Controllable Synthesis of Copper Oxide/Carbon Core/Shell Nanowire Arrays and Their Application for Electrochemical Energy Storage
Abstract
:1. Introduction
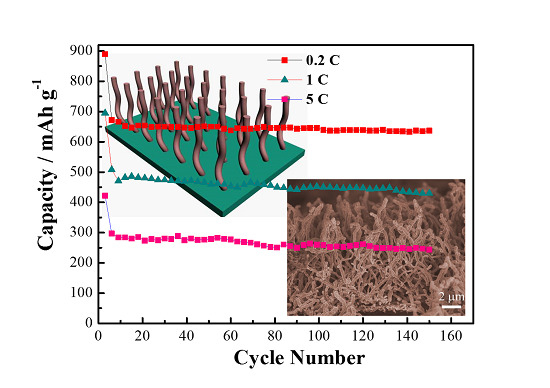
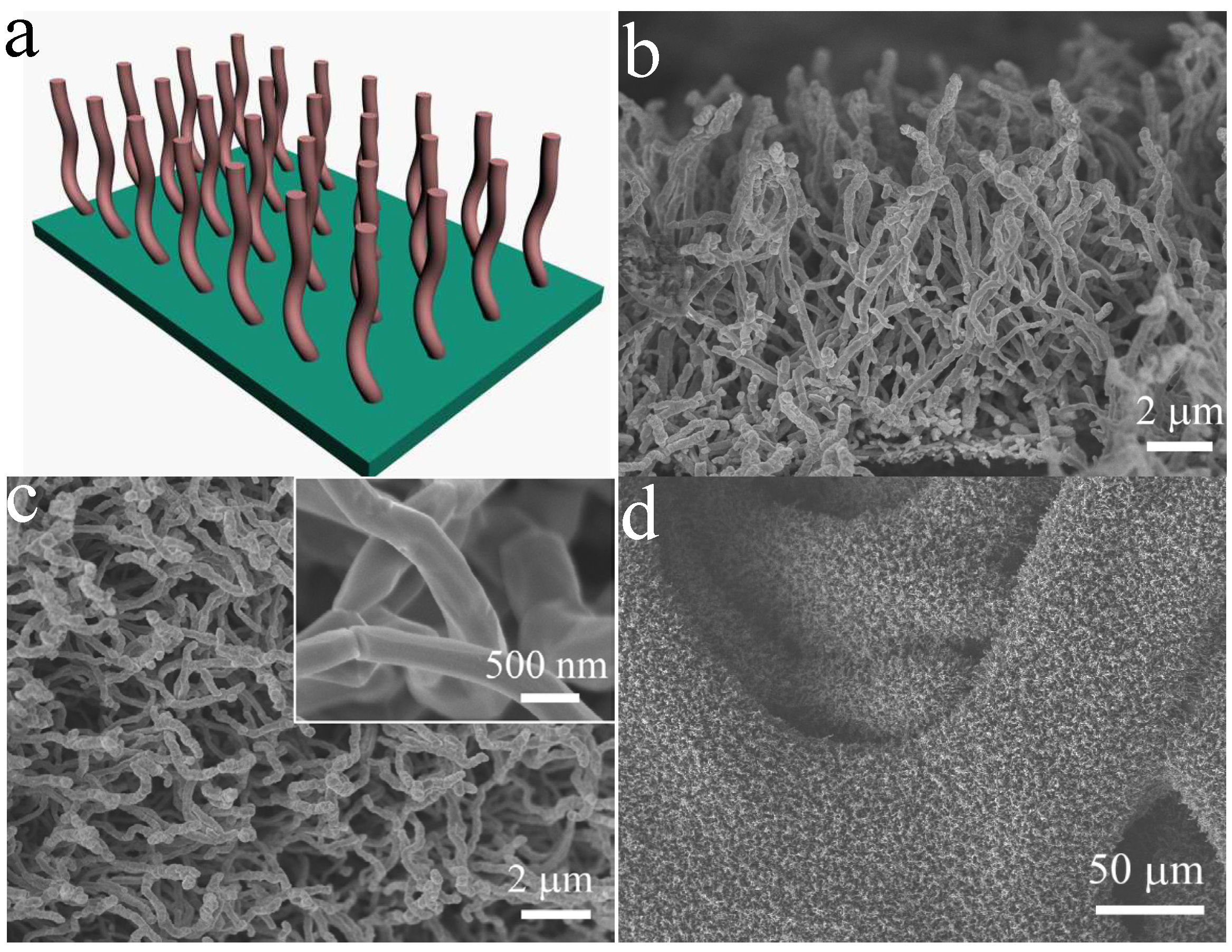
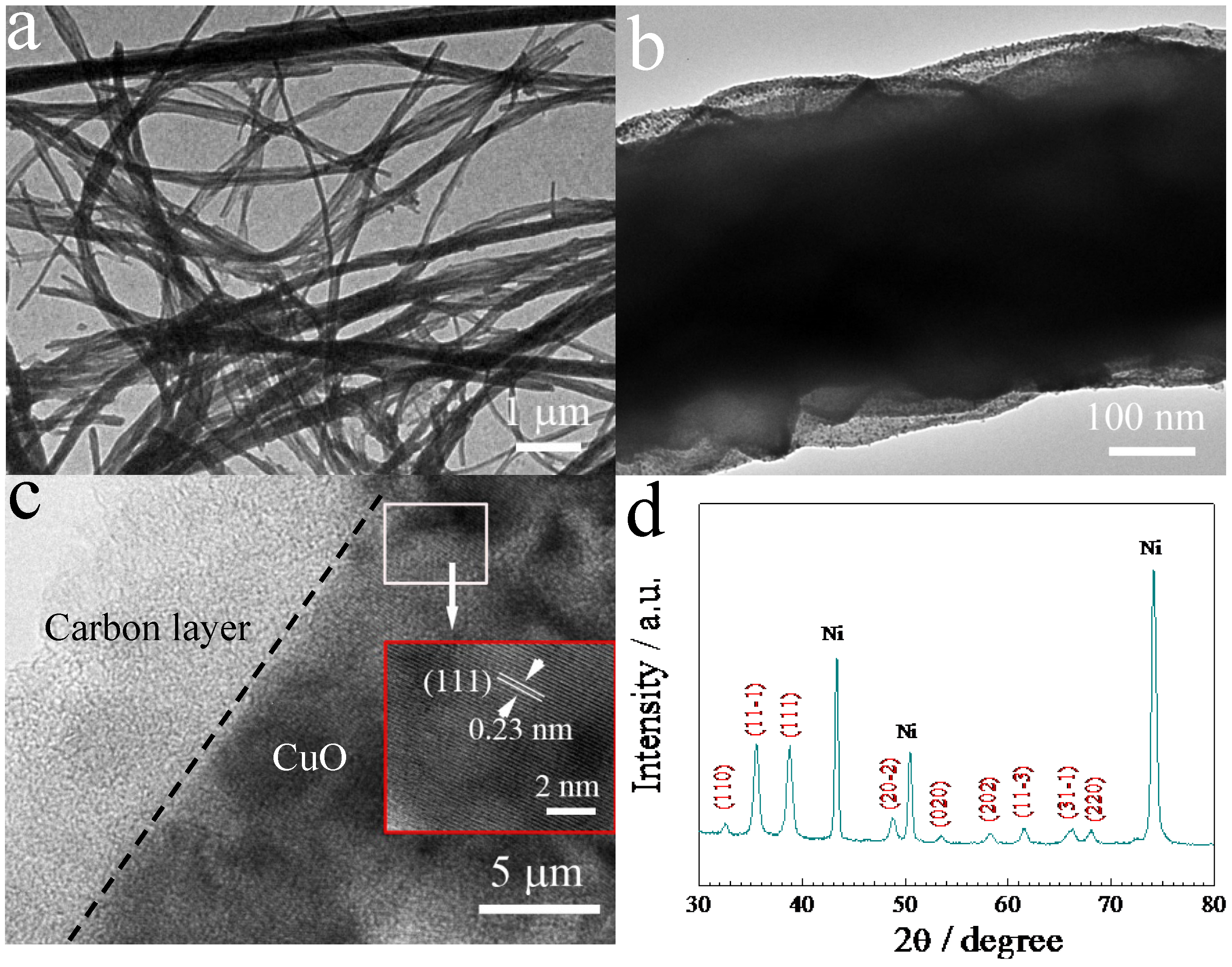
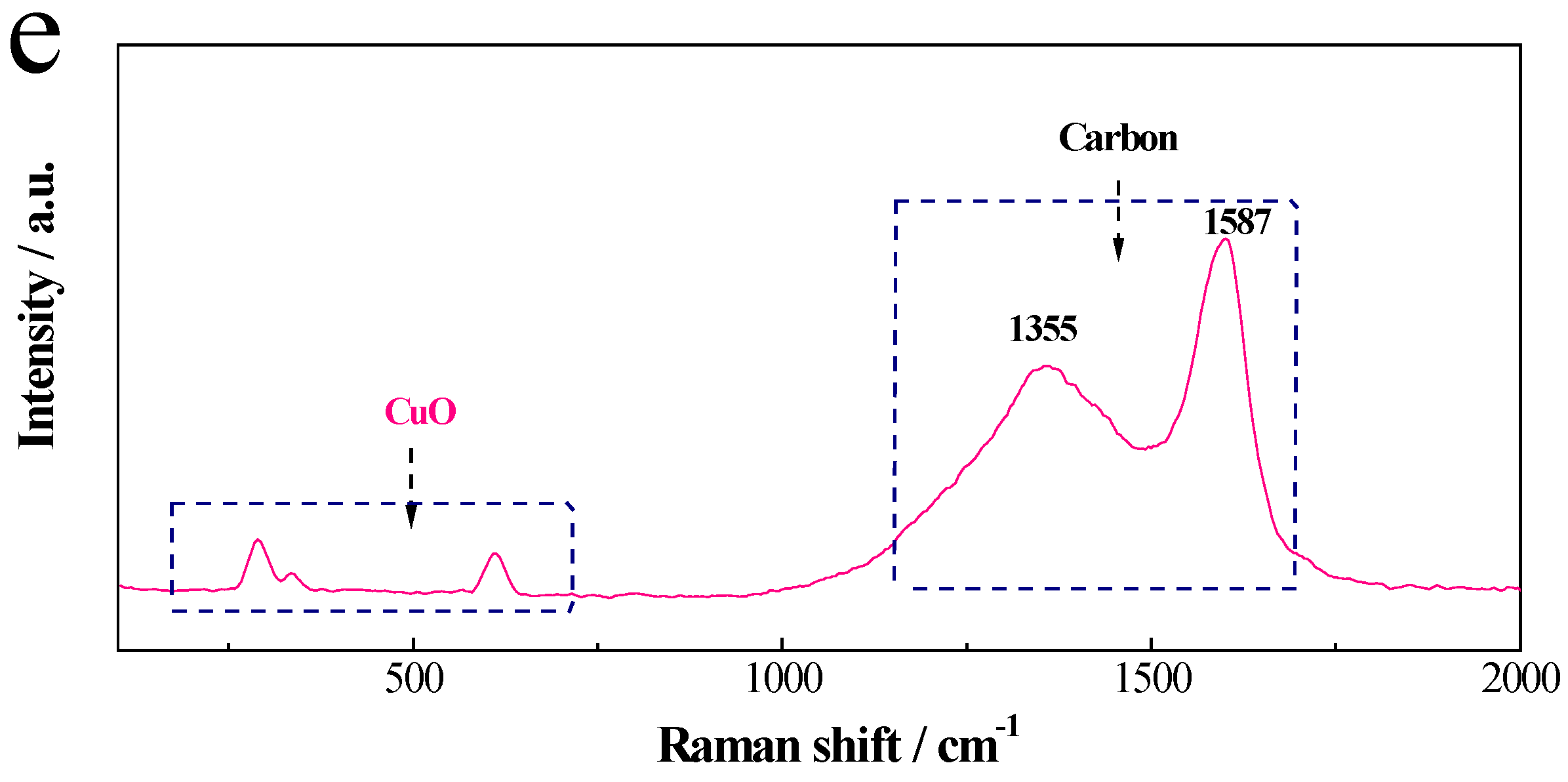
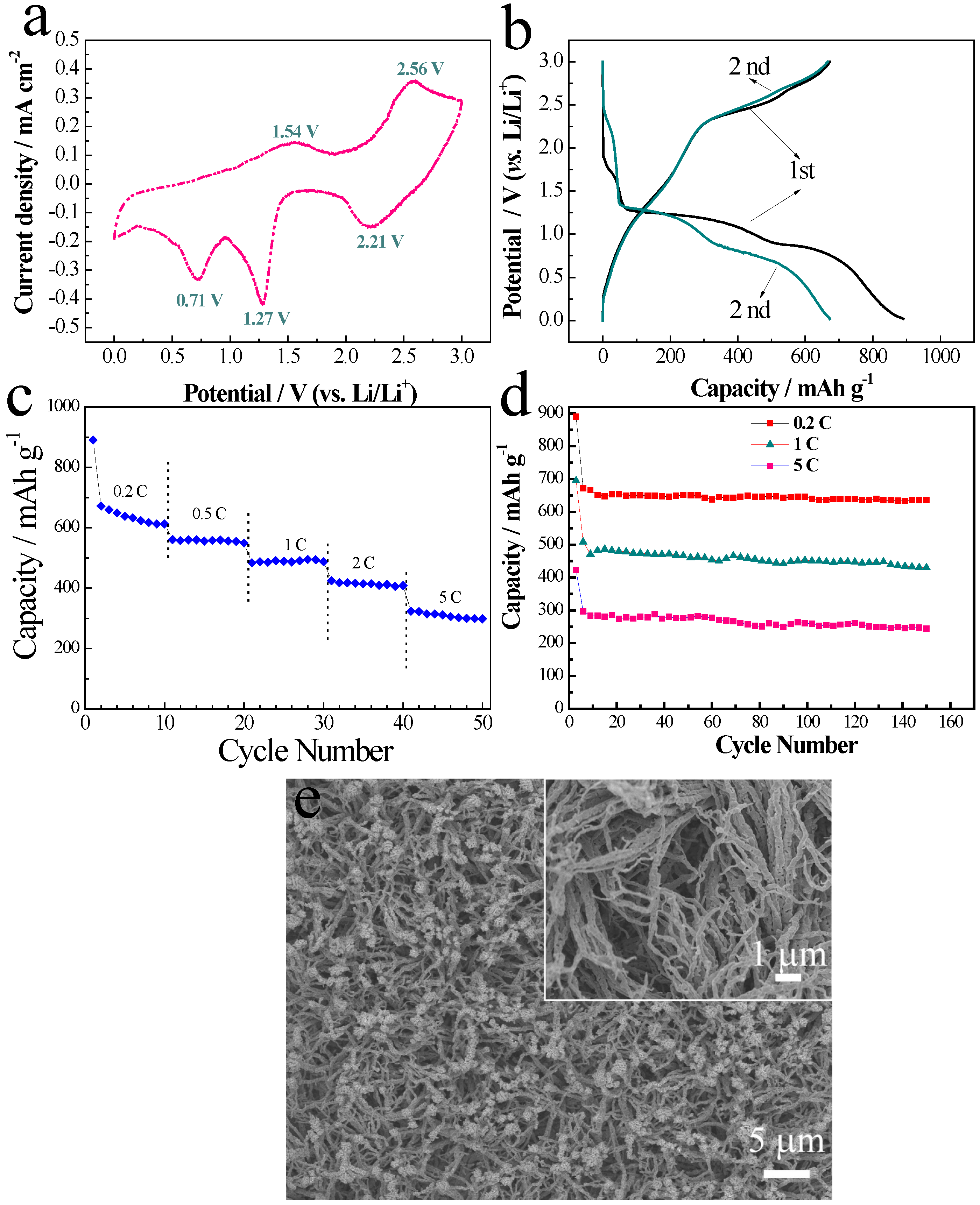
2. Results and Discussion
3. Experimental Section
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chae, B.M.; Oh, E.S.; Lee, Y.K. Conversion mechanisms of cobalt oxide anode for Li-ion battery: In situ X-ray absorption fine structure studies. J. Power Sources 2015, 274, 748–754. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Geng, D.; Zhang, Y.; Meng, X.; Li, R.; Sun, X. Superior cycle stability of nitrogen-doped graphene nanosheets as anodes for lithium ion batteries. Electrochem. Commun. 2011, 13, 822–825. [Google Scholar] [CrossRef]
- Magasinski, A.; Dixon, P.; Hertzberg, B.; Kvit, A.; Ayala, J.; Yushin, G. High-performance lithium-ion anodes using a hierarchical bottom-up approach. Nat. Mater. 2010, 9, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Poizot, P.; Laruelle, S.; Grugeon, S.; Dupont, L.; Tarascon, J.M. Nano-sized transition-metaloxides as negative-electrode materials for lithium-ion batteries. Nature 2000, 407, 496–499. [Google Scholar] [PubMed]
