Nanoscience Supporting the Research on the Negative Electrodes of Li-Ion Batteries
Abstract
:1. Introduction
2. Nano-Structured Carbon
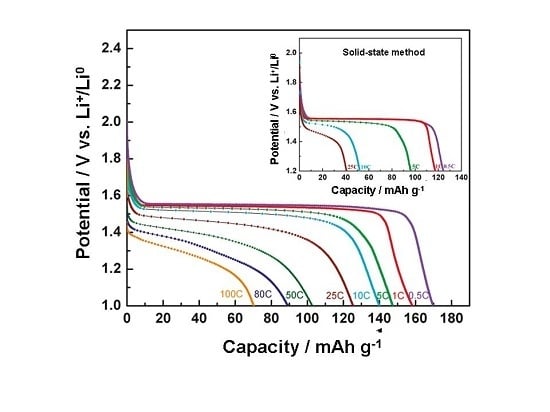
3. Silicon Anodes
3.1. Thin Films
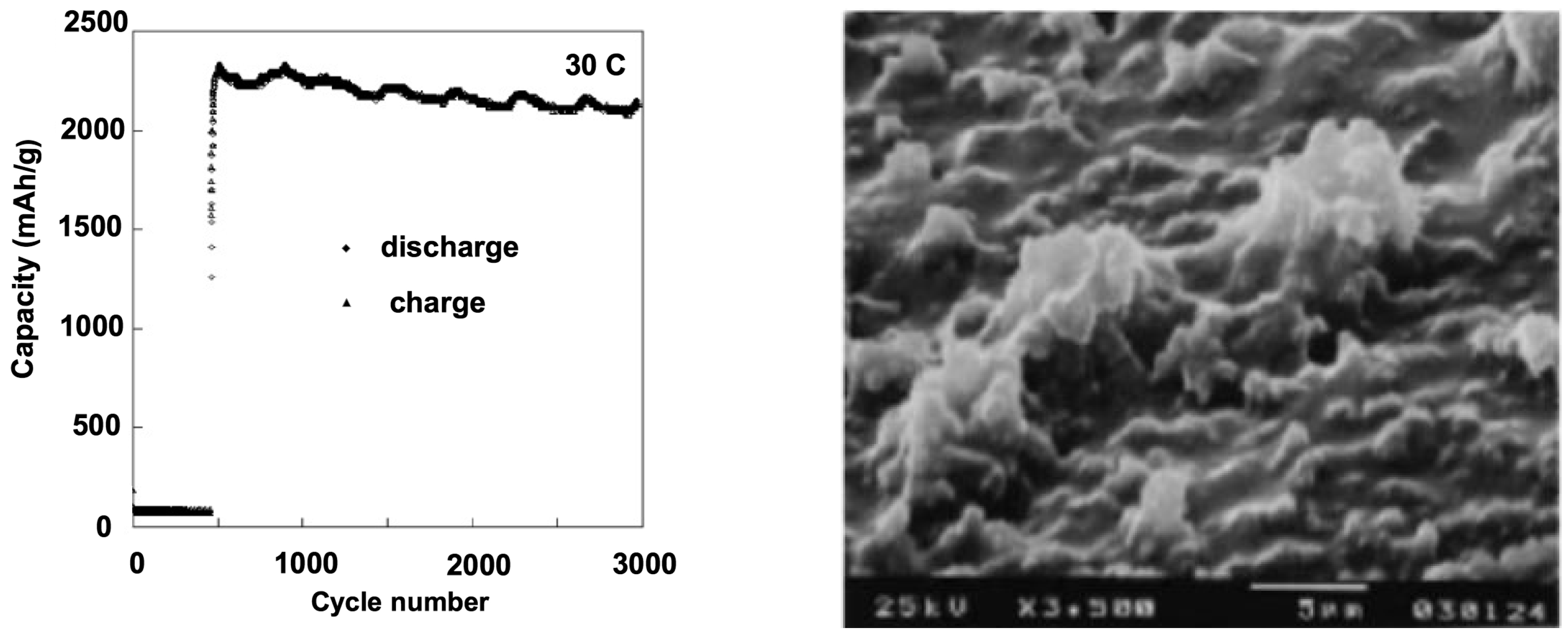

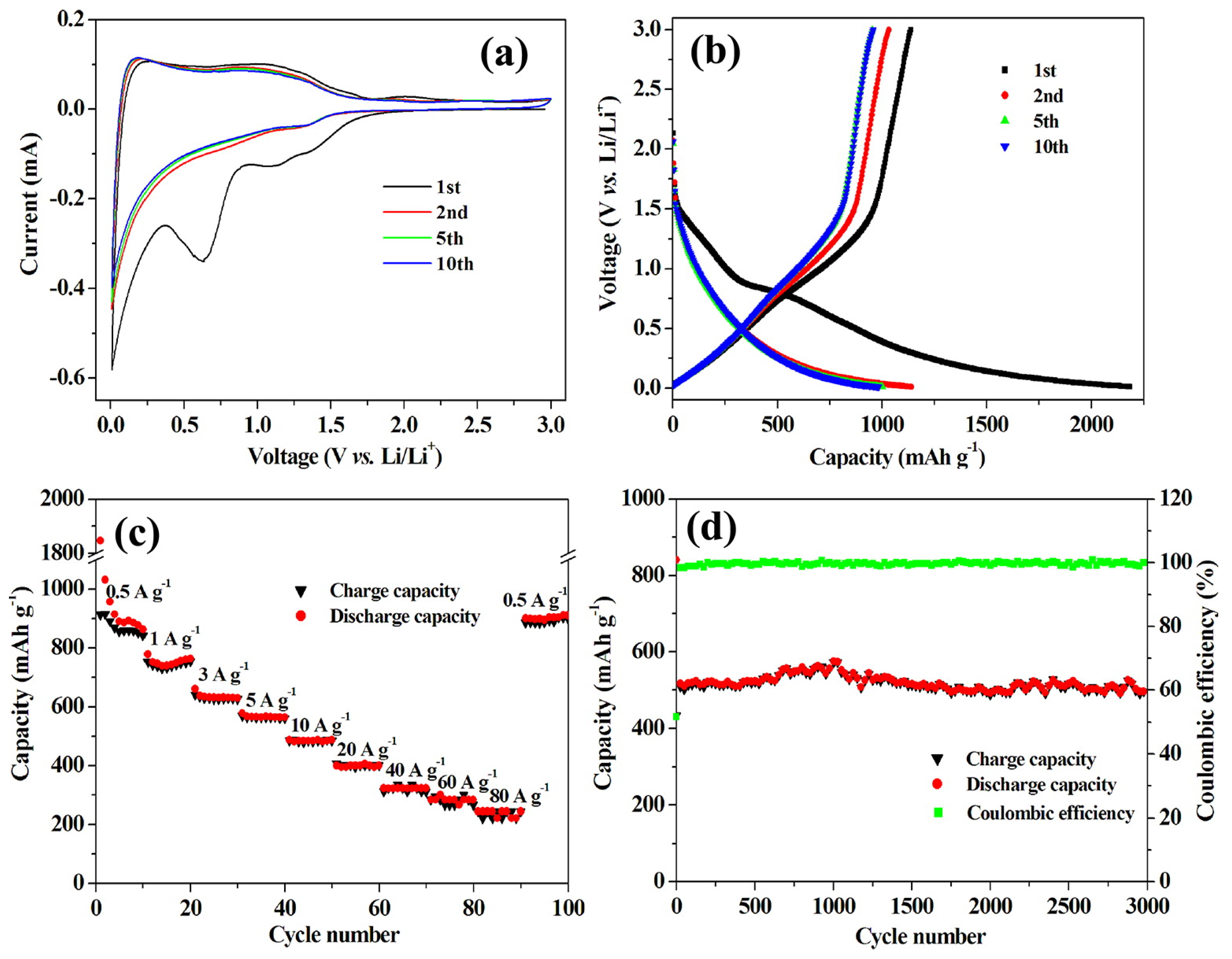
3.2. Nanowires and Nanotubes
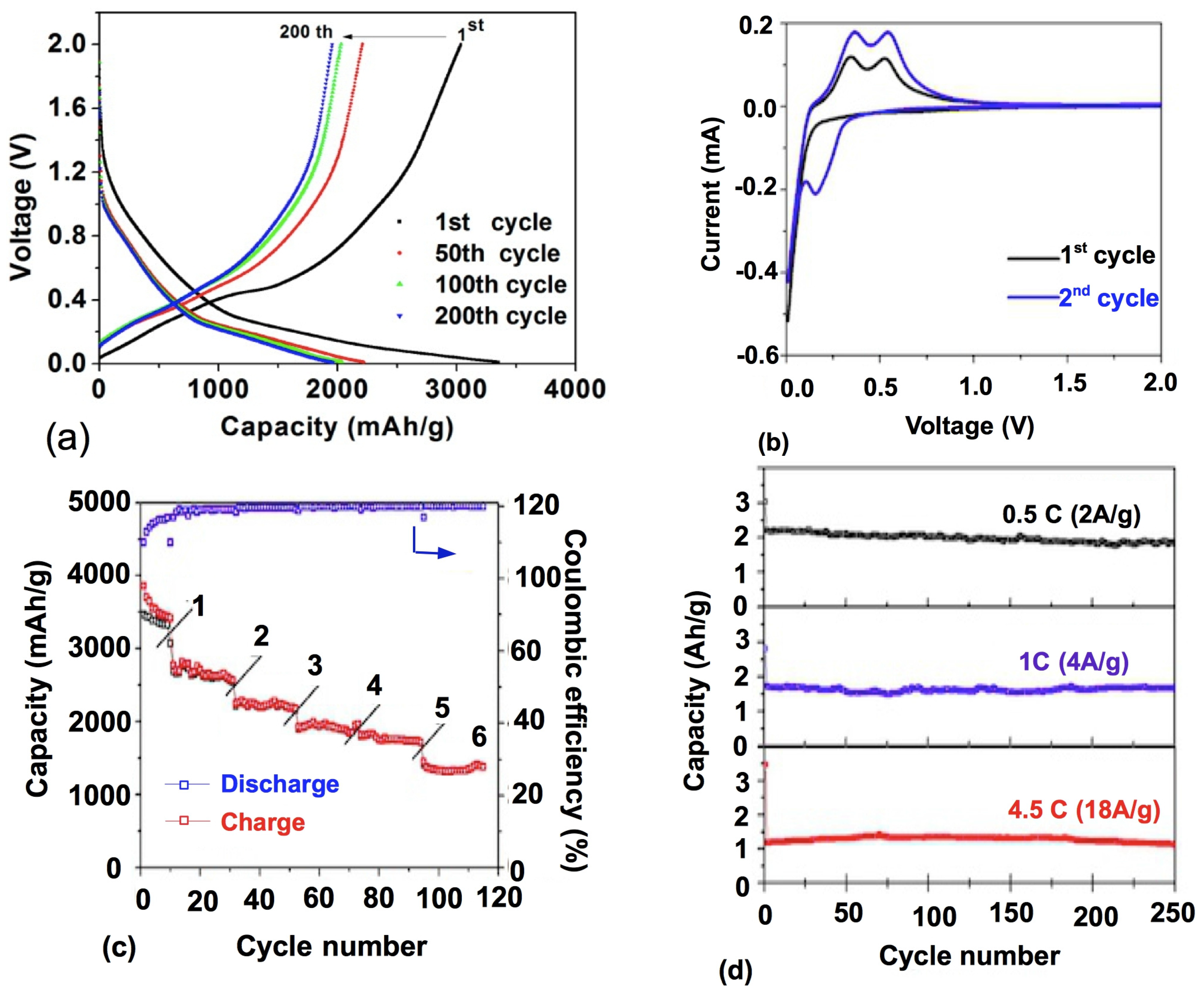
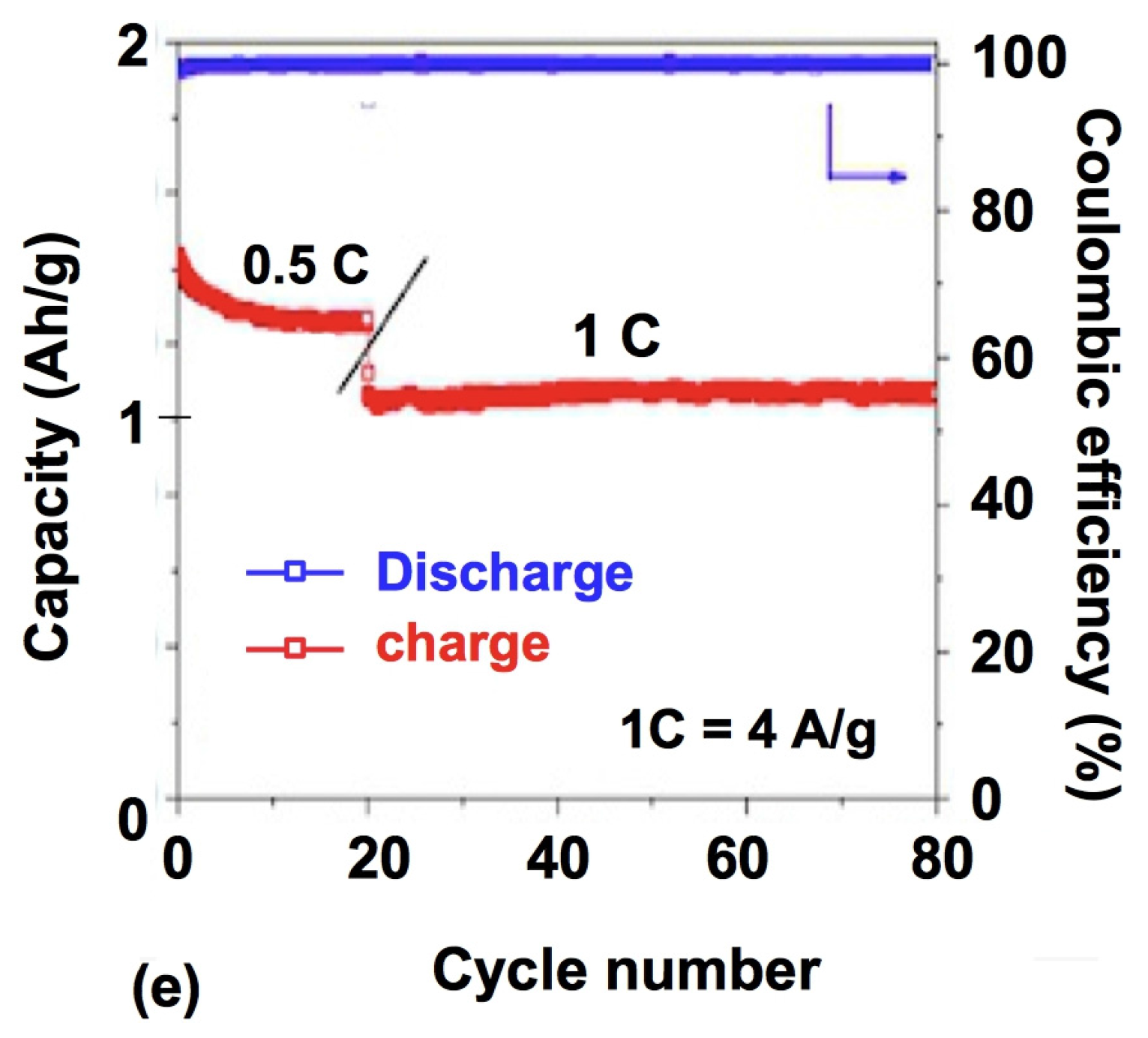
3.3. Porous Si
3.4. Coated Si Nanostructures and Stabilization of the SEI
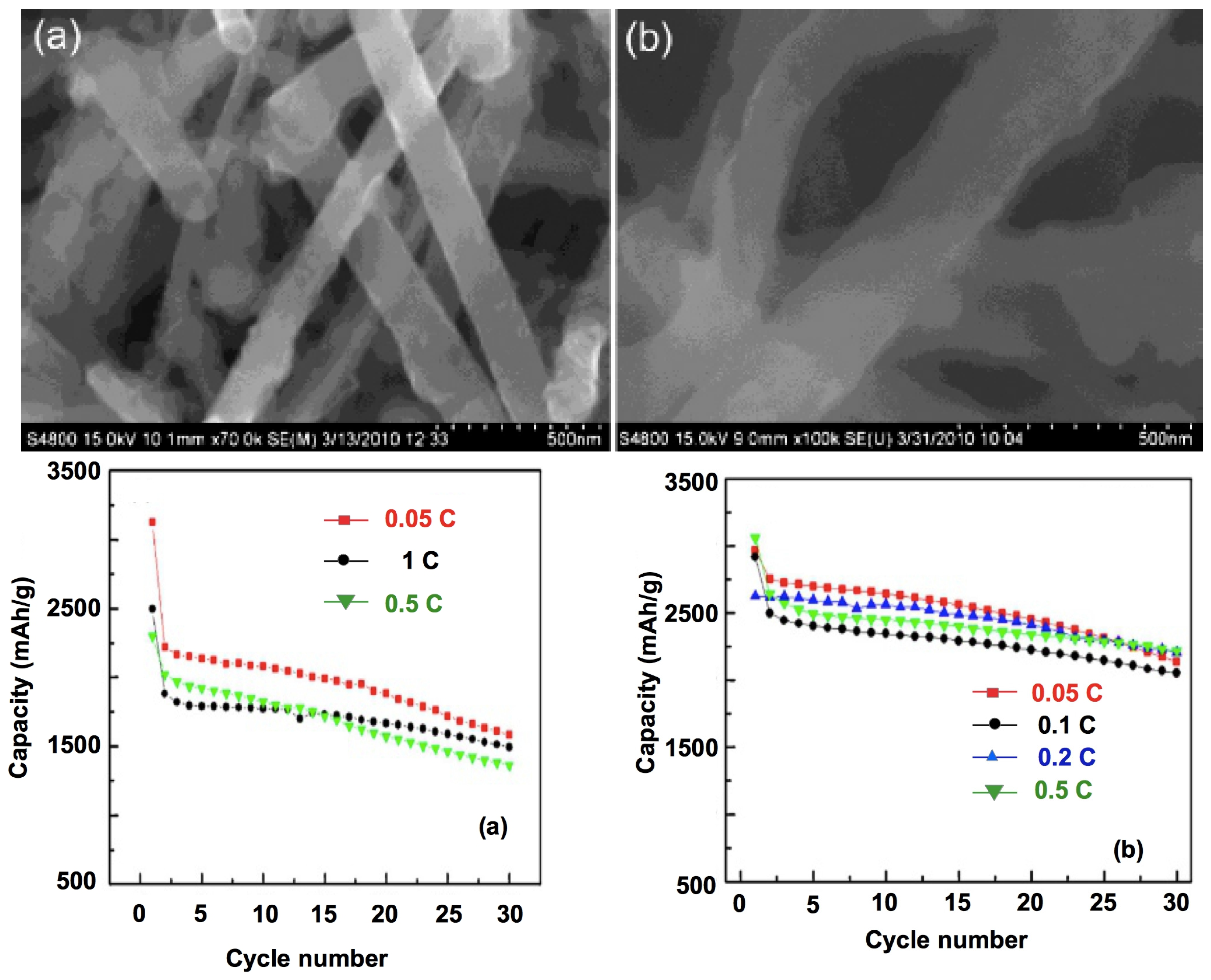
4. Li4Ti5O12
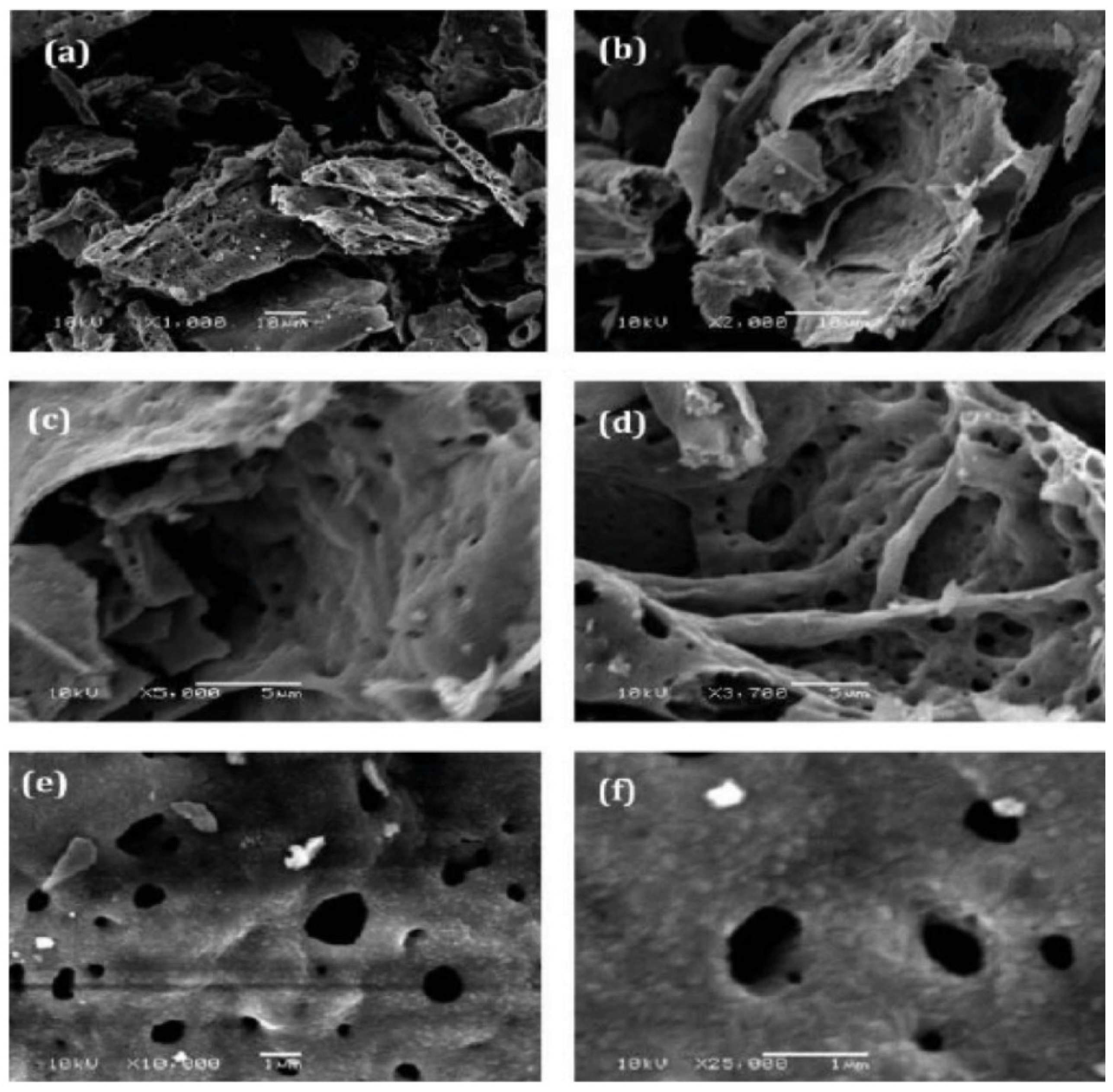
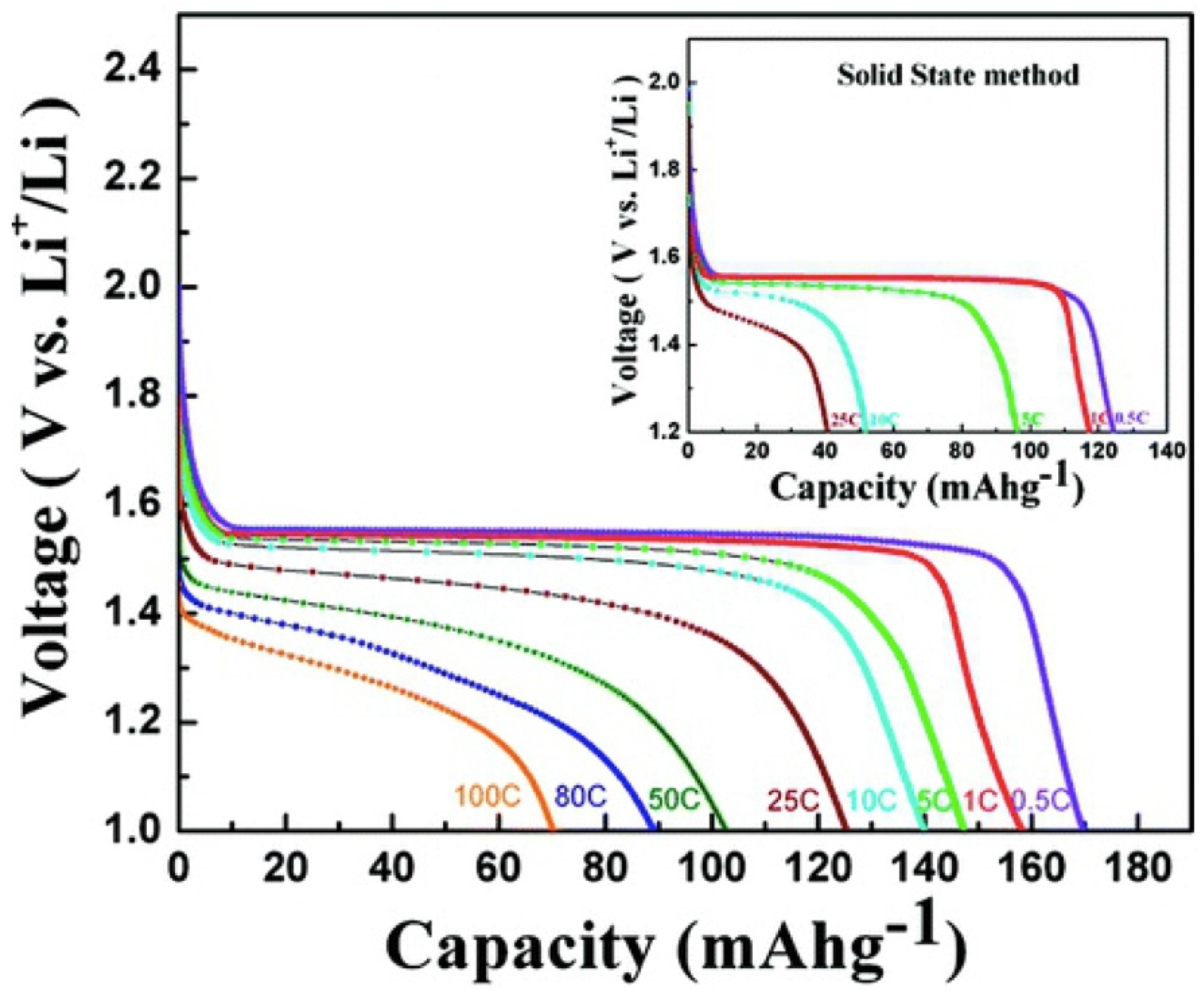
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Goriparti, S.; Miele, E.; De Angelis, F.; Di Fabrizio, E.; Zaccaria, R.P.; Capiglia, C. Review on recent progress of nanostructured anode materials for Li-ion batteries. J. Power Sour. 2014, 257, 421–443. [Google Scholar] [CrossRef]
- Julien, C.M.; Mauger, A.; Vijh, A.; Zaghib, K. Lithium Batteries: Science and Technology; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Li, C.C.; Orsini, F.; DuPasquier, A.; Beaudouin, B.; Tarascon, J.M.; Trentin, M.; Langenhuizen, N.; DeBeer, E.; Notten, P. In situ SEM study of the interfaces in plastic lithium cells. J. Power Sour. 1999, 81–82, 918–921. [Google Scholar]
- Landi, B.J.; Ganter, M.J.; Cress, C.D.; Di Leo, R.A.; Raffaelle, R.P. Carbon nanotubes for lithium ion batteries. Energy Environ. Sci. 2009, 2, 638–654. [Google Scholar] [CrossRef]
- Kim, C.K.; Yang, S.; Kojima, M.; Yoshida, K.; Kim, Y.J.; Kim, Y.A.; Endo, M. Fabrication of electrospinning-derived carbon nanofiber webs for the anode material of lithium-ion secondary batteries. Adv. Funct. Mater. 2006, 16, 2393–2397. [Google Scholar] [CrossRef]
- Wu, Y.P.; Rahm, E; Holze, R. Carbon anode materials for lithium ion batteries. J. Power Sources 2003, 114, 228–236. [Google Scholar] [CrossRef]
- Cui, G.; Gu, L.; Zhi, L.; Kaskhedikar, N.; Aken, P.A.; Mullen, K.; Maier, J. A germanium-carbon nanocomposite material for lithium batteries. Adv. Mater. 2008, 20, 3079–3083. [Google Scholar] [CrossRef]
- Candelaria, S.L.; Shao, Y.; Zhou, W.; Li, X.; Xiao, J.; Zhang, J.-G.; Wang, Y.; Liu, J.; Li, J.; Cao, G. Nanostructured carbon for energy storage and conversion. Nano Energy 2012, 1, 195–220. [Google Scholar] [CrossRef]
- Liu, Y.; Xue, J.S.; Zheng, T.; Dahn, J.R. Mechanism of lithium insertion in hard carbons prepared by pyrolysis of epoxy resins. Carbon 1996, 34, 193–200. [Google Scholar] [CrossRef]
- Mabuchi, A.; Tokumitsu, K.; Fujimoto, H.; Kasuh, T. Charge-discharge characteristics of the mesocarbon microbeads heat-treated at different temperatures. J. Electrochem. Soc. 1995, 142, 1041–1046. [Google Scholar] [CrossRef]
- Nagao, M.; Pitteloud, C.; Kamiyama, T.; Otomo, T.; Itoh, K.; Fukunaga, T.; Tatsumi, K.; Kanno, R. Structure characterization and lithiation mechanism of nongraphitized carbon for lithium secondary batteries. J. Electrochem. Soc. 2006, 153, A914–A919. [Google Scholar] [CrossRef]
- Sun, J.; Liu, H.; Chen, X.; Evans, D.G.; Yang, W.; Duan, X. Carbon nanorings and their enhanced lithium storage properties. Adv. Mater. 2013, 25, 1125–1130. [Google Scholar] [CrossRef] [PubMed]
- Meunier, V.; Kephart, J.; Roland, C.; Bernhole, J. Ab initio investigations of lithium diffusion in carbon nanotube systems. J. Phys. Rev. Lett. 2002, 88. [Google Scholar] [CrossRef] [PubMed]
- Schauerman, C.M.; Ganter, M.J.; Gaustad, G.; Babbitt, C.W.; Raffaelle, R.P.; Landi, B.J. Recycling single-wall carbon nanotube anodes from lithium ion batteries. J. Mater. Chem. 2012, 22, 12008–12015. [Google Scholar] [CrossRef]
- Nishidate, K.; Hasegawa, M. Energetics of lithium ion adsorption on defective carbon nanotubes. Phys. Rev. B 2005, 71, 245418. [Google Scholar] [CrossRef]
