Liquid-State and Solid-State Properties of Nanotube/Polypropylene Nanocomposites Elaborated via a Simple Procedure
Abstract
:1. Introduction
2. Results and Discussion
2.1. Thermal Properties
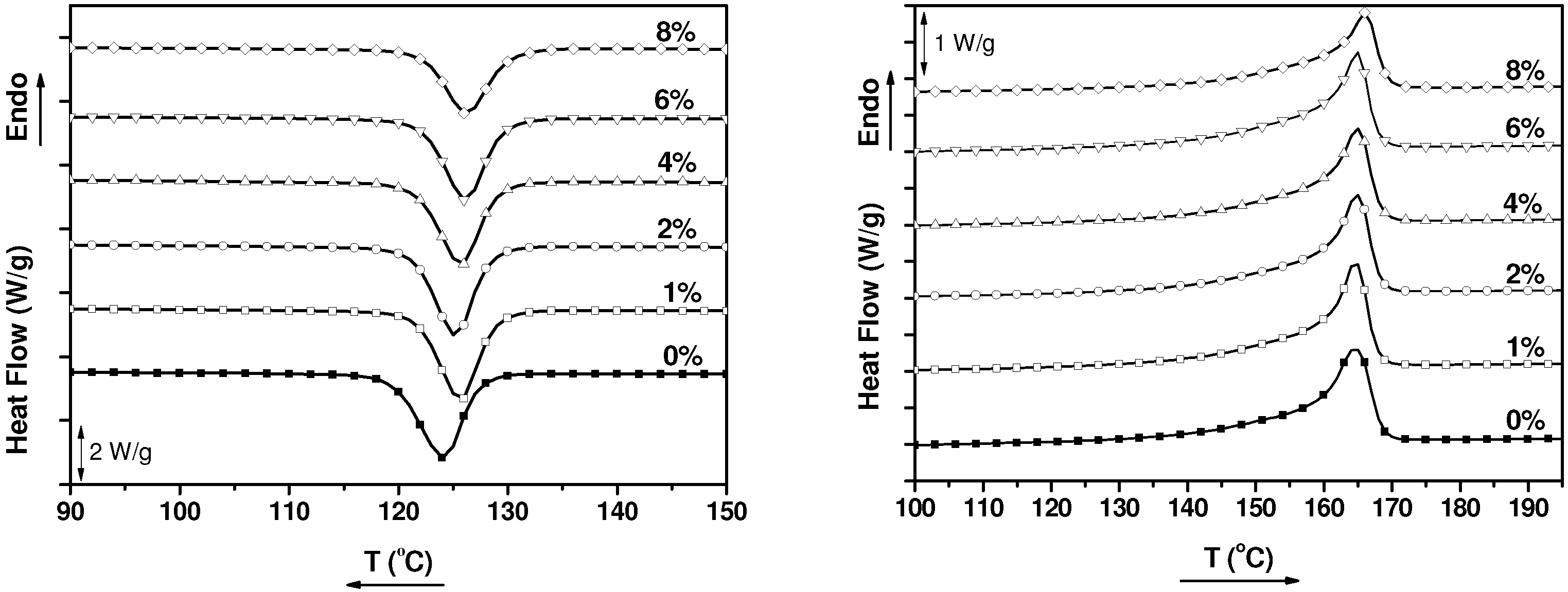
| Sample | Tc (°C) | Tm (°C) | Xc (%) |
|---|---|---|---|
| PP | 124.2 | 164.5 | 38 |
| PP1%MWCNT | 125.6 | 164.6 | 39 |
| PP2%MWCNT | 125.2 | 164.9 | 40 |
