Nanocarriers for DNA Vaccines: Co-Delivery of TLR-9 and NLR-2 Ligands Leads to Synergistic Enhancement of Proinflammatory Cytokine Release
Abstract
:1. Introduction
2. Results and Discussion
2.1. Nanoparticle Characterization
| Loaded with | TMC Nanoparticles | SWE06 | Cationorm® | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Size (nm) | PDI | ζ (mV) | Size (nm) | PDI | ζ (mV) | Size (nm) | PDI | ζ (mV) | |
| (blank) | 216 ± 2 | 0.1 | 31 ± 2 | 133 ± 2 | 0.1 | 27 ± 1 | 157 ± 3 | 0.2 | 18 ± 1 |
| pDNA | 252 ± 5 | 0.2 | 7 ± 5 | 188 ± 3 | 0.1 | −14 ± 1 | 205 ± 2 | 0.3 | −39 ± 0.5 |
| MDP | 215 ± 0.5 | 0.2 | 32 ± 4 | 134 ± 2 | 0.2 | 26 ± 1 | 156 ± 2 | 0.2 | 14 ± 0.2 |
| pDNA and MDP | 259 ± 3 | 0.2 | 4 ± 5 | 189 ± 3 | 0.2 | −16 ± 1 | 267 ± 12 | 0.5 | −41 ± 1 |
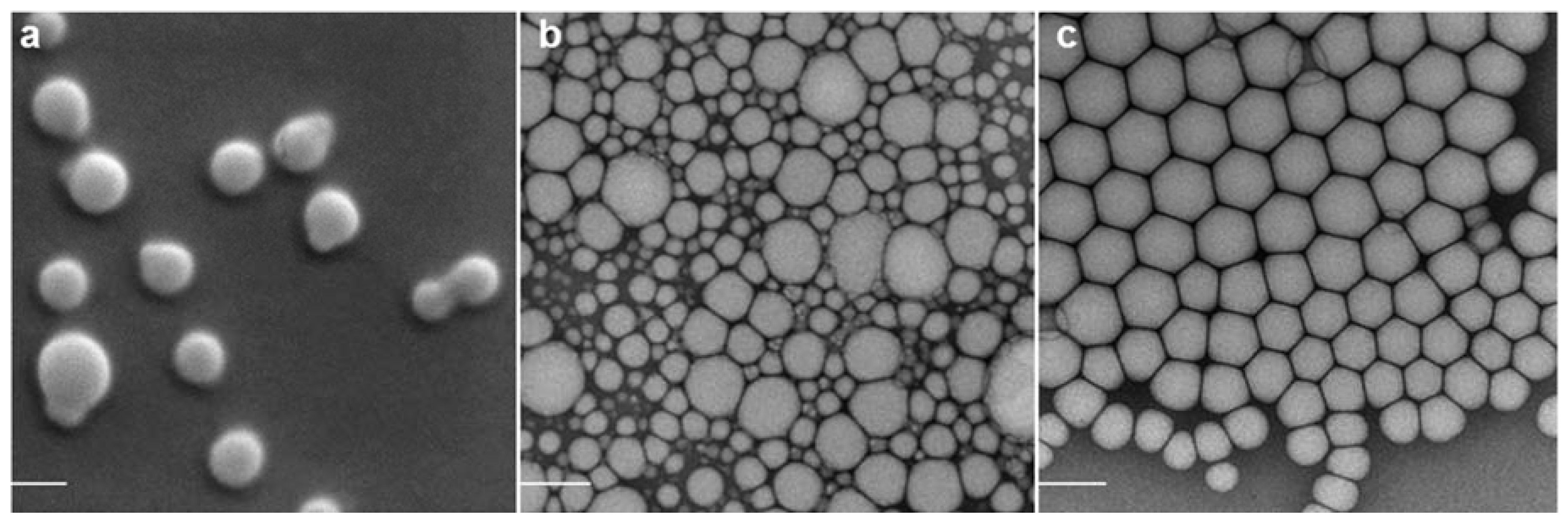
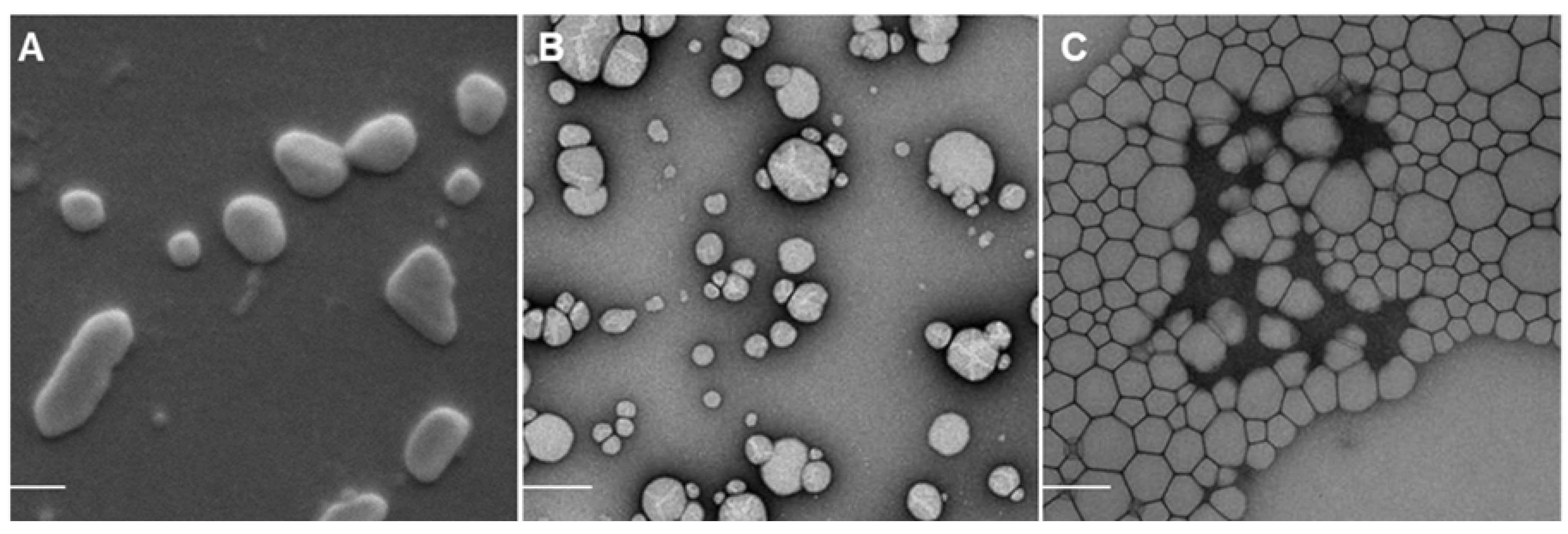
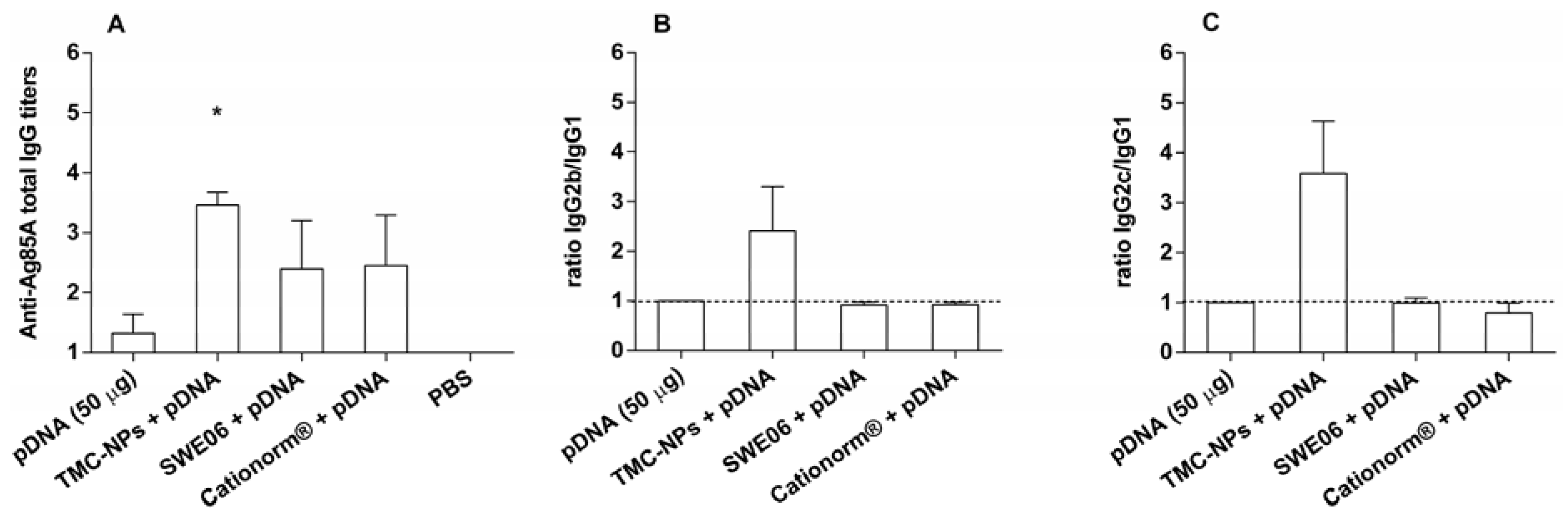
2.2. Adjuvant Effect of pDNA-Nanoformulations in Vivo
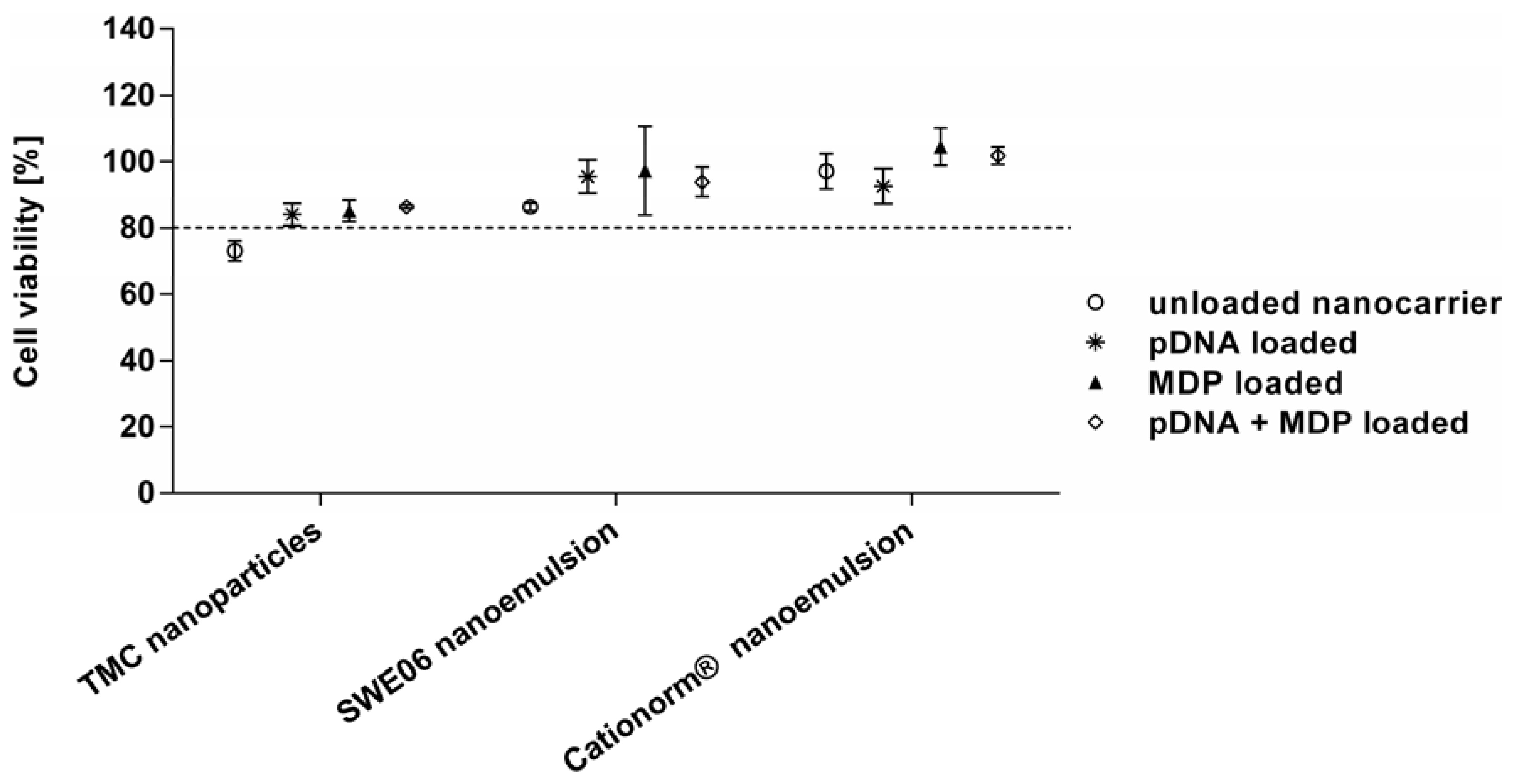