- Zhao, J.; Buldum, A.; Han, J.; Ping-Lu, J. First-principles study of Li-intercalated carbon nanotube ropes. Phys. Rev. Lett. 2000, 85, 1706–1709. [Google Scholar] [CrossRef] [PubMed]
- Di Leo, R.A.; Castiglia, A.; Ganter, M.J.; Rogers, R.E.; Cress, C.D.; Raffaelle, R.P.; Landi, B.J. Enhanced capacity and rate capability of carbon nanotube based anodes with titanium contacts for lithium ion batteries. ACS Nano 2010, 4, 6121–6131. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, I.; Oh, S.-M.; Hwang, J.Y.; Kang, C.; Choi, M.; Jeon, H.; Banerjee, R.; Sun, Y.K.; Choi, W. Ultrathin alumina-coated carbon nanotubes as an anode for high capacity Li-ion batteries. J. Mater. Chem. 2011, 21, 13621–13626. [Google Scholar] [CrossRef]
- Leung, K.; Qi, Y.; Zavadil, K.R.; Jung, Y.S.; Dillon, A.C.; Cavanagh, A.S.; Lee, S.-H.; George, S.M. Using atomic layer deposition to hinder solvent decomposition in lithium ion batteries: First-principles modeling and experimental studies. J. Am. Chem. Soc. 2011, 133, 14741–14754. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.S.; Cavanagh, A.S.; Riley, L.A.; Kang, S.-H.; Dillon, A.C.; Groner, M.D.; George, S.M.; Lee, S.H. Ultrathin direct atomic layer deposition on composite electrodes for highly durable and safe Li-ion batteries. Adv. Mater. 2010, 22, 2172–2176. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zheng, Z.; Liu, X.H.; Huang, S.; Zhu, T.; Wang, J.; Kushim, A.; Hudak, N.S.; Huang, X.; Zhang, S.; et al. Lithiation-induced embrittlement of multiwalled carbon nanotubes. ACS Nano 2011, 5, 7245–7253. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-S.; Zhou, G.; Yin, L.-C.; Ren, W.; Li, F.; Cheng, H.-M. Graphene/metal oxide composite electrode materials for energy storage. Nano Energy 2012, 1, 107–131. [Google Scholar] [CrossRef]
- Hou, J.; Shao, Y.; Ellis, M.W.; Moore, R.B.; Yi, B. Graphene-based electrochemical energy conversion and storage: fuel cells, supercapacitors and lithium ion batteries. Phys. Chem. Chem. Phys. 2011, 13, 15384–15402. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Shen, L.; Zheng, H.; Zhang, X. Ge-graphene-carbon nanotube composite anode for high performance lithium-ion batteries. J. Mater. Chem. A 2015, 3, 1498–1505. [Google Scholar] [CrossRef]
- Liang, M.; Zhi, L. Graphene-based electrode materials for rechargeable lithium batteries. J. Mater. Chem. 2009, 19, 5871–5878. [Google Scholar] [CrossRef]
- Brownson, D.A.C.; Kampouris, D.K.; Banks, C.E. An overview of graphene in energy production and storage applications. J. Power Sour. 2011, 196, 4873–4885. [Google Scholar] [CrossRef]
- Hwang, H.Y.; Koo, J.; Park, M.; Park, N.; Kwon, Y.; Lee, H. Multilayer graphynes for lithium ion battery anode. J. Phys. Chem. C 2013, 117, 6919–6923. [Google Scholar] [CrossRef]
- Pan, D.; Wang, S.; Zhao, B.; Wu, M.; Zhang, H.; Wang, Y.; Jiao, Z. Li storage properties of disordered grapheme nanosheets. Chem. Mater. 2009, 21, 3136–3142. [Google Scholar] [CrossRef]
- Lee, S.U.; Belosludov, R.V.; Mizuseki, H.; Kawazoe, Y. Designing nanogadgetry for nanoelectric devices with nitrogen-doped capped carbon nanotubes. Small 2009, 5, 1769–1775. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-L.; Xu, D.; Wang, H.G.; Wu, Z.; Zhang, X.-B. In situ fabrication of porous grapheme electrodes for high-performance energy storage. ACS Nano 2013, 7, 2422–2430. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, N.; Zhang, C.; Liu, F.; Zhu, J.; Hou, Y. Hybrid of Co3Sn2@Co nanoparticles and nitrogen-doped grapheme as a lithium battery anode. ACS Nano 2013, 7, 10307–10318. [Google Scholar] [CrossRef] [PubMed]
