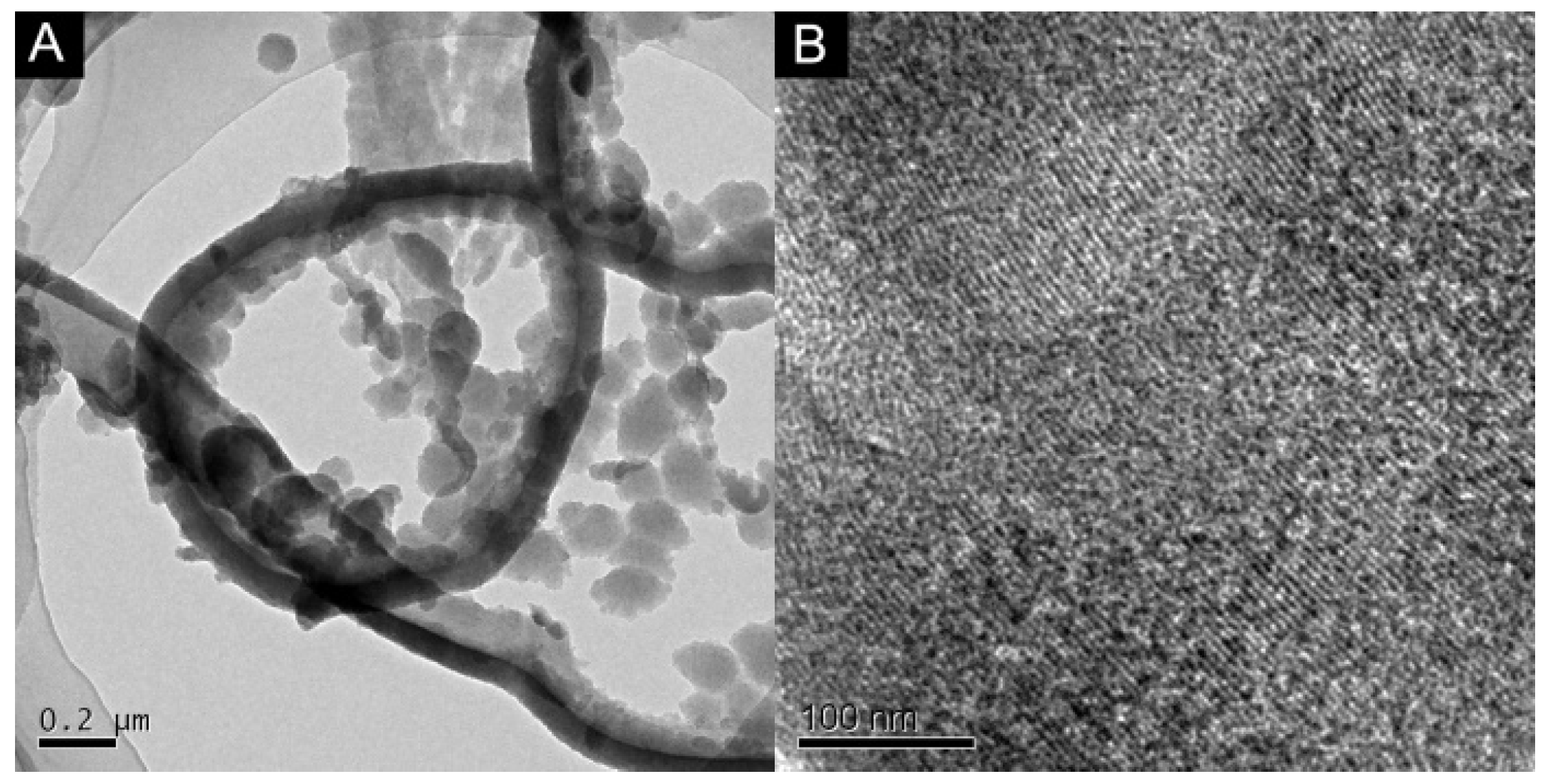
2.1. Morphology Characterization of SPAN Nanofibers and MCN
2.2. Electrochemical Behavior

2.3. Optimization of Experimental Parameters
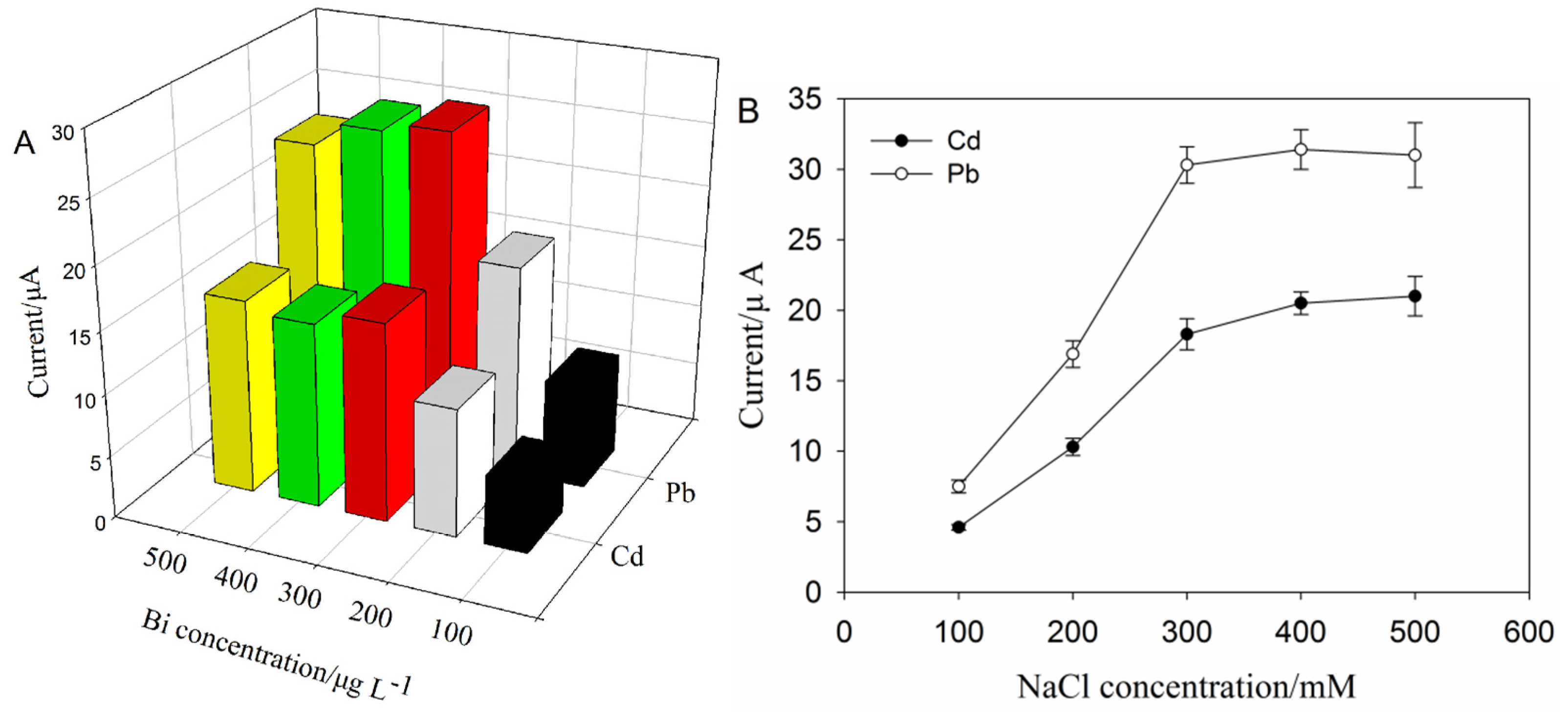
2.3.1. Optimization of Supporting Electrolyte
2.3.2. Effect of Deposition Time
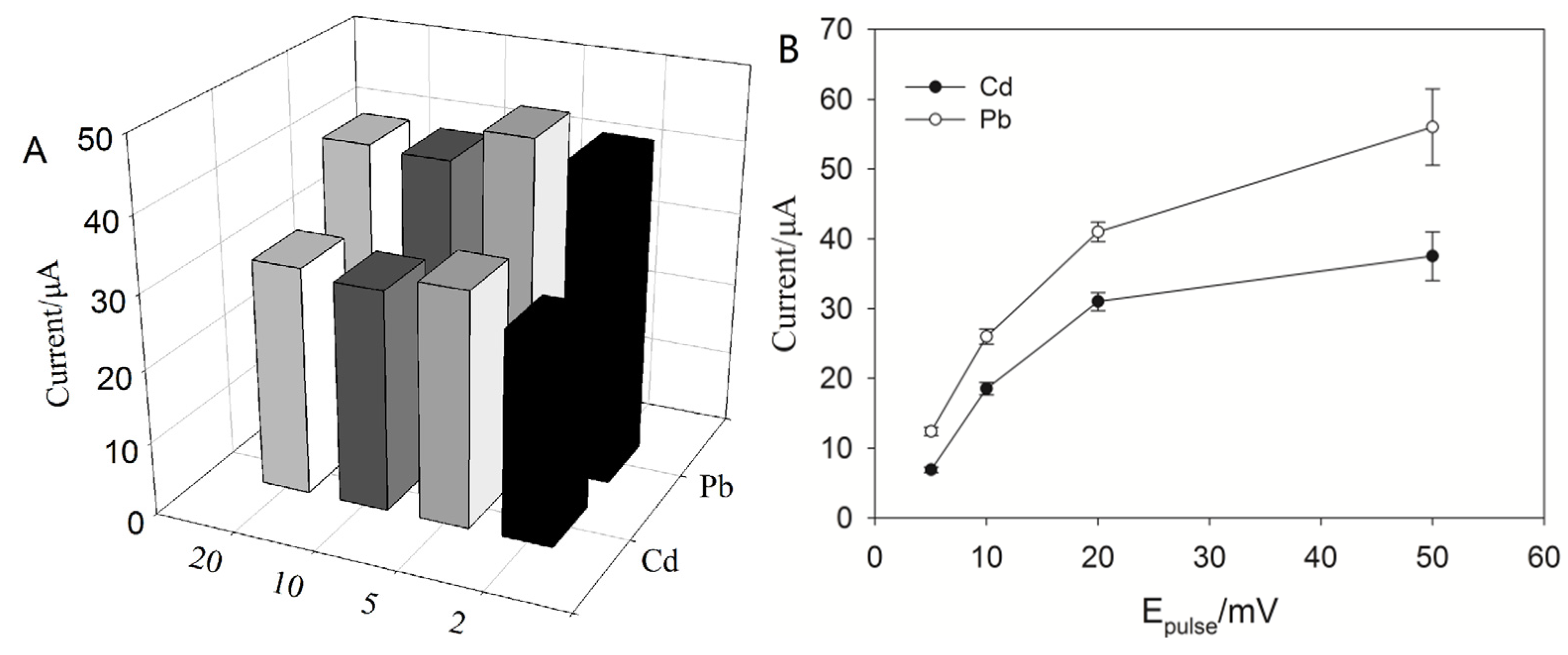
2.3.3. Effect of Frequency, SW Pulse Height and SW Step Increment
2.3.4. Effect of Equilibrium Time
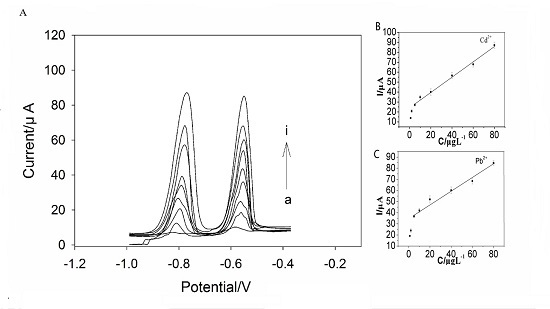
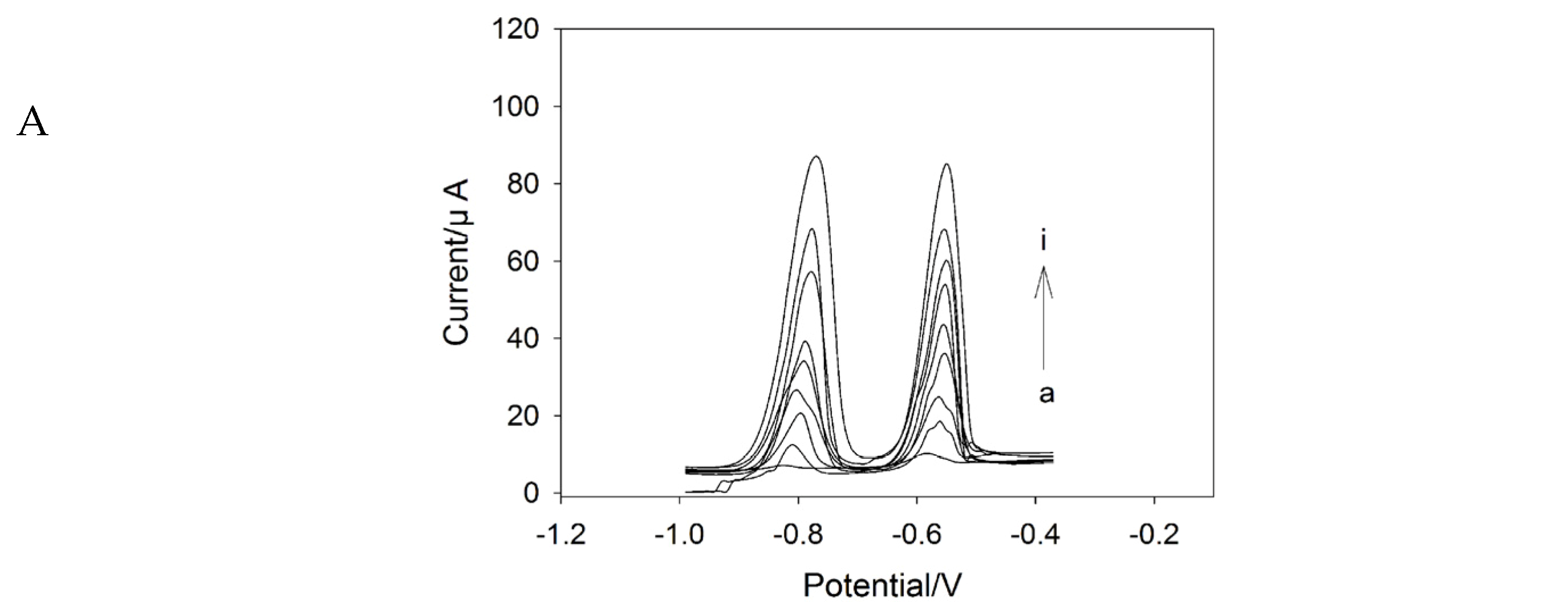
2.4. SWASV Analysis of Cd2+ and Pb2+


| Electrode | Analytical Technique | Linear Range (μg·L−1) | Detection Limit (μg·L−1) | Reference | ||
|---|---|---|---|---|---|---|
| Cd(II) | Pb(II) | Cd(II) | Pb(II) | |||
| Bi/SWNTs/GCE | SWASV | 0.5–11 | 0.5–11 | 0.076 | 0.18 | [33] |
| Bi/Au-GN-Cys/GCE | SWASV | 0.50–40 | 0.50–40 | 0.10 | 0.05 | [1] |
| Bi/ABTS-MWCNTs/GCE | DPSV | 0.5–35 | 0.2–50 | 0.2 | 0.1 | [5] |
| Bi/Nafion/PANI-MES/GCE | SWASV | 0.1–20 | 0.1–30 | 0.04 | 0.05 | [34] |
| RGO/Bi/GCE | SWASV | 20–120 | 20–120 | 2.8 | 0.55 | [35] |
| polymer/Bi/GCE | SWASV | 2–60 | 2–60 | 2 | 2 | [36] |
| Nafion/Bi/GCE | SWASV | 1–20 | 1–20 | 0.1 | 0.1 | [37] |
| SPAN/MCN/GCE | SWASV | 5–80 | 5–80 | 0.7 | 0.2 | This work |
2.5. Reproducibility, Stability and Interference
2.6. Application to Real Samples
| Sample | Added (μg L−1) | ICP-MS (μg L−1) | Proposed Sensor (nM) | Relative Concentration Deviation (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Cd2+ | Pb2+ | Cd2+ | Pb2+ | Cd2+ | Pb2+ | Cd2+ | Pb2+ | |
| River Water 1 | 5 | 5 | 5.3 ± 0.26 | 6.1 ± 0.43 | 5.5 ± 0.66 | 6.5 ± 0.53 | 2.60 | 4.49 |
| River Water 2 | 10 | 10 | 11.1 ± 0.96 | 10.9 ± 1.2 | 10.5 ± 0.49 | 11.3 ± 1.3 | 3.93 | 2.54 |