2.3. Cell Viability
2.4. In Vitro Activation of TLR-9 and NLR-2
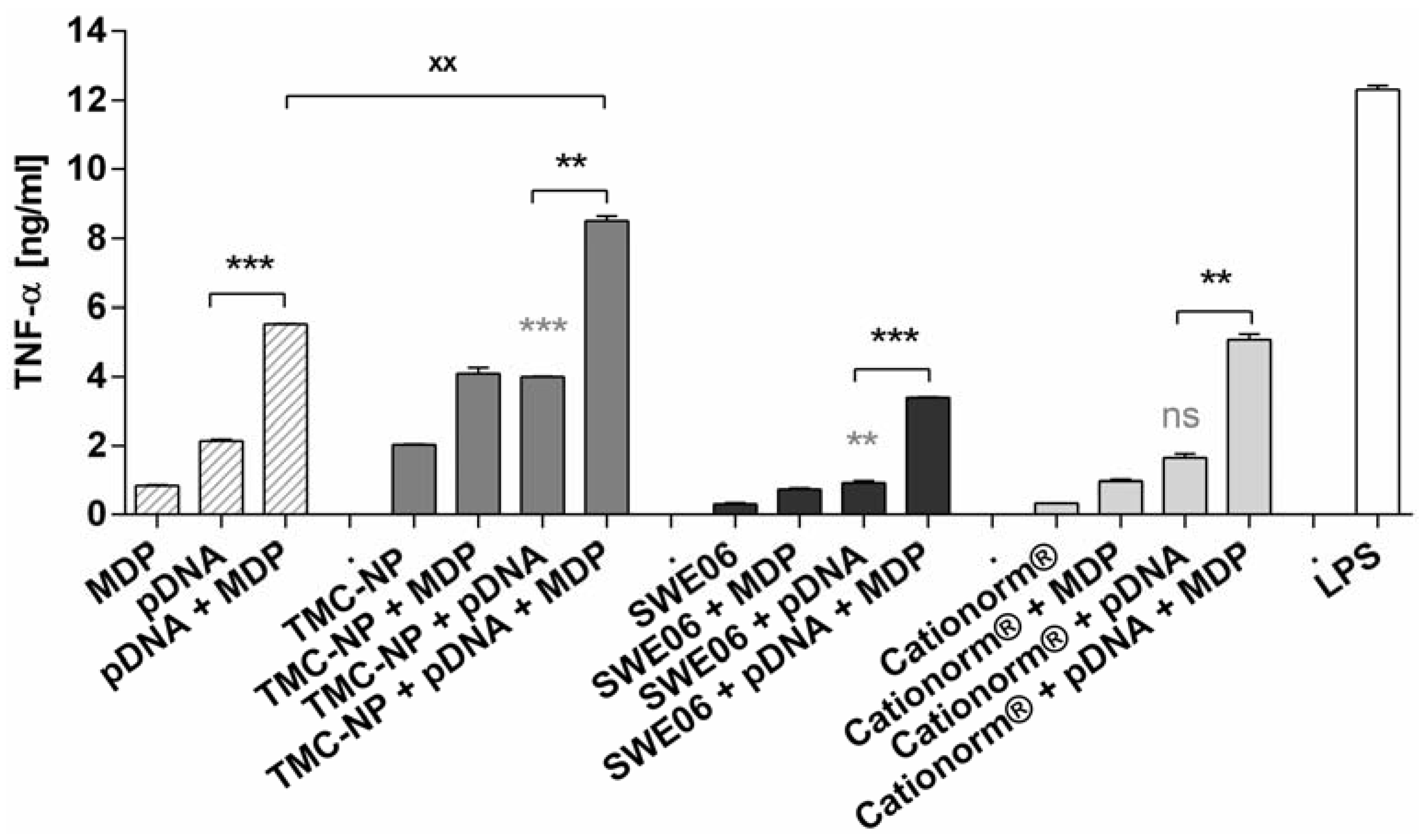
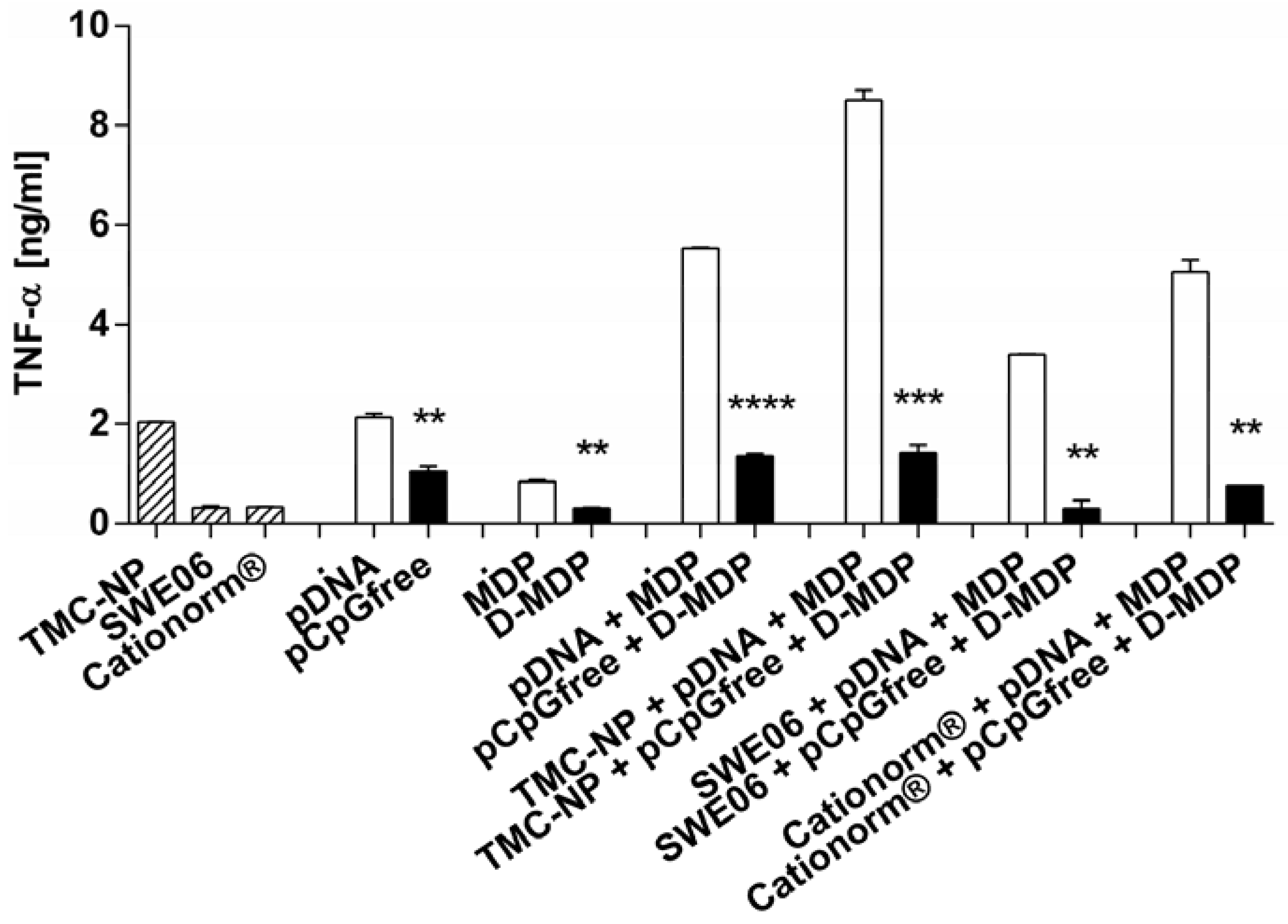
2.5. NLR-2 Dependent Synergistic Enhancement of TNF-α Release
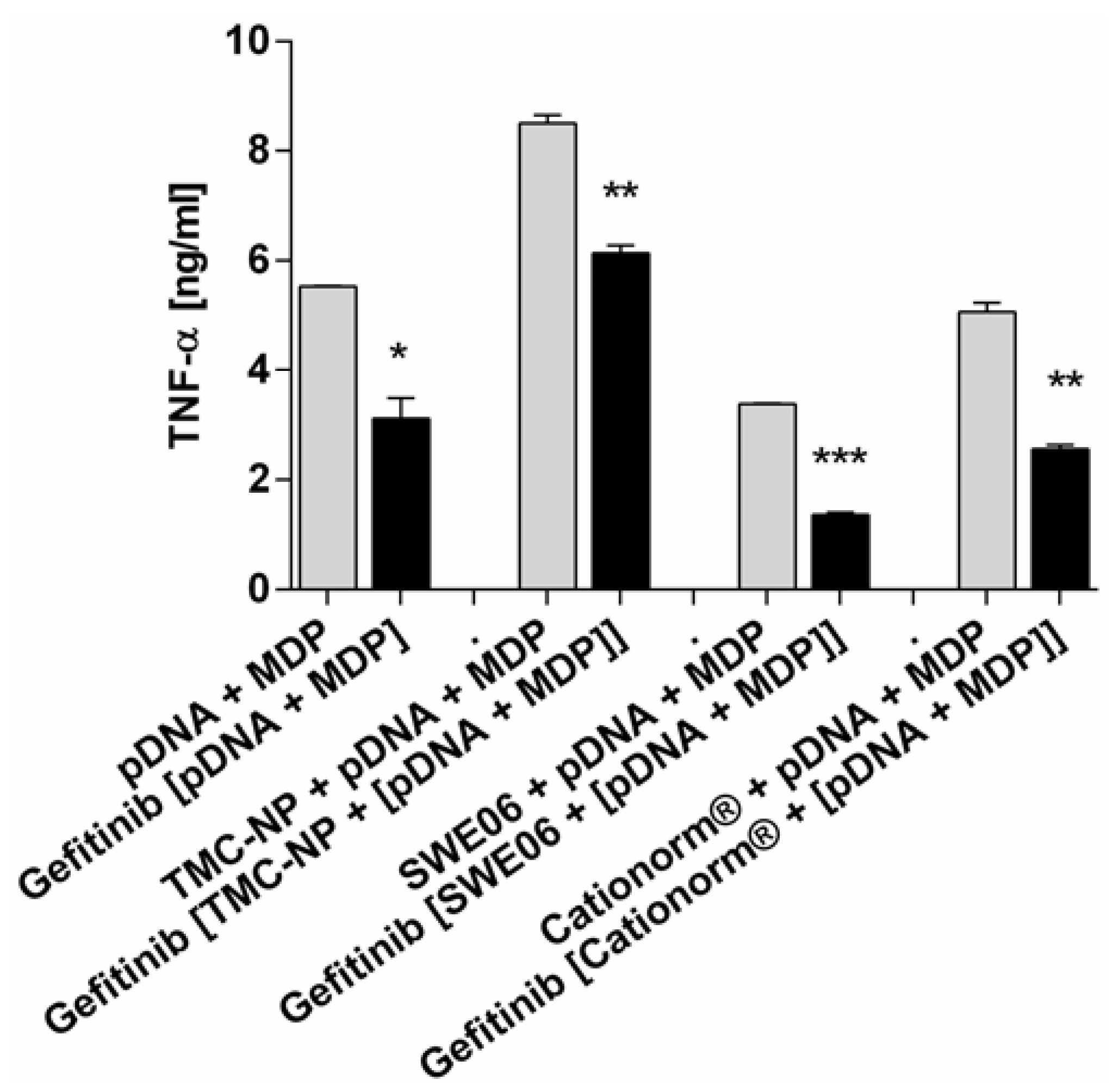
3. Experimental Section
3.1. Materials
3.2. Preparation of Nanoparticle Formulations
3.3. Characterization of Nanoparticles
3.3.1. Particle Size, Zeta Potential and Morphology of Nanocarriers
3.3.2. Loading Efficiency
3.3.3. In Vivo Immunogenicity of pDNA-nanoformulations
3.3.4. Cell Culture
3.3.5. In Vitro Cytotoxicity and Cytokine Release Assays
3.3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kutzler, M.A.; Weiner, D.B. DNA vaccines: Ready for prime time? Nat. Rev. Genet. 2008, 9, 776–788. [Google Scholar] [CrossRef] [PubMed]
- MacGregor, R.R.; Ginsberg, R.; Ugen, K.E.; Baine, Y.; Kang, C.U.; Tu, X.M.; Higgins, T.; Weiner, D.B.; Boyer, J.D. T-cell responses induced in normal volunteers immunized with a DNA-based vaccine containing HIV-1 env and rev. AIDS 2002, 16, 2137–2143. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Doolan, D.L.; Le, T.P.; Hedstrom, R.C.; Coonan, K.M.; Charoenvit, Y.; Jones, T.R.; Hobart, P.; Margalith, M.; Ng, J.; et al. Induction of antigen-specific cytotoxic T lymphocytes in humans by a malaria DNA vaccine. Science 1998, 282, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Le, T.P.; Coonan, K.M.; Hedstrom, R.C.; Charoenvit, Y.; Sedegah, M.; Epstein, J.E.; Kumar, S.; Wang, R.; Doolan, D.L.; Maguire, J.D.; et al. Safety, tolerability and humoral immune responses after intramuscular administration of a malaria DNA vaccine to healthy adult volunteers. Vaccine 2000, 18, 1893–1901. [Google Scholar] [CrossRef]
- Rottinghaus, S.T.; Poland, G.A.; Jacobson, R.M.; Barr, L.J.; Roy, M.J. Hepatitis B DNA vaccine induces protective antibody responses in human non-responders to conventional vaccination. Vaccine 2003, 21, 4604–4608. [Google Scholar] [CrossRef]
- Sasaki, S.; Sumino, K.; Hamajima, K.; Fukushima, J.; Ishii, N.; Kawamoto, S.; Mohri, H.; Kensil, C.R.; Okuda, K. Induction of systemic and mucosal immune responses to human immunodeficiency virus type 1 by a DNA vaccine formulated with QS-21 saponin adjuvant via intramuscular and intranasal routes. J. Virol. 1998, 72, 4931–4939. [Google Scholar] [PubMed]
- Giljohann, D.A.; Seferos, D.S.; Patel, P.C.; Millstone, J.E.; Rosi, N.L.; Mirkin, C.A. Oligonucleotide loading determines cellular uptake of DNA-modified gold nanoparticles. Nano Lett. 2007, 7, 3818–3821. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H. Topological conformational changes of human papillomavirus (HPV) DNA bound to an insoluble aluminum salt—A study by low temperature PCR. Adv. Biol. Chem. 2013, 3, 76–85. [Google Scholar] [CrossRef]
- O’Hagan, D.; Singh, M.; Ugozzoli, M.; Wild, C.; Barnett, S.; Chen, M.; Schaefer, M.; Doe, B.; Otten, G.R.; Ulmer, J.B. Induction of potent immune responses by cationic microparticles with adsorbed human immunodeficiency virus DNA vaccines. J. Virol. 