- Ge, M.Y.; Rong, J.; Fang, X.; Zhou, C. Porous doped silicon nanowires for lithium ion battery anode with long cycle life. Nano Lett. 2012, 12, 2318–2323. [Google Scholar] [CrossRef] [PubMed]
- Ge, M.Y.; Lu, Y.H.; Ercius, P.; Rong, J.P.; Fang, X.; Zhou, C.W.; Mecklenburg, M. Large-scale fabrication, 3D tomography and lithium-ion battery application of porous silicon. Nano Lett. 2014, 14, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Besenhard, J.O.; Yang, J.; Winter, M. Will advanced lithium-alloy anodes have a chance in lithium-ion batteries? J. Power Sour. 1997, 68, 87–90. [Google Scholar] [CrossRef]
- Beaulieu, L.Y.; Eberman, K.W.; Turner, R.L.; Krause, L.J.; Dahn, J.R. Collossal reversible volume changes in lithium alloys. Electrochem. Solid State Lett. 2001, 4, A137–A140. [Google Scholar] [CrossRef]
- Beaulieu, L.Y.; Hatchard, T.D.; Bonakdarpour, A.; Fleischauer, M.D.; Dahn, J.R. Reaction of Li with alloy thin films studied by in situ AFM. J. Electrochem. Soc. 2003, 150, A1457–A1464. [Google Scholar] [CrossRef]
- Zhang, X.W.; Patil, P.K.; Wang, C.S.; Appleby, A.J.; Little, F.E.; Cocke, D.L. Electrochemical performance of lithium ion battery, nano-silicon-based, disordered carbon composite anodes with different microstructures. J. Power Sour. 2004, 125, 206–213. [Google Scholar] [CrossRef]
- Chan, C.K.; Peng, H.L.; Liu, G.; McIlwrath, K.; Zhang, X.F.; Huggins, R.A.; Cui, Y. High-performance lithium battery anodes using silicon nanowires. Nat. Nanotechnol. 2008, 3, 31–35. [Google Scholar] [CrossRef] [PubMed]
- McDowell, M.T.; Lee, S.W.; Wang, C.; Cui, Y. The effect of metallic coatings and crystallinity on the volume expansion of silicon during electrochemical lithiation/delithiation. Nano Energy 2012, 1, 401–410. [Google Scholar] [CrossRef]
- Maver, U.; Znidarsic, A.; Gaberscek, M. An attempt to use atomic force microscopy for determination of bond type in lithium battery electrodes. J. Mater. Chem. 2011, 21, 4071–4075. [Google Scholar] [CrossRef]
- Soni, S.K.; Sheldon, B.W.; Xiao, X.C.; Verbrugge, M.W.; Ahn, D.; Haftbaradaran, H.; Gao, H.J. Stress migration during the lithiation of patterned amorphous Si islands. J. Electrochem. Soc. 2012, 159, A38–A43. [Google Scholar] [CrossRef]
- Lee, K.L.; Jung, J.Y.; Lee, S.W.; Moon, H.S.; Park, J.W. Electrochemical characteristics of a-Si thin film anode for Li-ion rechargeable batteries. J. Power Sour. 2004, 129, 270–274. [Google Scholar] [CrossRef]
- Park, M.S.; Wang, G.X.; Liu, H.K.; Dou, S.X. Electrochemical properties of Si thin film prepared by pulse laser deposition for lithium ion microbatteries. Electrochim. Acta 2006, 51, 5246–5249. [Google Scholar] [CrossRef]
- Raimann, P.R.; Hochgatterer, N.S.; Korepp, C.; Moller, K.C.; Winter, M.; Schrottner, H.; Hofer, F.; Besenhard, J.O. Monitoring dynamics of electrode reactions in Li-ion batteries by in situ ESEM. Ionics 2006, 12, 253–255. [Google Scholar] [CrossRef]
- Kim, H.; Choi, J.; Sohn, H.J.; Kang, T. The insertion mechanism of lithium Mg2Si anode material for Li-ion batteries. J. Electrochem. Soc. 1999, 146, 4401–4405. [Google Scholar] [CrossRef]
- Wachtler, M.; Winter, M.; Besenhard, J.O. Anodic materials for rechargeable Li-batteries. J. Power Sour. 2002, 105, 151–160. [Google Scholar] [CrossRef]
- Kim, J.W.; Ryu, J.H.; Lee, K.T.; Oh, S.M. Improvement of silicon powder negative electrodes by copper electroless deposition for lithium secondary batteries. J. Power Sour. 2005, 147, 227–233. [Google Scholar] [CrossRef]
- Goldman, J.L.; Long, B.R.; Gewirth, A.A.; Nuzzo, R.G. Strain anisotropies and self-limiting capacities in single-crystalline 3D silicon microstructures: Models for high energy density lithium-ion battery anodes. Adv. Funct. Mater. 2011, 21, 2412–2422. [Google Scholar] [CrossRef]