| River Water 3 | 15 | 15 | 16.4 ± 1.9 | 17.5 ± 2.1 | 15.1 ± 1.4 | 16.8 ± 1.7 | 5.37 | 2.89 |
3. Experimental Section
3.1. Reagents, Preparation of SPAN Nanofibers and MCN
3.2. Apparatus
3.3. Preparation of the Modified Electrodes
3.4. Heavy Metal Measurement Using the Electrochemical Sensor
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lian, Z.; Xu, L.; Huang, B.; Jia, N.; Liang, T.; Yao, S. Simultaneous determination of Cd(II) and Pb(II) using square wave anodic stripping voltammetry at a gold nanoparticle-graphene-cysteine composite modified bismuth film electrode. Electrochim. Acta 2014, 115, 471–477. [Google Scholar]
- Tang, L.; Chen, J.; Zeng, G.; Zhu, Y.; Zhang, Y.; Zhou, Y.; Xie, X.; Yang, G.; Zhang, S. Ordered mesoporous carbon and thiolated polyaniline modified electrode for simultaneous determination of cadmium(II) and lead(II) by anodic stripping voltammetry. Electroanalysis 2014, 26, 2283–2291. [Google Scholar] [CrossRef]
- Zhou, Y.; Tang, L.; Zeng, G.; Zhang, C.; Xie, X.; Liu, Y.; Wang, J.; Tang, J.; Zhang, Y.; Deng, Y. Label free detection of lead using impedimetric sensor based on ordered mesoporous carbon-gold nanoparticles and DNAzyme catalytic beacons. Talanta 2015, 146. [Google Scholar] [CrossRef]
- Bernard, A. Cadmium and its adverse effects on human health. Indian J. Med. Res. 2008, 128, 557–564. [Google Scholar] [PubMed]
- Deng, W.; Tan, Y.; Fang, Z.; Xie, Q.; Li, Y.; Liang, X.; Yao, S. ABTS-multiwalled carbon nanotubes nanocomposite/Bi film electrode for sensitive determination of Cd and Pb by differential pulse stripping voltammetry. Electroanalysis 2009, 21, 2477–2485. [Google Scholar] [CrossRef]
