Facile Attachment of TAT Peptide on Gold Monolayer Protected Clusters: Synthesis and Characterization
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of 14 nm Citrate-Capped Gold Nanoparticles, AuNPs
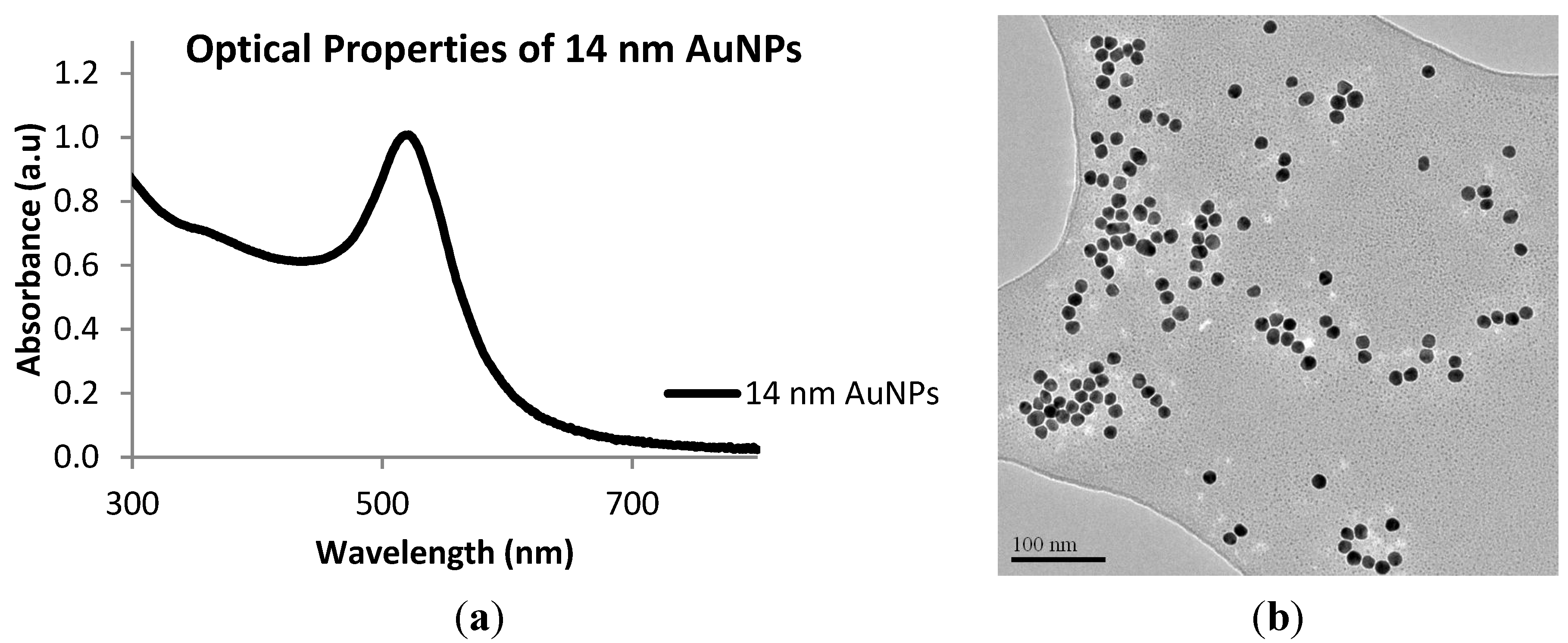
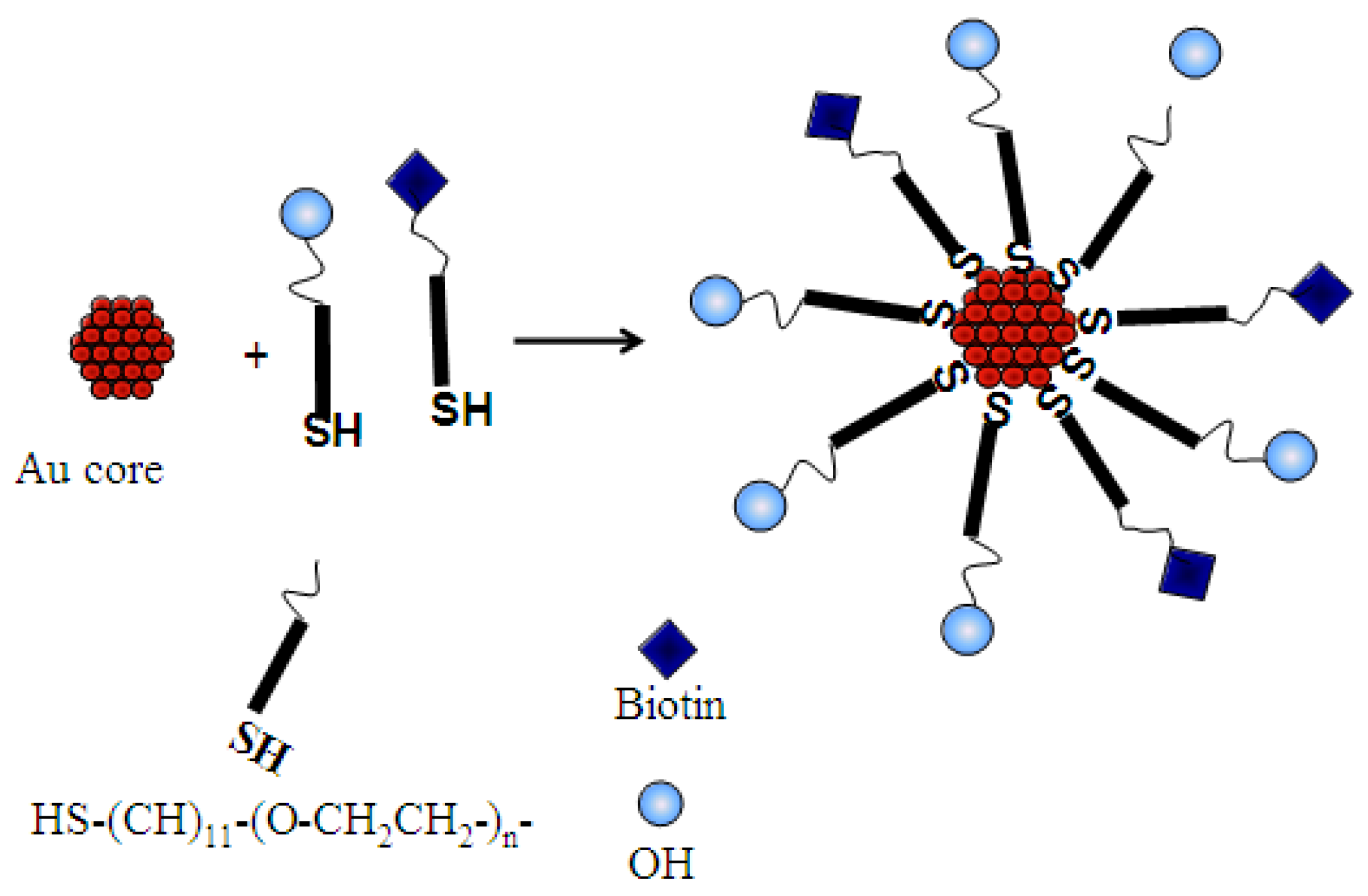
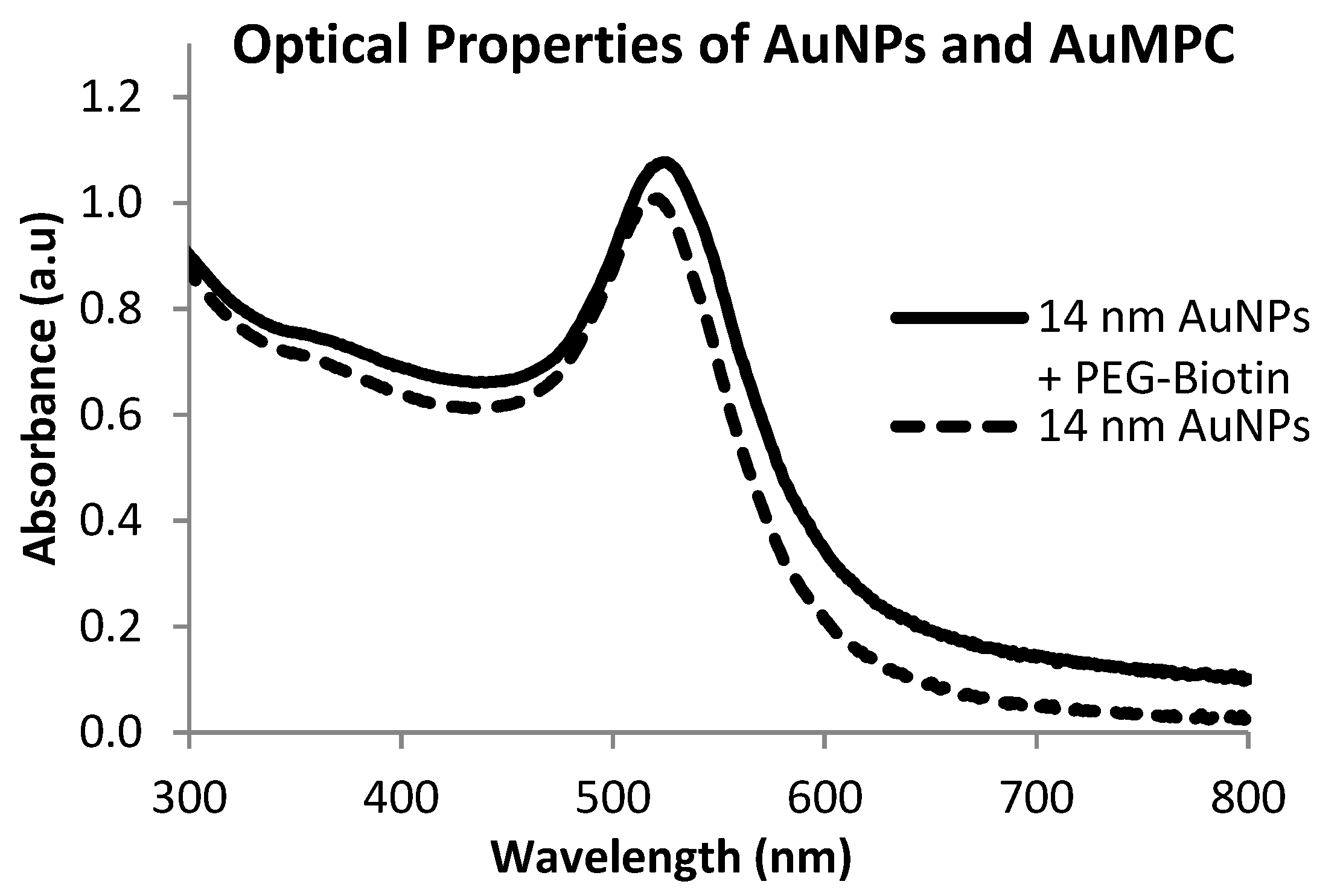
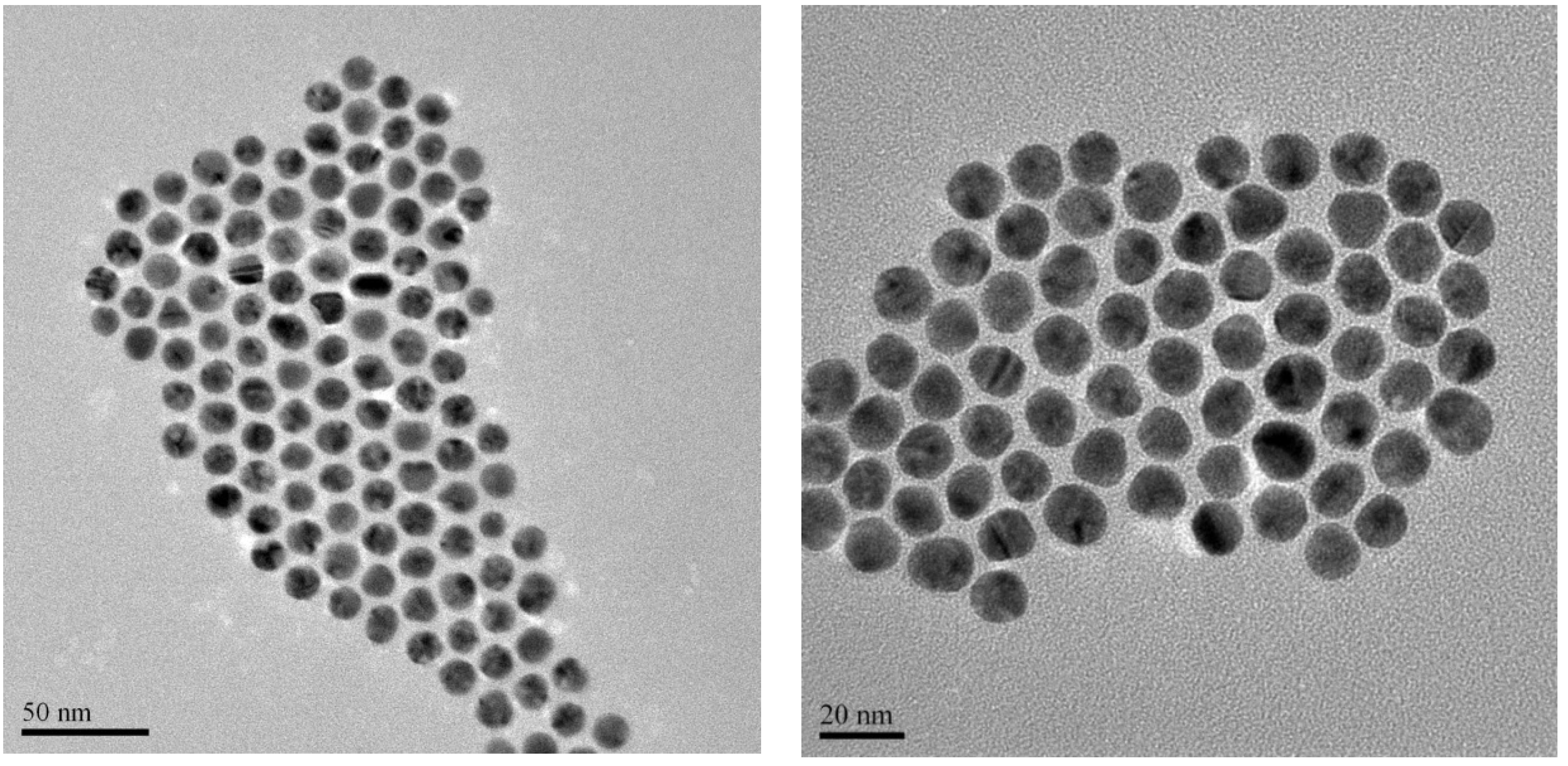
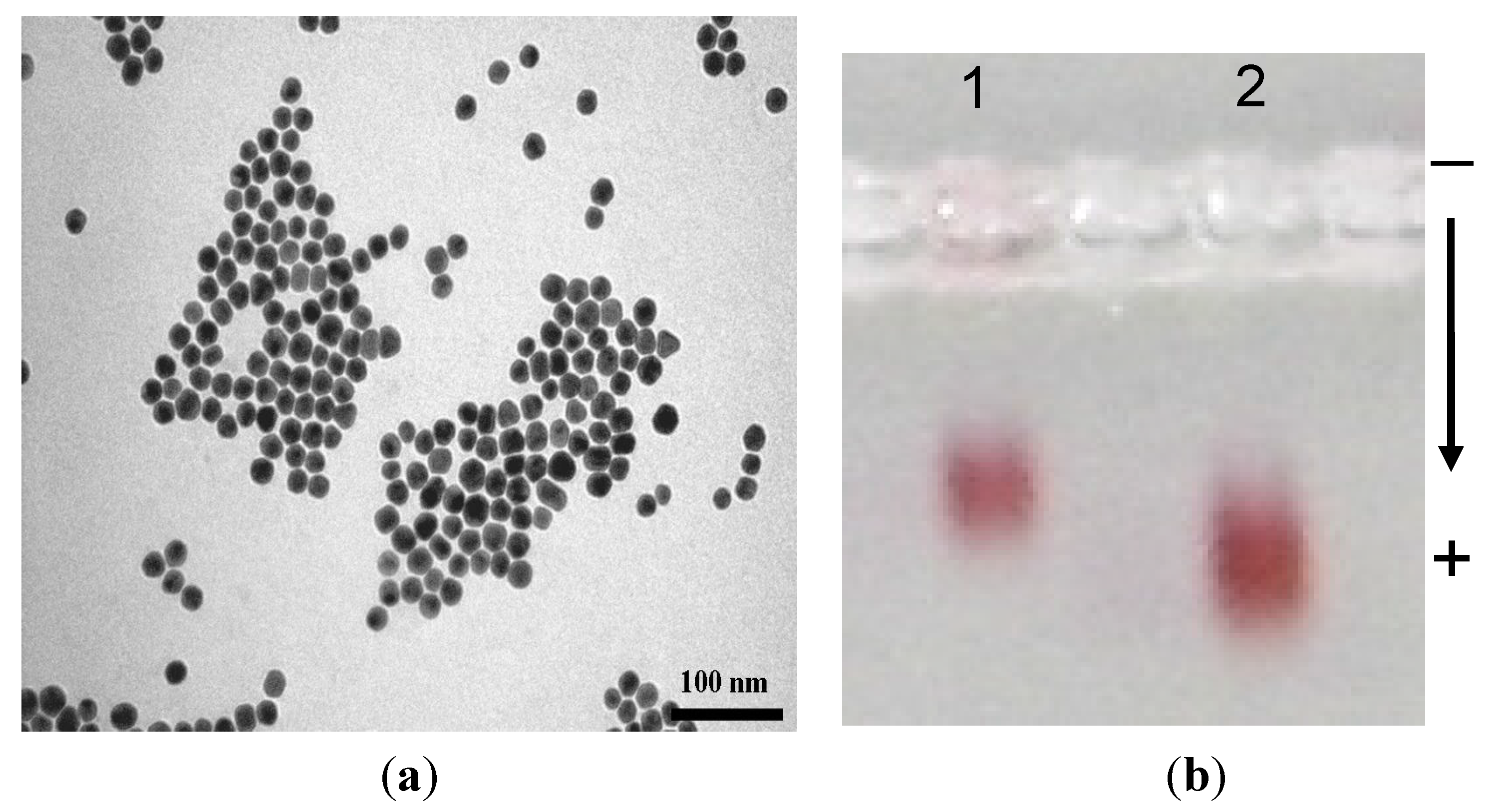
2.2. Biofunctionalization with Streptavidin
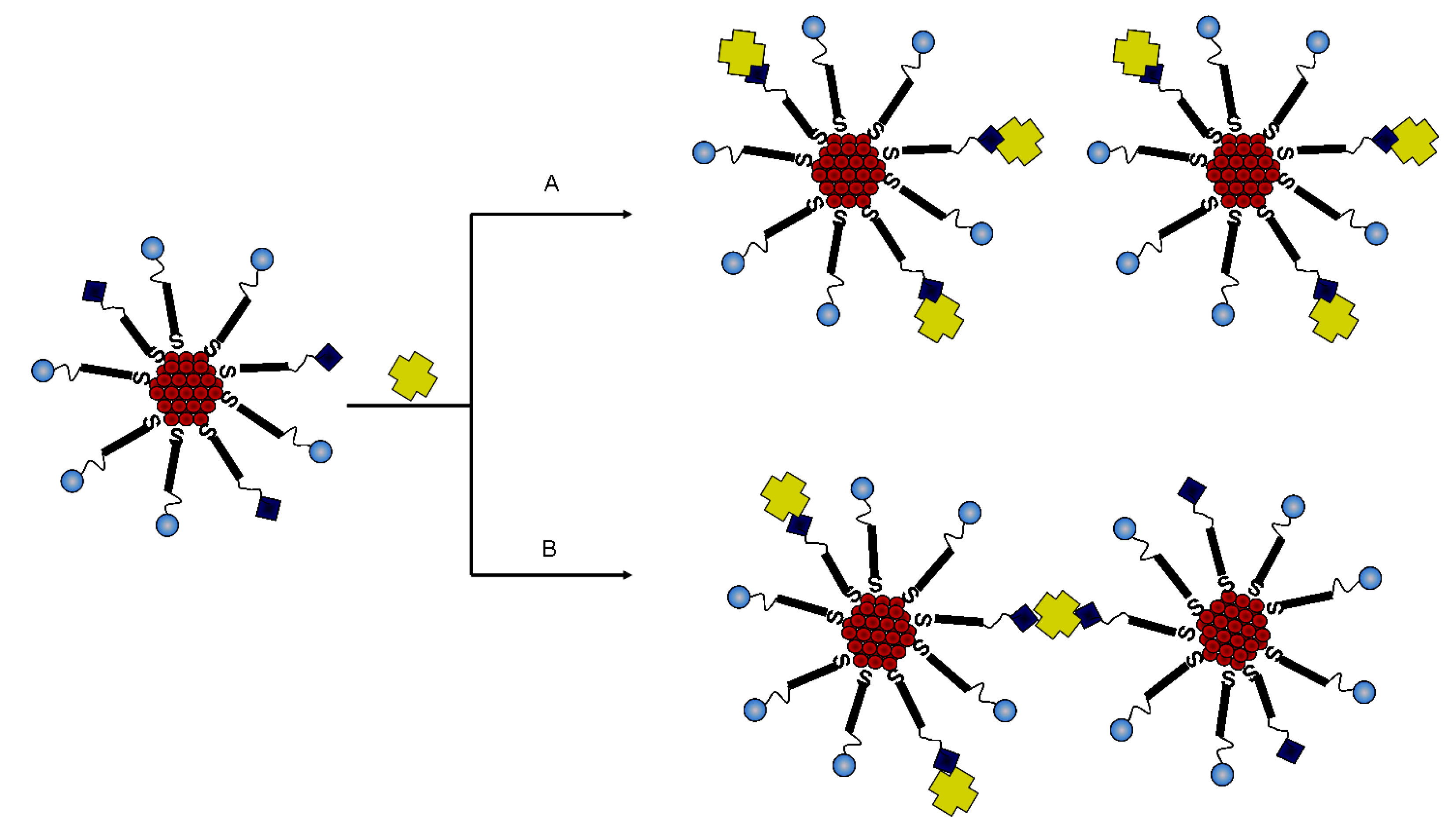
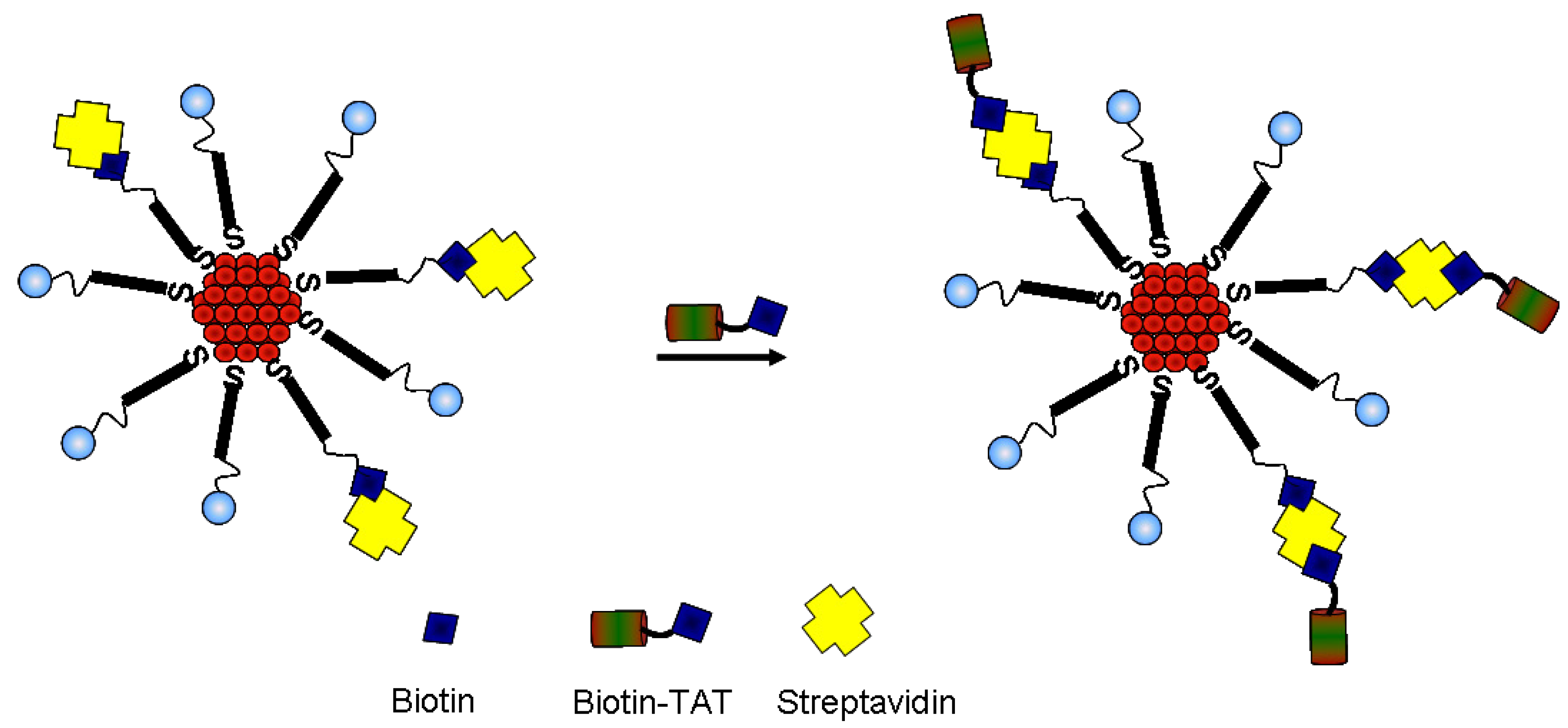
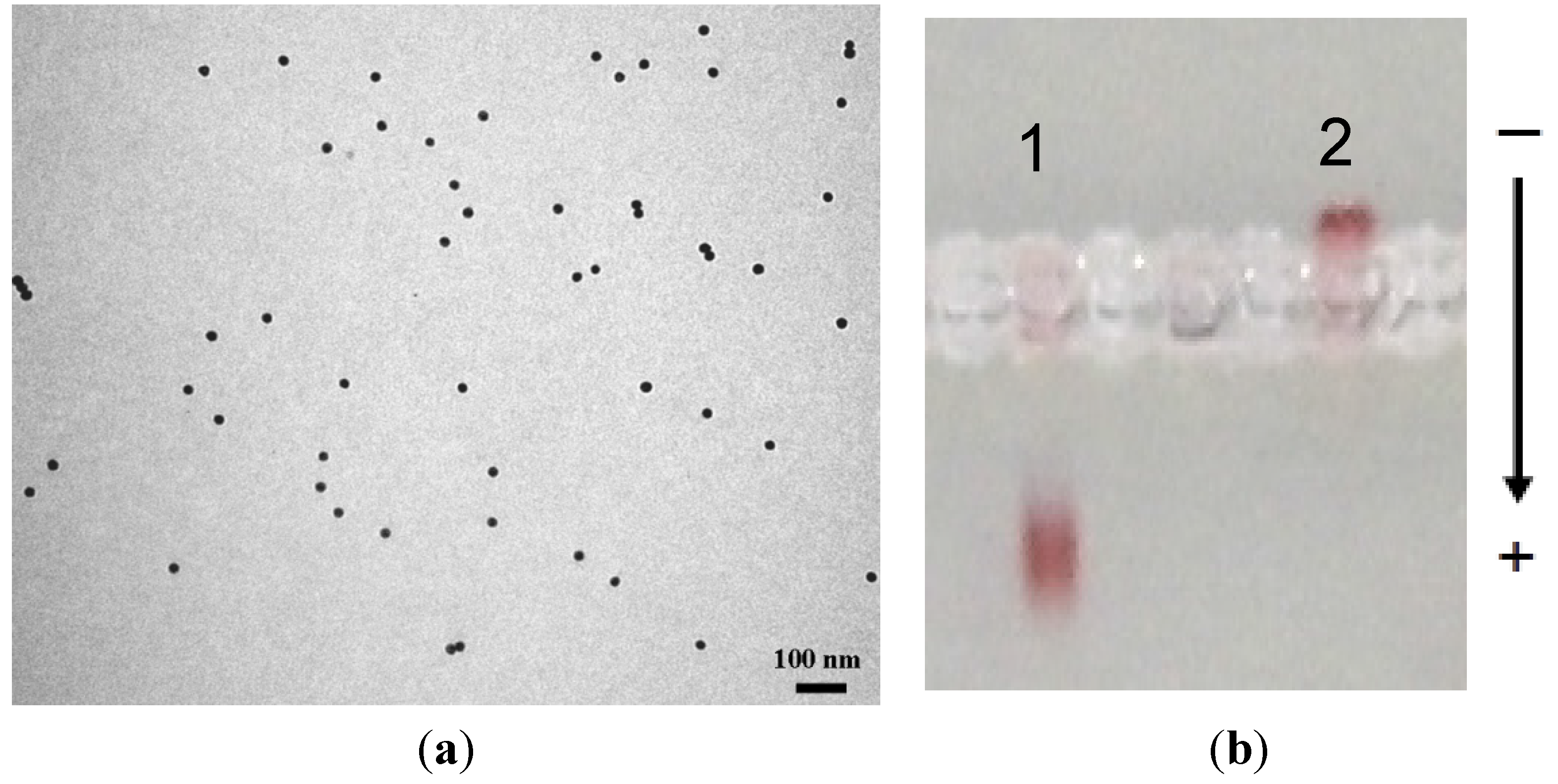
2.3. Immobilization of Biotinylated TAT Peptide
3. Experimental Section
3.1. Materials, Instrumentation and Methods
3.2. Synthesis
3.2.1. Synthesis of Citrate-Capped Gold Nanoparticles, AuNPs
3.2.2. Synthesis of PEG-Biotin Monolayer Protected Clusters, AuMPCs
3.3. Biofunctionalization with Streptavidin
3.4. Biomolecular Immobilization with Biotin-TAT
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dykman, L.; Khlebtsov, N. Gold nanoparticles in biomedical applications: Recent advances and perspectives. Chem. Soc. Rev. 2012, 41, 2256–2282. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Lin, Y. Nanomaterial labels in electrochemical immunosensors and immunoassays. Talanta 2012, 74, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Katz, E.; Willner, I. Integrated nanoparticle-biomolecule hybrid systems: Synthesis, properties, and applications. Angew. Chem. Int. Ed. 2004, 43, 6042–6108. [Google Scholar] [CrossRef] [PubMed]
- Elder, A.; Yang, H.; Gwiazda, R.; Teng, X.; Thurston, S.; He, H.; Oberdorster, G. Testing nanomaterials of unknown toxicity: An example based on platinum nanoparticles of different shapes. Adv. Mater. 2007, 19, 3124–3129. [Google Scholar] [CrossRef]
- Nath, N.; Chilkoti, A. A colorimetric gold nanoparticle sensor to interrogate biomolecular interactions in real time on a surface. Anal. Chem. 2002, 74, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Dykman, L.; Khlebtsov, N.G. Gold nanoparticles in biology and medicine: Recent advances and prospects. Acta Nat. 2011, 3, 34–55. [Google Scholar]
- Wang, J. Electrochemical biosensors: Towards point-of-care cancer diagnostics. Biosens. Bioelectron. 2006, 21, 1887–1892. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wang, J.; Wu, H.; Lin, Y. Nanovehicles based bioassay labels. Electroanalysis 2007, 19, 777–785. [Google Scholar] [CrossRef]