- Yang, J.; Winter, M.; Besenhard, J.O. Small particle size multiphase Li-alloy anodes for lithium-ion batteries. Solid State Ion. 1996, 90, 281–287. [Google Scholar] [CrossRef]
- Stjerndahl, M.; Bryngelsson, H.; Gustafsson, T.; Vaughey, J.T.; Tackeray, M.M.; Edstrom, K. Surface chemistry of intermetallic AlSb-anodes for Li-ion batteries. Electrochim. Acta 2007, 52, 4947–4955. [Google Scholar] [CrossRef]
- Lee, S.W.; McDowell, M.T.; Choi, J.W.; Cui, Y. Anomalous shape changes of silicon nanopillars by electrochemical lithiation. Nano Lett. 2011, 11, 3034–3039. [Google Scholar] [CrossRef] [PubMed]
- Wagesreither, S.; Lugstein, A.; Bertagnolli, E. Anisotropic lithiation behavior of crystalline silicon. Nanotechnology 2012, 23, 495716–495719. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Wang, J.W.; Huang, S.; Fan, F.F.; Huang, X.; Liu, Y.; Krylyuk, S.; Yoo, J.; Dayeh, S.A.; Davydov, A.V.; et al. In situ atomic-scale imaging of electrochemical lithiation in silicon. Nat. Nanotechnol. 2012, 7, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Takamura, T.; Ohara, S.; Uehara, M.; Suzuki, J.; Sekine, K. A vacuum deposited Si film having a Li extraction capacity over 2000 mAh/g with a long cycle life. J. Power Sour. 2004, 129, 96–100. [Google Scholar] [CrossRef]
- Ohara, S.; Suzuki, J.; Sekine, K.; Takamura, T. Li insertion/extraction reaction at a Si film evaporated on a Ni foil. J. Power Sour. 2003, 119–121, 591–596. [Google Scholar] [CrossRef]
- Chen, L.B.; Xie, J.Y.; Yu, H.C.; Wang, T.H. An amorphous Si thin film anode with high capacity and long cycling life for lithium ion batteries. J. Appl. Electrochem. 2009, 39, 1157–1162. [Google Scholar] [CrossRef]
- Maranchi, J.P.; Hepps, A.F.; Evans, A.G.; Nuhfer, N.T.; Kumta, P.N. Interfacial properties of the a-Si/Cu: Active-inactive thin-film anode system for lithium-ion batteries. J. Electrochem. Soc. 2006, 153, A1246–A1253. [Google Scholar] [CrossRef]
- Yu, C.; Li, X.; Ma, T.; Rong, J.; Zhang, R.; Shaffer, J.; An, Y.; Liu, Q.; Wei, B.; Jiang, H. Silicon thin films as anodes for high-performance lithium-ion batteries with effective stress relaxation. Adv. Energy Mater. 2012, 2, 68–73. [Google Scholar] [CrossRef]
- Schmidt, V.; Wittemann, J.V.; Gösele, U. Growth, thermodynamics and electrical properties of silicon nanowires. Chem. Rev. 2010, 110, 361–388. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.F.; Ruffo, R.; Chan, C.K.; Peng, H.L.; Cui, Y. Crystalline-amorphous core-shell silicon nanowires for high capacity and high current battery electrodes. Nano Lett. 2009, 9, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.K.; Patel, R.N.; O’Connell, M.J.; Korgel, B.A.; Cui, Y. Solution-grown silicon nanowires for lithium-ion battery anodes. ACS Nano 2010, 4, 1443–1450. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.; Yao, F.; Zamfir, M.R.; Biswas, C.; So, K.P.; Lee, Y.H.; Kim, S.M.; Cha, S.N.; Kim, J.M.; Pribat, D. Highly interconnected Si nanowires for improved stability Li-ion battery anodes. Adv. Energy Mater. 2011, 1, 1154–1161. [Google Scholar] [CrossRef]
- Bruce, P.G.; Scrosati, B.; Tarascon, J.M. Nanomaterials for rechargeable lithium batteries. Angew. Chem. Int. Ed. 2008, 47, 2930–2946. [Google Scholar] [CrossRef] [PubMed]
- Bang, B.M.; Kim, H.; Song, H.K.; Cho, J.; Park, S. Scalable approach to multi-dimensional bulk Si anodes via metal-assisted chemical etching. Energy Environ. Sci. 2011, 4, 5013–5019. [Google Scholar] [CrossRef] [Green Version]
- Zamfir, M.R.; Nguyen, H.T.; Moyen, E.; Lee, Y.H.; Pribat, D. Silicon nanowires for Li-based battery anodes: A review. J. Mater. Chem. A 2013, 1, 9566–9586. [Google Scholar] [CrossRef]