| PP4%MWCNT | 125.8 | 164.9 | 38 |
| PP6%MWCNT | 126.2 | 164.9 | 40 |
| PP8%MWCNT | 126.5 | 165.9 | 34 |

2.2. Analysis of the Molten State: Oscillatory Flow and PVT Measurements

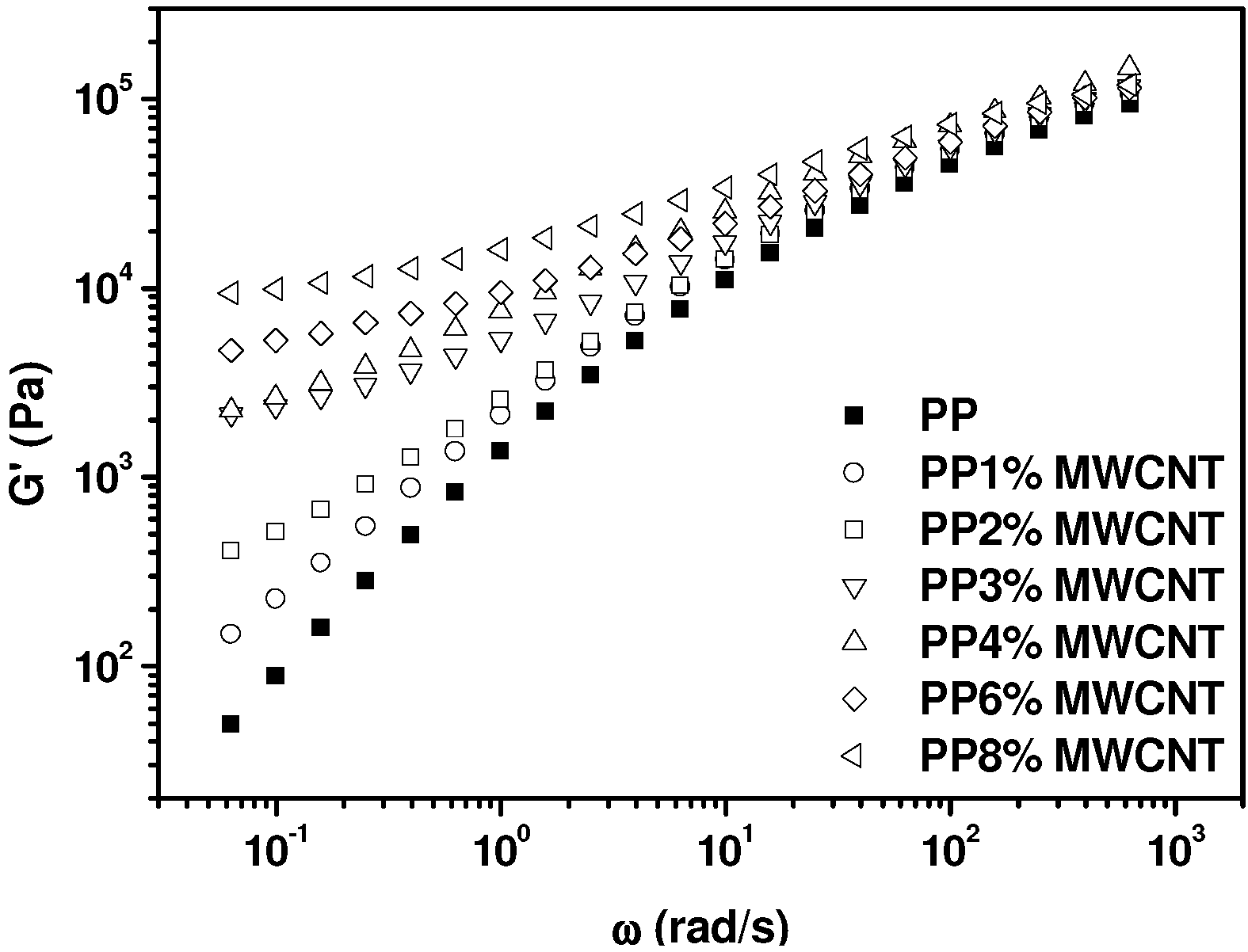