- Han, M.; Gao, X.; Su, J.; Nie, S. Quantum-dot-tagged microbeads for multiplexed optical coding of biomolecules. Nat. Biotechnol. 2001, 19, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wang, J.; Kim, J.; Jan, M.R.; Collins, G.E. Quantum-dot-tagged microbeads for multiplexed optical coding of biomolecules. Anal. Chem. 2004, 76, 7126–7130. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.; Park, T.J.; Kim, H.S.; Kim, J.H.; Cho, Y.J. Directed self-assembly of gold binding polypeptide-protein A fusion proteins for development of gold nanoparticle-based SPR immunosensors. Biosens. Bioelectron. 2009, 24, 2592–25927. [Google Scholar] [CrossRef] [PubMed]
- Brust, M.; Walker, M.; Bethell, D.; Schiffrin, J.; Whyman, R. Synthesis of thiol-derivatised gold nanoparticles in a two-phase Liquid–Liquid system. Chem. Commun. 1994, 801, 801–802. [Google Scholar] [CrossRef]
- Delong, R.K.; Reynolds, C.M.; Malcolm, Y.; Schaeffer, A.; Severs, T.; Wanekaya, A. Functionalized gold nanoparticles for the binding, stabilization, and delivery of therapeutic DNA, RNA, and other biological macromolecules. Nanotechnol. Sci. Appl. 2010, 3, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Goel, R.; Shah, N.; Visaria, R.; Paciotti, G.F.; Bischof, J.C. Biodistribution of TNF-α-coated gold nanoparticles in an in vivo model system. Nanomedicine 2009, 4, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Lees, E.; Amin, F.; Rivera, G.P.; Yang, F.; Mulvaney, P.; Parak, W.J. Polymer-coated nanoparticles: A universal tool for biolabelling experiments. Small 2011, 7, 3113–3127. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Baker, B.R.; Wachsmann-Hogiu, S.; Pagba, C.V.; Laurence, T.A.; Lane, S.M.; Lee, L.P.; Tok, J.B.H. Aptamer-based SERRS sensor for thrombin detection. Nano Lett. 2008, 8, 4386–4390. [Google Scholar] [CrossRef] [PubMed]
- Wuelfing, W.P.; Gross, S.M.; Miles, D.T.; Murray, R.W. Nanometer gold clusters protected by surface-bound monolayers of thiolated poly(ethylene glycol) polymer electrolyte. J. Am. Chem. Soc. 1998, 120, 12696–12697. [Google Scholar] [CrossRef]
- Tiwari, P.M.; Vig, K.; Dennis, V.A.; Singh, S.R. Functionalized gold nanoparticles and their biomedical applications. Nanomaterials 2011, 1, 31–63. [Google Scholar] [CrossRef]
- Ferrari, M. Cancer nanotechnology: Opportunities and challenges. Nat. Rev. Cancer 2005, 5, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Caswell, K.K.; Wilson, J.N.; Bunz, U.H.F.; Murphy, C.J. Preferential end-to-end assembly of gold nanorods by biotin-streptavidin connectors. J. Am. Chem. Soc. 2003, 125, 13914–13915. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.; Argarana, C.E.; Fidelio, G.D. Extremely high thermal stability of streptavidin and avidin upon biotin binding. Biomol. Eng. 1999, 16, 67–72. [Google Scholar] [CrossRef]