- Kim, H.; Han, B.; Choo, J.; Cho, J. Three-dimensional porous silicon particles for use in high-performance lithium secondary batteries. Angew. Chem. Int. Ed. 2008, 47, 10151–10154. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Cho, J. Superior lithium electroactive mesoporous Si@carbon core-shell nanowires for lithium battery anode material. Nano Lett. 2008, 8, 3688–3691. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Cheng, H.Y.; Choi, H.; Lee, J.H.; Han, H.; Lee, D.H.; Yoo, D.S.; Kwon, M.S.; Choi, J.M.; Doo, S.G.; et al. Si/Ge double-layered nanotube array as a lithium ion battery anode. ACS Nano 2012, 6, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Xia, J.L.; Lee, J.H.; Lee, D.H.; Kwon, M.S.; Choi, J.M.; Wu, J.; Doo, S.K.; Chang, H.; Park, W.; et al. Arrays of sealed silicon nanotubes as anodes for lithium ion batteries. Nano Lett. 2010, 10, 1710–1716. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H.; Kim, M.G.; Joo, J.; Kim, K.; Kim, J.; Ahn, S.; Cui, Y.; Cho, J. Silicon nanotube battery anodes. Nano Lett. 2009, 9, 3844–3847. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; McDowell, M.T.; Ryu, I.; Wu, H.; Liu, N.; Hu, L.; Nix, W.D.; Cui, Y. Interconnected silicon hollow nanospheres for lithium-ion battery anodes with long cycle life. Nano Lett. 2011, 11, 2949–2954. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Han, W.Q. Graphene enhances Li storage capacity of porous sigle-crystalline silicon nanowires. ACS Appl. Mater. Int. 2010, 2, 3709–3713. [Google Scholar] [CrossRef] [PubMed]
- Rong, J.; Masarapu, C.; Ni, J.; Zhang, Z.; Wei, B. Tandem structure of porous silicon film on single-walled carbon nanotube macrofilms for lithium-ion battery applications. ACS Nano 2010, 4, 4683–4690. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Sun, A.; Wang, C. A porous silicon-carbon anode with high overall capacity on carbon fiber current collector. Electrochem. Commun. 2010, 12, 981–984. [Google Scholar] [CrossRef]
- Ma, H.; Cheng, F.Y.; Chen, J.; Zhao, J.Z.; Li, C.S.; Tao, Z.L.; Liang, J. Nest-like silicon nanospheres for high-capacity lithium storage. Adv. Mater. 2007, 19, 4067–4070. [Google Scholar] [CrossRef]
- Ge, M.; Rong, J.; Fang, X.; Zhang, A.; Lu, Y.; Zhou, C. Scalable preparation of porous silicon nanoparticles and their application for lithium battery anodes. Nano Res. 2013, 6, 174–181. [Google Scholar] [CrossRef]
- Bang, B.M.; Lee, J.I.; Kim, H.; Cho, J.; Park, S. High-performance macroporous bulk silicon anodes synthesized by template-free chemical etching. Adv. Energy Mater. 2012, 2, 878–883. [Google Scholar] [CrossRef]
- Thakur, M.; Pernites, R.B.; Nitta, N.; Isaacson, M.; Sinsabaugh, S.L.; Wong, M.S.; Biswal, S.L. Freestanding macroporous silicon and pyrolyzed polyacrylonitrile as a composite anode for lithium ion batteries. Chem. Mater. 2012, 24, 2998–3003. [Google Scholar] [CrossRef]
- Thakur, M.; Isaacson, M.; Sinsabaugh, S.L.; Wong, M.S.; Biswal, S.L. Gold-coated porous silicon films as anodes for lithium ion batteries. J. Power Sour. 2012, 205, 426–432. [Google Scholar] [CrossRef]
- Thakur, M.; Sinsabaugh, S.L.; Isaacson, M.J.; Wong, M.S.; Biswal, S.L. Inexpensive method for producing macroporous silicon particulates (MPSPs) with pyrolyzed polyacrylonitrile for lithium ion batteries. Sci. Rep. 2012, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.G.; Braun, P.V. Three-dimensional metal scaffold supported bicontinuous silicon battery anodes. Nano Lett. 2012, 12, 2778–2783. [Google Scholar] [CrossRef] [PubMed]