- Xiong, Q.Q.; Tu, J.P.; Lu, Y.; Chen, J.; Yu, Y.X.; Qiao, Y.Q.; Wang, X.L.; Gu, C.D. Synthesis of Hierarchical Hollow-Structured Single-Crystalline Magnetite (Fe3O4) Microspheres: The Highly Powerful Storage versus Lithium as an Anode for Lithium Ion Batteries. J. Phys. Chem. C 2012, 116, 6495–6502. [Google Scholar] [CrossRef]
- Wang, X.; Fan, Y.; Susantyoko, R.A.; Xiao, Q.; Sun, L.; He, D.; Zhang, Q. High areal capacity Li ion battery anode based on thick mesoporous Co3O4 nanosheet networks. Nano Energy 2014, 5, 91–96. [Google Scholar] [CrossRef]
- Wang, X.; Qiao, L.; Sun, X.; Li, X.; Hu, D.; Zhang, Q.; He, D. Mesoporous NiO nanosheet networks as high performance anodes for Li ion batteries. J. Mater. Chem. A 2013, 1, 4173–4176. [Google Scholar] [CrossRef]
- Wang, X.; Sun, L.; Hu, X.; Susantyoko, R.A.; Zhang, Q. Ni–Si nanosheet network as high performance anode for Li ion batteries. J. Power Sources 2015, 280, 393–396. [Google Scholar] [CrossRef]
- Guan, X.; Nai, J.W.; Zhang, Y.P.; Wang, P.X.; Yang, J.; Zheng, L.R.; Zhang, J.; Guo, L. CoO Hollow Cube/Reduced Graphene Oxide Composites with Enhanced Lithium Storage Capability. Chem. Mater. 2014, 26, 5958–5964. [Google Scholar] [CrossRef]
- Xia, X.; Chao, D.; Fan, Z.; Guan, C.; Cao, X.; Zhang, H.; Fan, H.J. A New Type of Porous Graphite Foams and Their Integrated Composites with Oxide/Polymer Core/Shell Nanowires for Supercapacitors: Structural Design, Fabrication, and Full Supercapacitor Demonstrations. Nano Lett. 2014, 14, 1651–1658. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Zhang, Y.; Chao, D.; Guan, C.; Zhang, Y.; Li, L.; Ge, X.; Bacho, I.M.; Tu, J.; Fan, H.J. Solution synthesis of metal oxides for electrochemical energy storage applications. Nanoscale 2014, 6, 5008–5048. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.H.; Tu, J.P.; Zhang, Y.Q.; Wang, X.L.; Gu, C.D.; Zhao, X.B.; Fan, H.J. High-Quality Metal Oxide Core/Shell Nanowire Arrays on Conductive Substrates for Electrochemical Energy Storage. ACS Nano 2012, 6, 5531–5538. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.Q.; Lu, Y.; Wang, X.L.; Gu, C.D.; Qiao, Y.Q.; Tu, J.P. Improved electrochemical performance of porous Fe3O4/carbon core/shell nanorods as an anode for lithium-ion batteries. J. Alloy. Compd. 2012, 536, 219–225. [Google Scholar] [CrossRef]
- Xiong, Q.Q.; Tu, J.P.; Lu, Y.; Chen, J.; Yu, Y.X.; Wang, X.L.; Gu, C.D. Three-dimensional porous nano-Ni/Fe3O4 composite film: Enhanced electrochemical performance for lithium-ion batteries. J. Mater. Chem. 2012, 22, 18639–18645. [Google Scholar] [CrossRef]
- Su, P.P.; Liao, S.C.; Rong, F.; Wang, F.Q.; Chen, J.; Li, C.; Yang, Q.H. Enhanced lithium storage capacity of Co3O4 hexagonal nanorings derived from Co-based metal organic frameworks. J. Mater. Chem. A 2014, 2, 17408–17414. [Google Scholar] [CrossRef]
- Li, X.; Meng, X.; Liu, J.; Geng, D.; Zhang, Y.; Banis, M.N.; Li, Y.; Yang, J.; Li, R.; Sun, X.; et al. Tin Oxide with Controlled Morphology and Crystallinity by Atomic Layer Deposition onto Graphene Nanosheets for Enhanced Lithium Storage. Adv. Funct. Mater. 2012, 22, 1647–1654. [Google Scholar] [CrossRef]