- Bruchez, M., Jr.; Morone, M.; Gin, P.; Weiss, S.; Alivisatos, A.P. Semiconductor Nanocrystals as Fluorescent Biological Labels. Science 1998, 281, 2013–2016. [Google Scholar] [CrossRef] [PubMed]
- Niemeyer, C.M. Nanoparticles, Proteins, and Nucleic Acids: Biotechnology meets materials science. Angew. Chem. Int. Ed. 2001, 40, 4128–4158. [Google Scholar] [CrossRef]
- Zanchet, D.; Micheel, C.M.; Parak, W.J.; Gerion, D.; Alivisatos, A.P. Electrophoretic isolation of discrete Au nanocrystal/DNA conjugates. Nano Lett. 2001, 1, 32–35. [Google Scholar] [CrossRef]
- Brooks, H.; Lebleu, B.; Vives, E. TAT peptide-mediated cellular delivery: Back to basics. Adv. Drug Deliver. Rev. 2005, 57, 559–577. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Pei, W.; Ge, H.; Liang, Q.; Luo, Y.; Sharp, F.R.; Lu, A.; Ran, R.; Graham, S.H.; Chen, J. In vivo delivery of a Bcl-xL fusion protein containing the TAT Protein transduction domain protects against ischemic brain injury and neuronal apoptosis. J. Neurosci. 2002, 22, 5423–5431. [Google Scholar] [PubMed]
- Kaplan, I.M.; Wadia, J.S.; Dowdy, S.F. Cationic TAT peptide transduction domain enters cells by macropinocytosis. J. Control. Release 2005, 102, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Schwarze, S.R.; Ho, A.; Vocero-Akbani, A.; Dowdy, S.F. In vivo protein transduction: Delivery of a biologically active protein into the mouse. Science 1999, 285, 1569–1572. [Google Scholar] [CrossRef] [PubMed]