- Gowda, S.R.; Pushparaj, V.; Herle, S.; Girishkumar, G.; Gordon, J.G.; Gullapalli, H.; Zhan, X.B.; Ajayan, P.M.; Reddy, A.L.M. Three-dimensionally engineered porous silicon electrodes for Li ion batteries. Nano Lett. 2012, 12, 6060–6065. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Wang, G.; Huo, Y.; Zhang, S.; Cao, G.; Zhao, X. Hollow nano silicon prepared by a controlled template direction and magnesiothermic reduction reaction as anode for lithium ion batteries. New J. Chem. 2014, 38, 4177–4181. [Google Scholar] [CrossRef]
- Cui, L.-F.; Hu, L.B.; Wu, H.; Choi, J.W.; Cui, Y. Inorganic glue enabling high performance of silicon particles as lithium ion battery anode. J. Electrochem. Soc. 2011, 158, A592–A596. [Google Scholar] [CrossRef]
- Nadimpalli, S.P.; Sethuraman, V.A.; Dalavi, S.; Lucht, B.; Chon, M.J.; Shenoy, V.B.; Guduru, P.R. Quantifying capacity loss due to solid-electrolyte-interphase layer formation on silicon negative electrodes in lithium-ion batteries. J. Power Sour. 2012, 215, 145–151. [Google Scholar] [CrossRef]
- Kovalenko, I.; Zdyrko, B.; Magasinski, A.; Hertzberg, B.; Milicev, Z.; Burtovyy, R.; Luzinov, I.; Yushin, G. A major constituent of brown algae for use in high-capacity Li-ion batteries. Science 2011, 334, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Sethuraman, V.A.; Kowolik, K.; Srinivasan, V. Increased cycling efficiency and rate capability of copper-coated silicon anodes in lithium-ion batteries. J. Power Sour. 2011, 196, 393–398. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Tang, J.J.; Yang, J.; Xie, J.; Ma, L.L. Silicon@carbon hollow core-shell heterostructures novel anode materials for lithium ion batteries. Electrochim. Acta 2013, 87, 663–668. [Google Scholar] [CrossRef]
- Chen, S.; Gordin, M.L.; Yi, R.; Howlett, G.; Sohn, H.; Wang, D. Silicon core-hollow carbon shell nanocomposites with tunable buffer voids for high capacity anodes of lithium-ion batteries. Phys. Chem. Chem. Phys. 2012, 14, 12741–12745. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Meduri, P.; Chen, X.; Qi, W.; Engelhard, M.H.; Xu, W.; Ding, F.; Xiao, J.; Wang, W.; Wang, C. Hollow core-shell structured porous Si-C nanocomposites for Li-ion battery anodes. J. Mater. Chem. 2012, 22, 11014–11017. [Google Scholar] [CrossRef]
- Jung, D.S.; Hwang, T.H.; Park, S.B.; Choi, J.W. Spray drying method for large-scale and high-performance silicon negative electrodes in Li-ion batteries. Nano Lett. 2013, 13, 2092–2097. [Google Scholar] [CrossRef] [PubMed]
- Magasinski, A.; Dixon, P.; Hertzberg, B.; Kvit, A.; Ayala, J.; Yushin, G. High-performance lithium-ion anodes using a hierarchical bottom-up approach. Nat. Mater. 2010, 9, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Chan, G.; Choi, J.W.; Ryu, I.; Yao, Y.; McDowell, M.T.; Lee, S.W.; Jackson, A.; Yang, Y.; Hu, L.B.; et al. Stable cycling of double-walled silicon nanotube battery anodes through solid-electrolyte interphase control. Nat. Nanotechnol. 2012, 7, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Nyholm, L.; Nyström, G.; Mihranyan, A.; Stromme, M. Toward flexible polymer and paper-based energy storage devices. Adv. Mater. 2011, 23, 3751–3769. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Wu, H.; McDowell, M.T.; Yao, Y.; Wang, C.; Cui, Y. A yolk-shell design for stabilized and scalable Li-ion battery alloy anodes. Nano Lett. 2012, 12, 3315–3322. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.X.; Dong, Z.X.; Fu, Y.P.; Yang, Y. Silicon nanowires with and without carbon coating as anode materials for lithium-ion batteries. J. Solid State Electrochem. 2010, 14, 1829–1834. [Google Scholar] [CrossRef]
- Chen, H.; Xiao, Y.; Wang, L.; Yang, Y. Silicon nanowires coated with copper layer as anode materials for lithium-ion batteries. J. Power Sour. 2011, 196, 6657–6662. [Google Scholar] [CrossRef]