- He, X.; Su, Z.; Xie, Q.; Chen, C.; Fu, Y.; Chen, L.; Liu, Y.; Ma, M.; Deng, L.; Qin, D. Differential pulse anodic stripping voltammetric determination of Cd and Pb at a bismuth glassy carbon electrode modified with Nafion, poly(2,5-dimercapto-1,3,4-thiadiazole) and multiwalled carbon nanotubes. Microchim. Acta 2011, 173, 95–102. [Google Scholar] [CrossRef]
- Bagheri, H.; Afkhami, A.; Saber-Tehrani, M.; Khoshsafar, H. Preparation and characterization of magnetic nanocomposite of Schiff base/silica/magnetite as a preconcentration phase for the trace determination of heavy metal ions in water, food and biological samples using atomic absorption spectrometry. Talanta 2012, 97, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Dias, L.F.; Saint’Pierre, T.D.; Maia, S.M.; Frescura, V.L.A.; Welz, B.; Curtius, A.J. Determination of arsenic, lead, selenium and tin in sediments by slurry sampling electrothermal vaporization inductively coupled plasma mass spectrometry using Ru as permanent modifier and NaCl as a carrier. Spectrochim. Acta Part B 2002, 57, 2003–2015. [Google Scholar] [CrossRef]
- Guzmán-Mar, J.L.; Hinojosa-Reyes, L.; Serra, A.M.; Hernández-Ramírez, A.; Cerdà, V. Applicability of multisyringe chromatography coupled to cold-vapor atomic fluorescence spectrometry for mercury speciation analysis. Anal. Chim. Acta 2011, 708, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Arpadjan, S.; Celik, G.; Taşkesen, S.; Güçer, S. Arsenic, cadmium and lead in medicinal herbs and their fractionation. Food Chem. Toxicol. 2008, 46, 2871–2875. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, Z.; Meng, Y.; Zhang, P.; Su, Z.; Liu, Y.; Huang, Y.; Zhou, Y.; Xie, Q.; Yao, S. Sensitive square wave anodic stripping voltammetric determination of Cd2+ and Pb2+ ions at Bi/Nafion/overoxidized 2-mercaptoethanesulfonate-tethered polypyrrole/glassy carbon electrode. Sens. Actuators B 2014, 191, 94–101. [Google Scholar] [CrossRef]