- Ko, S.; Lee, J.I.; Yang, H.S.; Park, S.; Jeong, U. Mesoporous CuO Particles Threaded with CNTs for High-Performance Lithium-Ion Battery Anodes. Adv. Mater. 2012, 24, 4451–4456. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Li, C.Z.; Sun, T.; Ma, J. High-performance supercapacitor material based on Ni(OH)(2) nanowire-MnO2 nanoflakes core-shell nanostructures. Chem. Commun. 2012, 48, 2606–2608. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Liu, J.; Guo, H.; Tong, X. Synthesis of CuO nanowire arrays as high-performance electrode for lithium ion batteries. Mater. Lett. 2015, 139, 55–58. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, P.; Chao, D.; Wang, X.; Zhang, X.; Chen, S.; Tay, B.K.; Huang, H.; Zhang, H.; Mai, W.; et al. All Metal Nitrides Solid-State Asymmetric Supercapacitors. Adv. Mater. 2015, 27, 4566–4571. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.B.; Wang, D.S.; Li, F.; Liu, D.Q.; Wang, P.; Li, X.W.; Yue, H.W.; Peng, S.L.; He, D.Y. Facile synthesis of CuO nanorod for lithium storage application. Mater. Lett. 2013, 90, 4–7. [Google Scholar] [CrossRef]
- Xiang, J.Y.; Tu, J.P.; Zhang, L.; Zhou, Y.; Wang, X.L.; Shi, S.J. Self-assembled synthesis of hierarchical nanostructured CuO with various morphologies and their application as anodes for lithium ion batteries. J. Power Sources 2010, 195, 313–319. [Google Scholar] [CrossRef]