- Ryu, I.; Choi, J.W.; Cui, Y.; Nix, W.D. Size-dependent fracture of Si nanowire battery anodes. J. Mech. Phys. Solids 2011, 59, 1717–1730. [Google Scholar] [CrossRef]
- Liu, X.H.; Zheng, H.; Zhong, L.; Huang, S.; Karki, K.; Zhang, L.Q.; Liu, Y.; Kushima, A.; Liang, W.T.; Wang, J.W.; et al. Anisotropic swelling and fracture of silicon nanowires during lithiation. Nano Lett. 2011, 11, 3312–3318. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Liu, N.; McDowell, M.T.; Pasta, M.; Cui, Y. Improving the cycling stability of silicon nanowire anodes with conducting polymer coatings. Energy Environ. Sci. 2012, 5, 7927–7930. [Google Scholar] [CrossRef]
- Xiao, X.; Lu, P.; Ahn, D. Ultrathin multifunctional oxide coatings for lithium ion batteries. Adv. Mater. 2011, 23, 3911–3915. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yu, X.; Wang, Y.; Li, H.; Huang, X. Alumina-coated patterned amorphous silicon as the anode for a lithium-ion battery with high coulombic efficiency. Adv. Mater. 2011, 23, 4938–4941. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.; Zamfir, M.R.; Duong, L.D.; Lee, Y.H.; Bondavalli, P.; Pribat, D. Alumina-coated silicon-based nanowire arrays for high quality Li-ion battery anodes. J. Mater. Chem. 2012, 22, 24618–24626. [Google Scholar] [CrossRef]
- Liu, Y.; Hudak, N.S.; Huber, D.L.; Limmer, S.J.; Sullivan, J.P.; Huang, J.Y. In situ transmission electron microscopy observation of pulverization of aluminum nanowires and evolution of the thin surface Al2O3 layers during lithiation-delithiation cycles. Nano Lett. 2011, 11, 4188–4194. [Google Scholar] [CrossRef] [PubMed]
- Evanoff, K.; Kahn, J.; Balandin, A.; Magasinski, A.; Ready, W.J.; Fuller, T.F.; Yushin, G. Towards ultrathick battery electrodes: Aligned carbon nanotube enabled architecture. Adv. Mater. 2012, 24, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Lu, G.; Mao, S.; Kim, H.; Cui, S.; Yu, K.; Huang, X.; Hurle, P.T.; Mao, O.; Chen, J. Silicon nanotube anode for lithium-ion batteries. Electrochem. Commun. 2013, 29, 67–70. [Google Scholar] [CrossRef]
- Zaghib, K.; Dontigny, M.; Guerfi, A.; Trottier, J.; Hamel-Paquet, J.; Gariepy, V.; Galoutov, K.; Hovington, P.; Mauger, A.; Groult, H.; et al. An improved high-power battery with increased thermal operating range: C-LiFePO4//C-Li4Ti5O12. J. Power Sour. 2012, 216, 192–200. [Google Scholar] [CrossRef]
- Zaghib, K.; Dontigny, M.; Perret, P.; Guerfi, A.; Ramanathan, M.; Prakash, J.; Mauger, A.; Julien, C.M. Electrochemical and thermal characterization of lithium titanate spinel in C-LiFePO4//C-Li4Ti5O12 cells at sub-zero temperatures. J. Power Sour. 2014, 248, 1050–1057. [Google Scholar] [CrossRef]
- Yi, T.F.; Jiang, L.J.; Shu, J.; Yue, C.B.; Zhu, R.S.; Qiao, H.B. Recent development and application of Li4Ti5O12 as anode material of lithium ion battery. J. Phys. Chem. Solids 2010, 71, 1236–1242. [Google Scholar] [CrossRef]
- Reddy, M.V.; Subba Rao, G.V.; Chowdari, B.V.R. Metal oxides and oxysalts as anode materials for Li ion batteries. Chem. Rev. 2013, 113, 5364–5457. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.G.; Choi, D.; Kerisit, S.; Rosso, K.M.; Wang, D.H.; Zhang, J.; Graff, G.; Liu, J. Nanostructures and lithium electrochemical reactivity of lithium titanites and titanium oxides: A review. J. Power Sour. 2009, 192, 588–598. [Google Scholar] [CrossRef]
- Prakash, A.S.; Manikandan, P.; Ramesha, K.; Sathiya, M.; Tarascon, J.M.; Shukla, A.K. Solution-combustion synthesized nanocrystalline Li4Ti5O12 as high-rate performance Li-ion battery anode. Chem. Mater. 2010, 22, 2857–2863. [Google Scholar] [CrossRef]
- Chen, J.Z.; Yang, L.; Fang, S.H.; Hirano, S.; Tachibana, K. Synthesis of hierarchical mesoporous nest-like Li4Ti5O12 for high-rate lithium ion batteries. J. Power Sour. 2012, 200, 59–66. [Google Scholar] [CrossRef]