- Gemma, A.; Josefina, P.; Arben, M.I. Recent trends in macro-, micro-, and nanomaterial-based tools and strategies for heavy-metal detection. Chem. Rev. 2011, 111, 3433–3458. [Google Scholar]
- Daniela, N.; Fabiana, A.; Josefina Calvo, Q.; Patrizia, D.C.; Cinzia, F.; Danila, M. Disposable electrochemical sensor to evaluate the phytoremediation of the aquatic plant Lemna minor L. toward Pb2+ and/or Cd2+. Environ. Sci. Technol. 2014, 48, 7477–7485. [Google Scholar]
- Hwang, G.H.; Han, W.K.; Park, J.S.; Kang, S.G. Determination of trace metals by anodic stripping voltammetry using a bismuth-modified carbon nanotube electrode. Talanta 2008, 76, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Angeles Granado, R.M.; Mara, O.M.; Eduardo Pinilla, G. Modification of carbon screen-printed electrodes by adsorption of chemically synthesized Bi nanoparticles for the voltammetric stripping detection of Zn(II), Cd(II) and Pb(II). Talanta 2009, 80, 631–635. [Google Scholar]
- Huang, D.; Niu, C.; Wang, X.; Lv, X.; Zeng, G. “Turn-on” fluorescent sensor for Hg2+ based on single-stranded DNA functionalized Mn:CdS/ZnS quantum dots and gold nanoparticles by time-gated mode. Anal. Chem. 2013, 85, 1164–1170. [Google Scholar] [CrossRef] [PubMed]
- Kong, R.M.; Zhang, X.B.; Zhang, L.L.; Jin, X.Y.; Huan, S.Y.; Shen, G.L.; Yu, R.Q. An ultrasensitive electrochemical “turn-on” label-free biosensor for Hg2+ with AuNP-functionalized reporter DNA as a signal amplifier. Chem. Commun. 2009, 37, 5633–5635. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zeng, G.; Tang, L.; Chen, J.; Zhu, Y.; He, X.; Yan, H. Electrochemical Sensor Based on Electrodeposited Graphene-Au Modified Electrode and NanoAu Carrier Amplified Signal Strategy for Attomolar Mercury Detection. Anal. Chem. 2014, 87, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Yin, N.; Nie, D.; Fu, F.; Chen, G. An ultrasensitive electrochemical sensor for the mercuric ion via controlled assembly of SWCNTs. Chem. Commun. 2011, 47, 10665–10667. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Lin, T.; Zeng, G.; Chen, J.; Ye, C.; Yi, Z.; Yang, G.; Liu, Y.; Chen, Z.; Tang, W. Mesoporous carbon nitride based biosensor for highly sensitive and selective analysis of phenol and catechol in compost bioremediation. Biosens. Bioelectron. 2014, 61, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tang, L.; Xie, X.; Zeng, G.; Wang, J.; Deng, Y.; Yang, G.; Zhang, C.; Zhang, Y.; Chen, J. Sensitive impedimetric biosensor based on duplex-like DNA scaffolds and ordered mesoporous carbon nitride for silver(I) ion detection. Analyst 2014, 139, 6529–6535. [Google Scholar] [CrossRef] [PubMed]
- Song, E.; Choi, J.W. Conducting polyaniline nanowire and its applications in chemiresistive sensing. Nanomaterials 2013, 3, 498–523. [Google Scholar] [CrossRef]
- Brett, C.M.A.; Thiemann, C. Conducting polymers from aminobenzoic acids and aminobenzenesulphonic acids: Influence of pH on electrochemical behaviour. J. Electroanal. Chem. 2002, 538, 215–222. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Z.; Zhu, J.; Xu, C.; Yan, W.; Yao, C. A novel H2O2 amperometric biosensor based on gold nanoparticles/self-doped polyaniline nanofibers. Bioelectrochemistry 2011, 82, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, T.; Li, X.; Jiao, K. Three-step electrodeposition synthesis of self-doped polyaniline nanofiber-supported flower-like Au microspheres for high-performance biosensing of DNA hybridization recognition. Biosens. Bioelectron. 