- Liang, J.; Hu, H.; Park, H.; Xiao, C.; Ding, S.; Paik, U.; Lou, X.W. Construction of hybrid bowl-like structures by anchoring NiO nanosheets on flat carbon hollow particles with enhanced lithium storage properties. Energy Environ. Sci. 2015, 8, 1707–1711. [Google Scholar] [CrossRef]
- Kumar, P.R.; Jung, Y.H.; Bharathi, K.K.; Lim, C.H.; Kim, D.K. High capacity and low cost spinel Fe3O4 for the Na-ion battery negative electrode materials. Electrochim. Acta 2014, 146, 503–510. [Google Scholar] [CrossRef]
- Chen, J.S.; Li, C.M.; Zhou, W.W.; Yan, Q.Y.; Archer, L.A.; Lou, X.W. One-pot formation of SnO2 hollow nanospheres and alpha-Fe2O3@SnO2 nanorattles with large void space and their lithium storage properties. Nanoscale 2009, 1, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Liu, J.; Chao, D.; Wang, J.; Yin, J.; Lin, J.; Fan, H.; Shen, Z. Porous α-Fe2O3 nanorods supported on carbon nanotubes-graphene foam as superior anode for lithium ion batteries. Nano Energy 2014, 9, 364–372. [Google Scholar] [CrossRef]
- Cabana, J.; Monconduit, L.; Larcher, D.; Palacín, M.R. Beyond Intercalation-Based Li-Ion Batteries: The State of the Art and Challenges of Electrode Materials Reacting Through Conversion Reactions. Adv. Mater. 2010, 22, E170–E192. [Google Scholar] [CrossRef] [PubMed]