- Wadia, J.S.; Stan, R.V.; Dowdy, S.F. Transducible TAT-HA fusogenic peptide enhances escape of TAT-fusion proteins after lipid raft micropinocytosis. Nat. Med. 2004, 10, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Conner, S.D.; Schmid, S.L. Regulated portals of entry into the cell. Nature 2003, 422, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Sosibo, N.M.; Tshikhudo, R.T.; Revaprasadu, N. Stable hydrophilic nitrilotriacetic capped gold monolayer protected clusters. MRS Proc. 2008, 1064. [Google Scholar] [CrossRef]
- Tshikhudo, T.R.; Wang, Z.; Brust, M. Biocompatible gold nanoparticles. Mater. Sci. Technol. 2004, 20, 980–984. [Google Scholar] [CrossRef]
- Kanaras, A.G.; Kamounah, F.S.; Schaumburg, K.; Kiely, C.J.; Brust, M. Thioalkylated tetraethylene glycol: A new ligand for water soluble monolayer protected gold clusters. Chem. Commun. 2002, 20, 2294–2295. [Google Scholar] [CrossRef]
- De la Fuente, J.M.; Fandel, M.; Berry, C.C.; Riehle, M.; Cronin, L.; Aitchison, G.; Curtis, A.S.G. Quantum Dots Protected with Tiopronin: A new fluorescence system for cell-biology studies. Chem. Bio. Chem. 2005, 6, 989–991. [Google Scholar] [CrossRef] [PubMed]