- Lin, Y.S.; Tsai, M.C.; Duh, J.G. Self-assembled synthesis of nanoflower-like Li4Ti5O12 for ultrahigh rate lithium-ion batteries. J. Power Sour. 2012, 214, 314–318. [Google Scholar] [CrossRef]
- Cheng, L.; Yan, J.; Zhu, G.-N.; Luo, J.Y.; Wang, C.X.; Xia, Y.Y. General synthesis of carbon-coated nanostructure Li4Ti5O12 as a high rate electrode material for Li-ion intercalation. J. Mat. Chem. 2010, 20, 595–602. [Google Scholar] [CrossRef]
- Zhu, G.N.; Liu, H.J.; Zhang, J.H.; Wang, C.X.; Wang, Y.G.; Xia, Y.Y. Carbon-coated nano-sized Li4Ti5O12 nanoporous micro-sphere as anode material for high-rate lithium-ion batteries. Energy Environ. Sci. 2011, 4, 4016–4022. [Google Scholar] [CrossRef]
- Shen, L.; Yuan, C.; Luo, H.; Zhang, X.; Chen, L.; Li, H. Facile synthesis of hierarchically porous Li4Ti5O12 microspheres for high rate lithium ion batteries. J. Mater. Chem. 2010, 20, 6998–7004. [Google Scholar] [CrossRef]
- Kim, H.K.; Bak, S.M.; Kim, K.B. Li4Ti5O12/reduced graphite oxide nano-hybrid material for high rate lithium-ion batteries. Electrochem. Commun. 2010, 12, 1768–1771. [Google Scholar] [CrossRef]
- Ariyoshi, K.; Yamamoto, S.; Ohzuku, T. Three-volt lithium-ion battery with Li[Ni1/2Mn3/2]O4 and the zero-strain insertion material of Li[Li1/3Ti5/3]O4. J. Power Sour. 2003, 119, 959–963. [Google Scholar] [CrossRef]
- Ariyoshi, K.; Ohzuku, T. Conceptual design for 12 V “lead-free” accumulators for automobile and stationary applications. J. Power Sour. 2007, 174, 1258–1262. [Google Scholar] [CrossRef]
- Du Pasquier, A.; Huang, C.C.; Spitler, T. Nano Li4Ti5O12-LiMn2O4 batteries with high power capacity and improved cycle-life. J. Power Sour. 2009, 186, 508–514. [Google Scholar] [CrossRef]
- Amine, K.; Belharouak, I.; Chen, Z.H.; Tran, T.; Yumoto, H.; Ota, N.; Myung, S.T.; Sun, Y.K. Nanostructured anode material for high-power battery system in electric vehicles. Adv. Mater. 2010, 22, 3052–3057. [Google Scholar] [CrossRef] [PubMed]
- Jansen, A.N.; Kahaian, A.J.; Kepler, K.D.; Nelson, P.A.; Amine, K.; Dees, D.W.; Vissers, D.R.; Thackeray, M.M. Development of a high-power lithium-ion battery. J. Power Sour. 1999, 81–82, 902–905. [Google Scholar] [CrossRef]
- Sawai, K.; Yamato, R.; Ohzuku, T. Impedance measurements on lithium-ion battery consisting of Li[Li1/3Ti5/3]O4 and LiCo1/2Ni1/2O2. Electrochim. Acta 2006, 51, 1651–1655. [Google Scholar] [CrossRef]
- Lu, W.; Liu, J.; Sun, Y.K.; Amine, K. Electrochemical performance of Li4/3Ti5/3O4/Li1+x(Ni1/3Co1/3Mn1/3)1−xO2 cell for high power applications. J. Power Sour. 2007, 167, 212–216. [Google Scholar] [CrossRef]
- Jaiswal, A.; Horne, C.R.; Chang, O.; Zhang, W.; Kong, W.; Wang, E.; Chern, T.; Doeff, M.M. Nanoscale LiFePO4 and Li4Ti5O12 for high rate Li-ion batteries. J. Electrochem. Soc. 2009, 156, A1041–A1047. [Google Scholar] [CrossRef]
- Zaghib, K.; Dontigny, M.; Guerfi, A.; Charest, P.; Rodrigues, I.; Mauger, A.; Julien, C.M. Safe and fast-charging Li-ion battery with long shelf life for power applications. J. Power Sour. 2011, 196, 3949–3954. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mauger, A.; Julien, C.M. Nanoscience Supporting the Research on the Negative Electrodes of Li-Ion Batteries. Nanomaterials 2015, 5, 2279-2301. https://doi.org/10.3390/nano5042279
Mauger A, Julien CM. Nanoscience Supporting the Research on the Negative Electrodes of Li-Ion Batteries. Nanomaterials. 2015; 5(4):2279-2301. https://doi.org/10.3390/nano5042279
Chicago/Turabian StyleMauger, Alain, and Christian M. Julien. 2015. "Nanoscience Supporting the Research on the Negative Electrodes of Li-Ion Batteries" Nanomaterials 5, no. 4: 2279-2301. https://doi.org/10.3390/nano5042279