- Ryu, W.-H.; Shin, J.; Jung, J.-W.; Kim, I.-D. Cobalt(II) monoxide nanoparticles embedded in porous carbon nanofibers as a highly reversible conversion reaction anode for Li-ion batteries. J. Mater. Chem. A 2013, 1, 3239–3243. [Google Scholar] [CrossRef]
- Hu, Y.-Y.; Liu, Z.; Nam, K.-W.; Borkiewicz, O.J.; Cheng, J.; Hua, X.; Dunstan, M.T.; Yu, X.; Wiaderek, K.M.; Du, L.-S.; et al. Origin of additional capacities in metal oxide lithium-ion battery electrodes. Nat. Mater. 2013, 12, 1130–1136. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Xia, X.H.; Pan, G.X.; Chen, J.; Zhang, Y.J. Construction of carbon nanoflakes shell on CuO nanowires core as enhanced core/shell arrays anode of lithium ion batteries. Electrochim. Acta 2015, 178, 574–579. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhan, J.; Chen, M.; Xia, X. Controllable Synthesis of Copper Oxide/Carbon Core/Shell Nanowire Arrays and Their Application for Electrochemical Energy Storage. Nanomaterials 2015, 5, 1610-1619. https://doi.org/10.3390/nano5041610
Zhan J, Chen M, Xia X. Controllable Synthesis of Copper Oxide/Carbon Core/Shell Nanowire Arrays and Their Application for Electrochemical Energy Storage. Nanomaterials. 2015; 5(4):1610-1619. https://doi.org/10.3390/nano5041610
Chicago/Turabian StyleZhan, Jiye, Minghua Chen, and Xinhui Xia. 2015. "Controllable Synthesis of Copper Oxide/Carbon Core/Shell Nanowire Arrays and Their Application for Electrochemical Energy Storage" Nanomaterials 5, no. 4: 1610-1619. https://doi.org/10.3390/nano5041610