- Turkevitch, J.; Stevenson, P.C.; Hillie, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Frens, G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nat. Phys. Sci. 1973, 241, 20–22. [Google Scholar] [CrossRef]
- Shalkevich, N.; Shalkevich, A.; Ahmed, L.S.; Burgi, T. Reversible formation of gold nanoparticle-surfactant composite assemblies for the preparation of concentrated colloidal solutions. Phys. Chem. Chem. Phys. 2009, 11, 10175–10179. [Google Scholar] [CrossRef] [PubMed]
- Savage, D.; Matson, G.; Desai, S.; Nielander, G.; Morgensen, S.; Conklin, E.E. Avidin-Biotin Chemistry: A Handbook; Pierce Chemical Co.: Rockford, IL, USA, 1992. [Google Scholar]
- Green, N.M. Avidin and streptavidin. Methods Enzymol. 1990, 184, 51–67. [Google Scholar] [PubMed]
- Henrickson, W.A.; Pahler, A.; Smith, J.L.; Satow, Y.; Merritt, E.A.; Phizackerley, R.P. Crystal structure of core streptavidin determined from multiwavelength anomalous diffraction of synchrotron radiation. Proc. Natl. Acad. Sci. USA. 1989, 86, 2190–2194. [Google Scholar] [CrossRef]
- Manson, J.; Kumar, D.; Meenan, B.J.; Dixon, D. Polyethylene glycol functionalized gold nanoparticles: The influence of capping density on stability in various media. Gold Bull. 2011, 44, 99–105. [Google Scholar] [CrossRef]