2001, 75, 9037–9043. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Mumper, R.J. The effect of co-administration of adjuvants with a nanoparticle-based genetic vaccine delivery system on the resulting immune responses. Eur. J. Pharm. Biopharm. 2003, 55, 11–18. [Google Scholar] [CrossRef]
- Poecheim, J.; Borchard, G. Immunotherapy and vaccines. In Controlled Release Systems: Advances in Nanobottles and Active Nanoparticles; Forcada, J., van Herk, A., Pastorin, G., Eds.; Pan Stanford Publishing: Singapore, 2015; pp. 523–551. [Google Scholar]
- Denis-Mize, K.S.; Dupuis, M.; Singh, M.; Woo, C.; Ugozzoli, M.; O’Hagan, D.T.; Donnelly, J.J., III; Ott, G.; McDonald, D.M. Mechanisms of increased immunogenicity for DNA-based vaccines adsorbed onto cationic microparticles. Cell. Immunol. 2003, 225, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Heuking, S.; Borchard, G. Toll-like receptor-7 agonist decoration enhances the adjuvanticity of chitosan-DNA nanoparticles. J. Pharm. Sci. 2012, 101, 1166–1177. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Behera, A.K.; Lockey, R.F.; Zhang, J.; Bhullar, G.; de la Cruz, C.P.; Chen, L.-C.; Leong, K.W.; Huang, S.-K.; Mohapatra, S.S. Intranasal gene transfer by chitosan-DNA nanospheres protects BALB/c mice against acute respiratory syncytial virus infection. Hum. Gene Ther. 2002, 13, 1415–1425. [Google Scholar] [CrossRef] [PubMed]
- Egli, A.; Santer, D.; Barakat, K.; Zand, M.; Levin, A.; Vollmer, M.; Weisser, M.; Khanna, N.; Kumar, D.; Tyrrell, L. Vaccine adjuvants-understanding molecular mechanisms to improve vaccines. Swiss Med. Wkly. 2014, 144. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration (FDA). FDA Approves First Adjuvanted Vaccine for Prevention of H5N1 Avian Influenza. Available online: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm376444.htm (accessed on 26 March 2015).