2011, 26, 2953–2959. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yang, T.; Li, X.; Wang, D.; Jiao, K. Conductive architecture of Fe2O3 microspheres/self-doped polyaniline nanofibers on carbon ionic liquid electrode for impedance sensing of DNA hybridization. Biosens. Bioelectron. 2009, 25, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Ghenaatian, H.; Mousavi, M.; Kazemi, S.; Shamsipur, M. Electrochemical investigations of self-doped polyaniline nanofibers as a new electroactive material for high performance redox supercapacitor. Synth. Met. 2009, 159, 1717–1722. [Google Scholar] [CrossRef]
- Malinauskas, A. Self-doped polyanilines. J. Power Sources 2004, 126, 214–220. [Google Scholar] [CrossRef]
- Zeng, G.; Li, Z.; Tang, L.; Wu, M.; Lei, X.; Liu, Y.; Liu, C.; Pang, Y.; Yi, Z. Gold nanoparticles/water-soluble carbon nanotubes/aromatic diamine polymer composite films for highly sensitive detection of cellobiose dehydrogenase gene. Electrochim. Acta 2011, 56, 4775–4782. [Google Scholar] [CrossRef]
- Zou, L.; Zhang, Y.; Qin, H.; Ye, B. Simultaneous Determination of Thallium and Lead on a Chemically Modified Electrode with Langmuir—Blodgett Film of a p-tert-Butylcalix[4]arene Derivative. Electroanalysis 2009, 21, 2563–2568. [Google Scholar] [CrossRef]
- Kachoosangi, R.T.; Banks, C.E.; Ji, X.; Compton, R.G. Electroanalytical Determination of Cadmium(II) and Lead(II) Using an in-situ Bismuth Film Modified Edge Plane Pyrolytic Graphite Electrode. Anal. Sci. 2007, 23, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Kefala, G.; Economou, A.; Voulgaropoulos, A.; Sofoniou, M. A study of bismuth-film electrodes for the detection of trace metals by anodic stripping voltammetry and their application to the determination of Pb and Zn in tapwater and human hair. Talanta 2003, 61, 603–610. [Google Scholar] [CrossRef]