- Elzey, S.; Tsai, D.-H.; Rabb, S.A.; Yu, L.L.; Winchester, M.R.; Hackley, V.A. Quantification of ligand packing density on gold nanoparticles using ICP_OES. Anal. Bioanal. Chem. 2012, 403, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Hinterwirth, H.; Kappel, S.; Waitz, T.; Prohaska, T.; Lindner, W.; Lämmerhofer, M. Quantifying thiol ligand density of self-assembled monolayers on gold nanoparticles by inductively coupled plasma_mass spectrometry. ACS Nano 2013, 7, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sosibo, N.M.; Keter, F.K.; Skepu, A.; Tshikhudo, R.T.; Revaprasadu, N. Facile Attachment of TAT Peptide on Gold Monolayer Protected Clusters: Synthesis and Characterization. Nanomaterials 2015, 5, 1211-1222. https://doi.org/10.3390/nano5031211
Sosibo NM, Keter FK, Skepu A, Tshikhudo RT, Revaprasadu N. Facile Attachment of TAT Peptide on Gold Monolayer Protected Clusters: Synthesis and Characterization. Nanomaterials. 2015; 5(3):1211-1222. https://doi.org/10.3390/nano5031211
Chicago/Turabian StyleSosibo, Ndabenhle M., Frankline K. Keter, Amanda Skepu, Robert T. Tshikhudo, and Neerish Revaprasadu. 2015. "Facile Attachment of TAT Peptide on Gold Monolayer Protected Clusters: Synthesis and Characterization" Nanomaterials 5, no. 3: 1211-1222. https://doi.org/10.3390/nano5031211