- Lallemand, F.; Daull, P.; Benita, S.; Buggage, R.; Garrigue, J.-S. Successfully improving ocular drug delivery using the cationic nanoemulsion, novasorb. J. Drug Deliv. 2012. [Google Scholar] [CrossRef] [PubMed]
- Bruffaerts, N.; Huygen, K.; Romano, M. DNA vaccines against tuberculosis. Expert Opin. Biol. Ther. 2014, 14, 1801–1813. [Google Scholar] [CrossRef] [PubMed]
- Appelberg, R. Protective role of interferon gamma, tumor necrosis factor alpha and interleukin-6 in Mycobacterium tuberculosis and M. Avium infections. Immunobiology 1994, 191, 520–525. [Google Scholar] [CrossRef]
- Cavalcanti, Y.V.; Brelaz, M.C.; Neves, J.K.; Ferraz, J.C.; Pereira, V.R. Role of TNF-Alpha, IFN-Gamma, and IL-10 in the development of pulmonary tuberculosis. Pulm. Med. 2012. [Google Scholar] [CrossRef] [PubMed]
- Meager, A.; Wadhwa, M. An Overview of Cytokine Regulation of Inflammation and Immunity. eLS 2013, a0024658. [Google Scholar]
- Ahmed, R.; Pulendran, B. Yellow fever vaccine YF-17D activates multiple dendritic cell subsets via TLR2, 7, 8, and 9 to stimulate polyvalent immunity. J. Exp. Med. 2006, 203, 413–424. [Google Scholar]
- West, A.P. Recognition and signaling by Toll-like receptors. Annu. Rev. Cell Dev. Biol. 2006, 22, 409–437. [Google Scholar] [CrossRef] [PubMed]
- Coulombe, F. NOD2 and Mycobacteria. Master’ Thesis, McGill University, Montreal, QC, Canada, August 2009. [Google Scholar]
- Chin, A.I.; Dempsey, P.W.; Bruhn, K.; Miller, J.F.; Xu, Y.; Cheng, G. Involvement of receptor-interacting protein 2 in innate and adaptive immune responses. Nature 2002, 416, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Inohara, N.; Hernandez, L.D.; Galán, J.E.; Núñez, G.; Janeway, C.A.; Medzhitov, R.; Flavell, R.A. RICK/Rip2/CARDIAK mediates signalling for receptors of the innate and adaptive immune systems. Nature 2002, 416, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Uehara, A.; Yang, S.; Fujimoto, Y.; Fukase, K.; Kusumoto, S.; Shibata, K.; Sugawara, S.; Takada, H. Muramyldipeptide and diaminopimelic acid-containing desmuramylpeptides in combination with chemically synthesized Toll-like receptor agonists synergistically induced production of interleukin-8 in a NOD2- and NOD1-dependent manner, respectively, in human monocytic cells in culture. Cell. Microbiol. 2005, 7, 53–61. [Google Scholar] [PubMed]
- Archer, K.A.; Ader, F.; Kobayashi, K.S.; Flavell, R.A.; Roy, C.R. Cooperation between multiple microbial pattern recognition systems is important for host protection against the intracellular pathogen Legionella pneumophila. Infect. Immun. 2010, 78, 2477–2487. [Google Scholar] [CrossRef] [PubMed]
- Gadad, A.; Chandra, P.S.; Dandagi, P.; Mastiholimath, V. Moxifloxacin loaded polymeric nanoparticles for sustained ocular drug delivery. Int. J. Pharm. Sci. Nanotechol. 2012, 5, 1727–1734. [Google Scholar]
- Bootz, A.; Vogel, V.; Schubert, D.; Kreuter, J. Comparison of scanning electron microscopy, dynamic light scattering and analytical ultracentrifugation for the sizing of poly(butyl cyanoacrylate) nanoparticles. Eur. J. Pharm. Biopharm. 2004, 57, 369–375. [Google Scholar] [CrossRef]
- Ott, G.; Radhakrishnan, R.; Fang, J.-H.; Hora, M. The Adjuvant MF59: A 10-Year Perspective. In Vaccine Adjuvants; O’Hagan, D., Ed.; Springer: New York, NY, USA, 2000; Volume 42, pp. 211–228. [Google Scholar]
- Ioannou, X.P.; Gomis, S.M.; Karvonen, B.; Hecker, R.; Babiuk, L.A.; van Drunen Littel-van den Hurk, S. CpG-containing oligodeoxynucleotides, in combination with conventional adjuvants, enhance the magnitude and change the bias of the immune responses to a herpesvirus glycoprotein. Vaccine 2002, 21, 127–137. [Google Scholar] [CrossRef]
- Li, X.; Min, M.; Du, N.; Gu, Y.; Hode, T.; Naylor, M.; Chen, D.; Nordquist, R.E.; Chen, W.R. Chitin, chitosan, and glycated chitosan regulate immune responses: The novel adjuvants for cancer vaccine. Clin. Dev. Immunol. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Borchard, G.; Esmaeili, F.; Heuking, S. Chitosan-Based Delivery Systems for Mucosal Vaccination. In Chitosan-based Systems for Biopharmaceuticals; Sarmento, B., Neves, J.d., Eds.; Wiley: Chichester, UK, 2012; pp. 211–224. [Google Scholar]
- Tokura, S.; Hiroshi, T.; Ichiro, A. Immunological aspects of chitin and chitin derivatives administered to animals. In Chitin and Chitinases; Jollès, P., Ed.; Birkhäuser: Basel, Switzerland, 1999; Volume 87, pp. 279–292. [Google Scholar]
- Slütter, B.; Plapied, L.; Fievez, V.; Alonso Sande, M.; des Rieux, A.; Schneider, Y.-J.; van Riet, E.; Jiskoot, W.; Préat, V. Mechanistic study of the adjuvant effect of biodegradable nanoparticles in mucosal vaccination. J. Control. Release 2009, 138, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Strong, P.; Clark, H.; Reid, K. Intranasal application of chitin microparticles down-regulates symptoms of allergic hypersensitivity to Dermatophagoides pteronyssinus and Aspergillus fumigatus in murine models of allergy. Clin. Exp. Allergy 2002, 32, 1794–1800. [Google Scholar] [CrossRef] [PubMed]