- Ouyang, R.; Zhu, Z.; Tatum, C.E.; Chambers, J.Q.; Xue, Z.L. Simultaneous stripping detection of Pb(II), Cd(II) and Zn(II) using a bimetallic Hg-Bi/single-walled carbon nanotubes composite electrode. J. Electroanal. Chem. 2011, 656, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Su, Z.; He, X.; Liu, Y.; Qin, C.; Zhou, Y. Square wave anodic stripping voltammetric determination of Cd and Pb ions at a Bi/Nafion/thiolated polyaniline/glassy carbon electrode. Electrochem. Commun. 2012, 15, 34–37. [Google Scholar] [CrossRef]
- Sahoo, P.K.; Panigrahy, B.; Sahoo, S.; Satpati, A.K.; Dan, L.; Bahadur, D. In situ synthesis and properties of reduced graphene oxide/Bi nanocomposites: As an electroactive material for analysis of heavy metals. Biosens. Bioelectron. 2013, 43, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Kefala, G.; Economou, A. Polymer-coated bismuth film electrodes for the determination of trace metals by sequential-injection analysis/anodic stripping voltammetry. Anal. Chim. Acta 2006, 576, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Kefala, G.; Economou, A.; Voulgaropoulos, A. A study of Nafion-coated bismuth-film electrodes for the determination of trace metals by anodic stripping voltammetry. Analyst 2004, 129, 1082–1090. [Google Scholar] [CrossRef] [PubMed]
- Vinu, A.; Ariga, K.; Mori, T.; Nakanishi, T.; Hishita, S.; Golberg, D.; Bo, Y. Preparation and characterization of well-ordered hexagonal mesoporous carbon nitride. Adv. Mater. 2005, 17, 1648–1652. [Google Scholar] [CrossRef]