- Bueter, C.L.; Specht, C.A.; Levitz, S.M. Innate sensing of chitin and chitosan. PLoS Pathog. 2013, 9. [Google Scholar] [CrossRef] [PubMed]
- Dan, J.M.; Wang, J.P.; Lee, C.K.; Levitz, S.M. Cooperative stimulation of dendritic cells by Cryptococcus neoformans mannoproteins and CpG oligodeoxynucleotides. PLoS ONE 2008, 3. [Google Scholar] [CrossRef] [PubMed]
- Hwang, T.-L.; Aljuffali, I.A.; Lin, C.-F.; Chang, Y.-T.; Fang, J.-Y. Cationic additives in nanosystems activate cytotoxicity and inflammatory response of human neutrophils: Lipid nanoparticles versus polymeric nanoparticles. Int. J. Nanomed. 2015, 10, 371–385. [Google Scholar]
- Kean, T.; Roth, S.; Thanou, M. Trimethylated chitosans as non-viral gene delivery vectors: Cytotoxicity and transfection efficiency. J. Control. Release 2005, 103, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Heuking, S.; Adam-Malpel, S.; Sublet, E.; Iannitelli, A.; Stefano, A.D.; Borchard, G. Stimulation of human macrophages (THP-1) using Toll-like receptor-2 (TLR-2) agonist decorated nanocarriers. J. Drug Target. 2009, 17, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Goodman, C.M.; McCusker, C.D.; Yilmaz, T.; Rotello, V.M. Toxicity of gold nanoparticles functionalized with cationic and anionic side chains. Bioconjug. Chem. 2004, 15, 897–900. [Google Scholar] [CrossRef] [PubMed]
- Petterson, T.; Jendholm, J.; Månsson, A.; Bjartell, A.; Riesbeck, K.; Cardell, L.-O. Effects of NOD-like receptors in human B lymphocytes and crosstalk between NOD1/NOD2 and Toll-like receptors. J. Leukoc. Biol. 2011, 89, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Krieg, A.M. CpG motifs in bacterial DNA and their immune effects. Annu. Rev. Immunol. 2002, 20, 709–760. [Google Scholar] [CrossRef] [PubMed]
- Gafvelin, G.; Grönlund, H. Chitosan-based adjuvants. In Molecular Vaccines; Giese, M., Ed.; Springer International Publishing: New York, NY, USA, 2014; pp. 623–631. [Google Scholar]
- Ishii, K.J.; Kawagoe, T.; Koyama, S.; Matsui, K.; Kumar, H.; Kawai, T.; Uematsu, S.; Takeuchi, O.; Takeshita, F.; Coban, C.; et al. TANK-binding kinase-1 delineates innate and adaptive immune responses to DNA vaccines. Nature 2008, 451, 725–729. [Google Scholar] [CrossRef] [PubMed]
- Mantile, F.; Basile, C.; Cicatiello, V.; de Falco, D.; Caivano, A.; de Berardinis, P.; Prisco, A. A multimeric immunogen for the induction of immune memory to beta-amyloid. Immunol. Cell Biol. 2011, 89, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Hart, P.H.; Vitti, G.F.; Burgess, D.R.; Whitty, G.A.; Piccoli, D.S.; Hamilton, J.A. Potential antiinflammatory effects of interleukin 4: Suppression of human monocyte tumor necrosis factor alpha, interleukin 1, and prostaglandin E2. Proc. Natl. Acad. Sci. USA 1989, 86, 3803–3807. [Google Scholar] [CrossRef] [PubMed]
- Tigno-Aranjuez, J.T.; Asara, J.M.; Abbott, D.W. Inhibition of RIP2’s tyrosine kinase activity limits NOD2-driven cytokine responses. Genes Dev. 2010, 24, 2666–2677. [Google Scholar] [CrossRef] [PubMed]
- Domard, A.; Rinaudo, M.; Terrassin, C. New method for the quaternization of chitosan. Int. J. Biol. Macromol. 1986, 8, 105–107. [Google Scholar] [CrossRef]
- Heuking, S.; Iannitelli, A.; di Stefano, A.; Borchard, G. Toll-like receptor-2 agonist functionalized biopolymer for mucosal vaccination. Int. J. Pharm. 2009, 381, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Schatz, C.; Domard, A.; Viton, C.; Pichot, C.; Delair, T. Versatile and efficient formation of colloids of biopolymer-based polyelectrolyte complexes. Biomacromolecules 2004, 5, 1882–1892. [Google Scholar] [CrossRef] [PubMed]
- HAS Home Page. Decision by the National Committee for the Evaluation of Medical Devices and Health Technologies. Available online: http://www.has-sante.fr/portail/jcms/c_1696520/fr/cationorm (accessed on 4 December 2013).
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poecheim, J.; Heuking, S.; Brunner, L.; Barnier-Quer, C.; Collin, N.; Borchard, G. Nanocarriers for DNA Vaccines: Co-Delivery of TLR-9 and NLR-2 Ligands Leads to Synergistic Enhancement of Proinflammatory Cytokine Release. Nanomaterials 2015, 5, 2317-2334. https://doi.org/10.3390/nano5042317
Poecheim J, Heuking S, Brunner L, Barnier-Quer C, Collin N, Borchard G. Nanocarriers for DNA Vaccines: Co-Delivery of TLR-9 and NLR-2 Ligands Leads to Synergistic Enhancement of Proinflammatory Cytokine Release. Nanomaterials. 2015; 5(4):2317-2334. https://doi.org/10.3390/nano5042317
Chicago/Turabian StylePoecheim, Johanna, Simon Heuking, Livia Brunner, Christophe Barnier-Quer, Nicolas Collin, and Gerrit Borchard. 2015. "Nanocarriers for DNA Vaccines: Co-Delivery of TLR-9 and NLR-2 Ligands Leads to Synergistic Enhancement of Proinflammatory Cytokine Release" Nanomaterials 5, no. 4: 2317-2334. https://doi.org/10.3390/nano5042317