- Tang, L.; Zhou, Y.; Zeng, G.; Li, Z.; Liu, Y.; Zhang, Y.; Chen, G.; Yang, G.; Lei, X.; Wu, M. A tyrosinase biosensor based on ordered mesoporous carbon–Au/l-lysine/Au nanoparticles for simultaneous determination of hydroquinone and catechol. Analyst 2013, 138, 3552–3560. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Lin, T.; Zeng, G.; Chen, J.; Wang, J.; Fan, C.; Yang, G.; Yi, Z.; Xia, X. Amplified and selective detection of manganese peroxidase genes based on enzyme-scaffolded-gold nanoclusters and mesoporous carbon nitride. Biosens. Bioelectron. 2014, 65, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tang, L.; Zeng, G.; Zhu, J.; Dong, H.; Zhang, Y.; Xie, X.; Wang, J.; Deng, Y. A novel biosensor for silver(I) ion detection based on nanoporous gold and duplex-like DNA scaffolds with anionic intercalator. RSC Adv. 2015, 5, 69738–69744. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Zhou, Y.; Tang, L.; Zeng, G.; Zhang, J.; Peng, B.; Xie, X.; Lai, C.; Long, B.; Zhu, J. Determination of Cd2+ and Pb2+ Based on Mesoporous Carbon Nitride/Self-Doped Polyaniline Nanofibers and Square Wave Anodic Stripping Voltammetry. Nanomaterials 2016, 6, 7. https://doi.org/10.3390/nano6010007
Zhang C, Zhou Y, Tang L, Zeng G, Zhang J, Peng B, Xie X, Lai C, Long B, Zhu J. Determination of Cd2+ and Pb2+ Based on Mesoporous Carbon Nitride/Self-Doped Polyaniline Nanofibers and Square Wave Anodic Stripping Voltammetry. Nanomaterials. 2016; 6(1):7. https://doi.org/10.3390/nano6010007
Chicago/Turabian StyleZhang, Chang, Yaoyu Zhou, Lin Tang, Guangming Zeng, Jiachao Zhang, Bo Peng, Xia Xie, Cui Lai, Beiqing Long, and Jingjing Zhu. 2016. "Determination of Cd2+ and Pb2+ Based on Mesoporous Carbon Nitride/Self-Doped Polyaniline Nanofibers and Square Wave Anodic Stripping Voltammetry" Nanomaterials 6, no. 1: 7. https://doi.org/10.3390/nano6010007