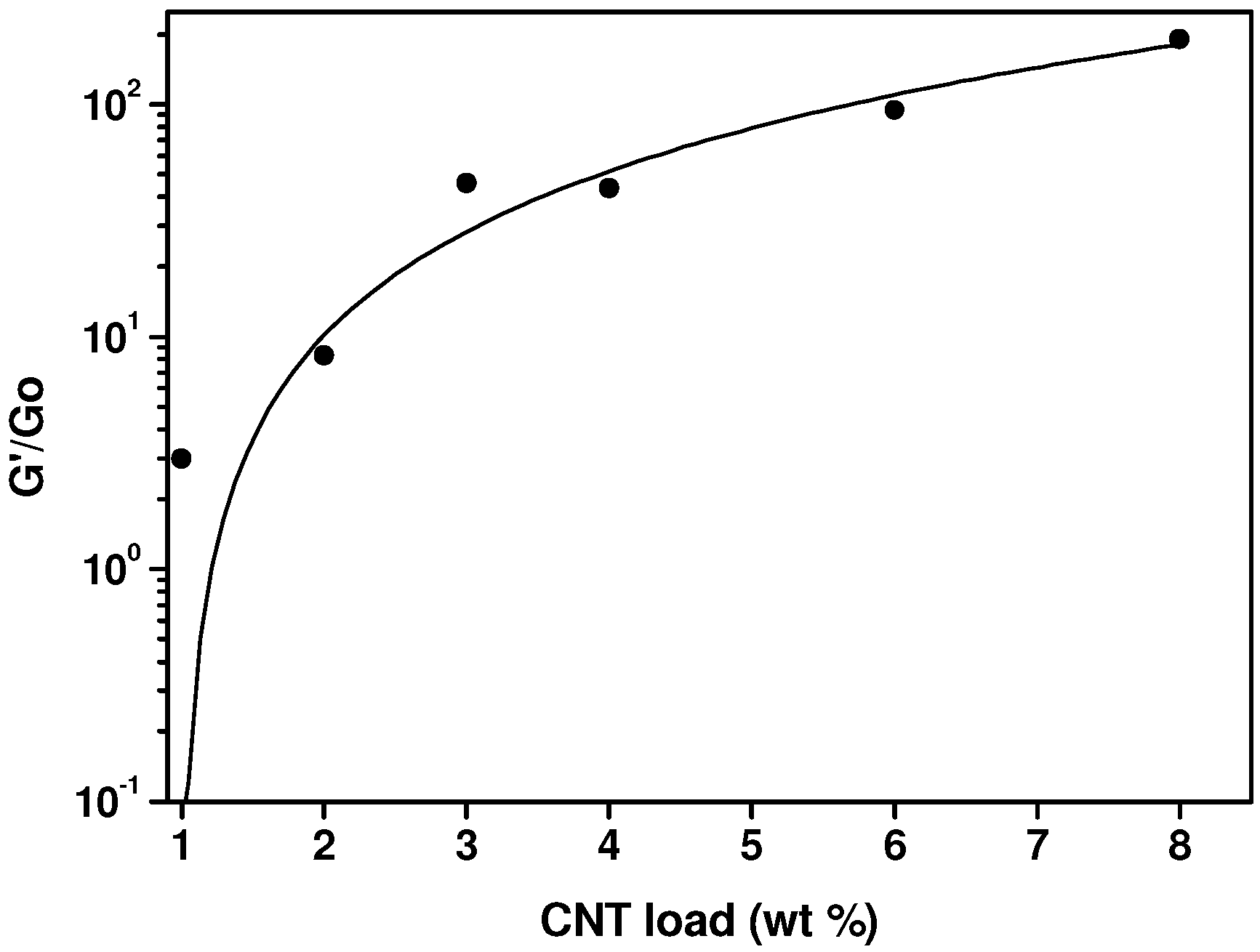
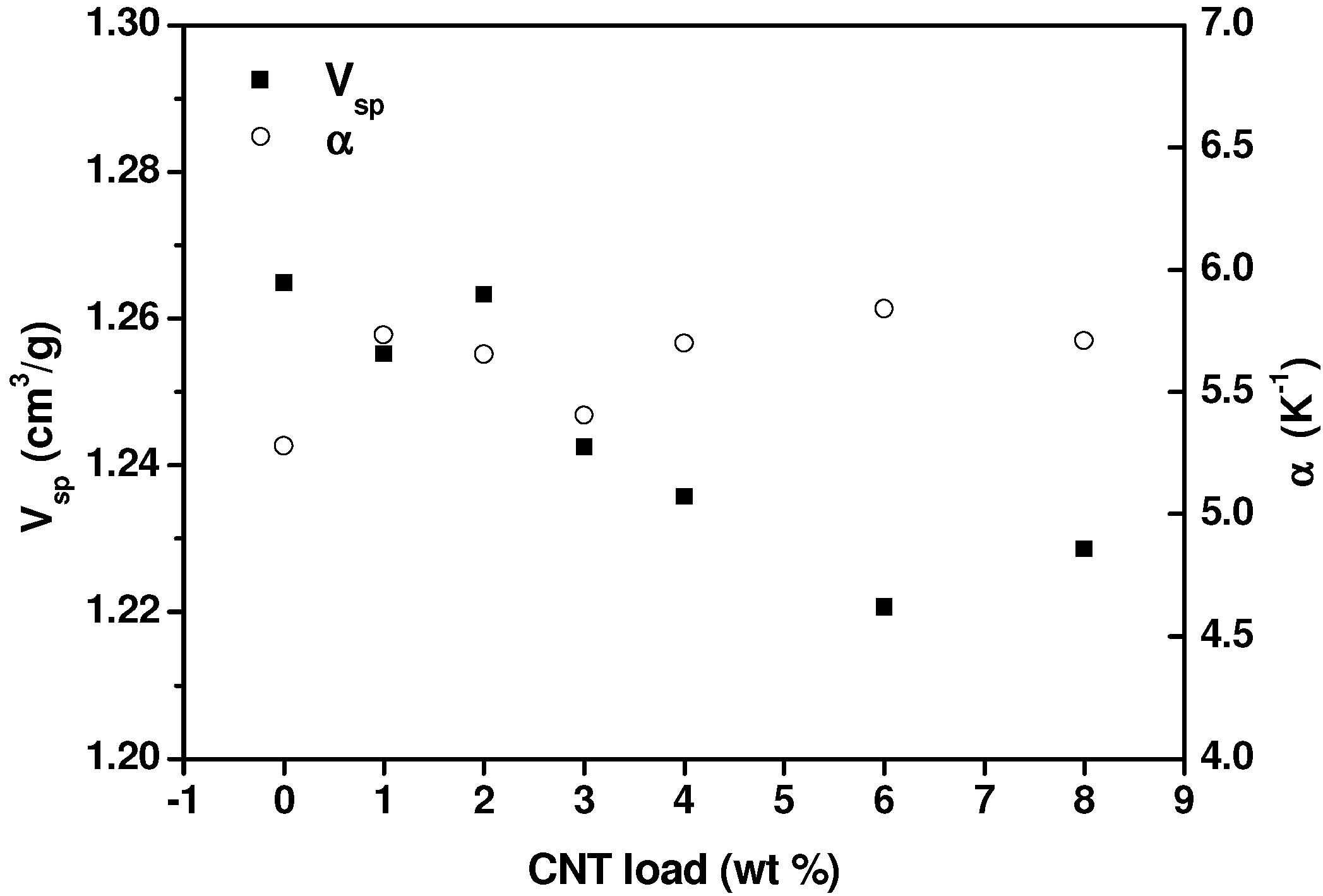
2.3. Electrical Conductivity Measurements in the Molten State, during Crystallization and in the Solid State

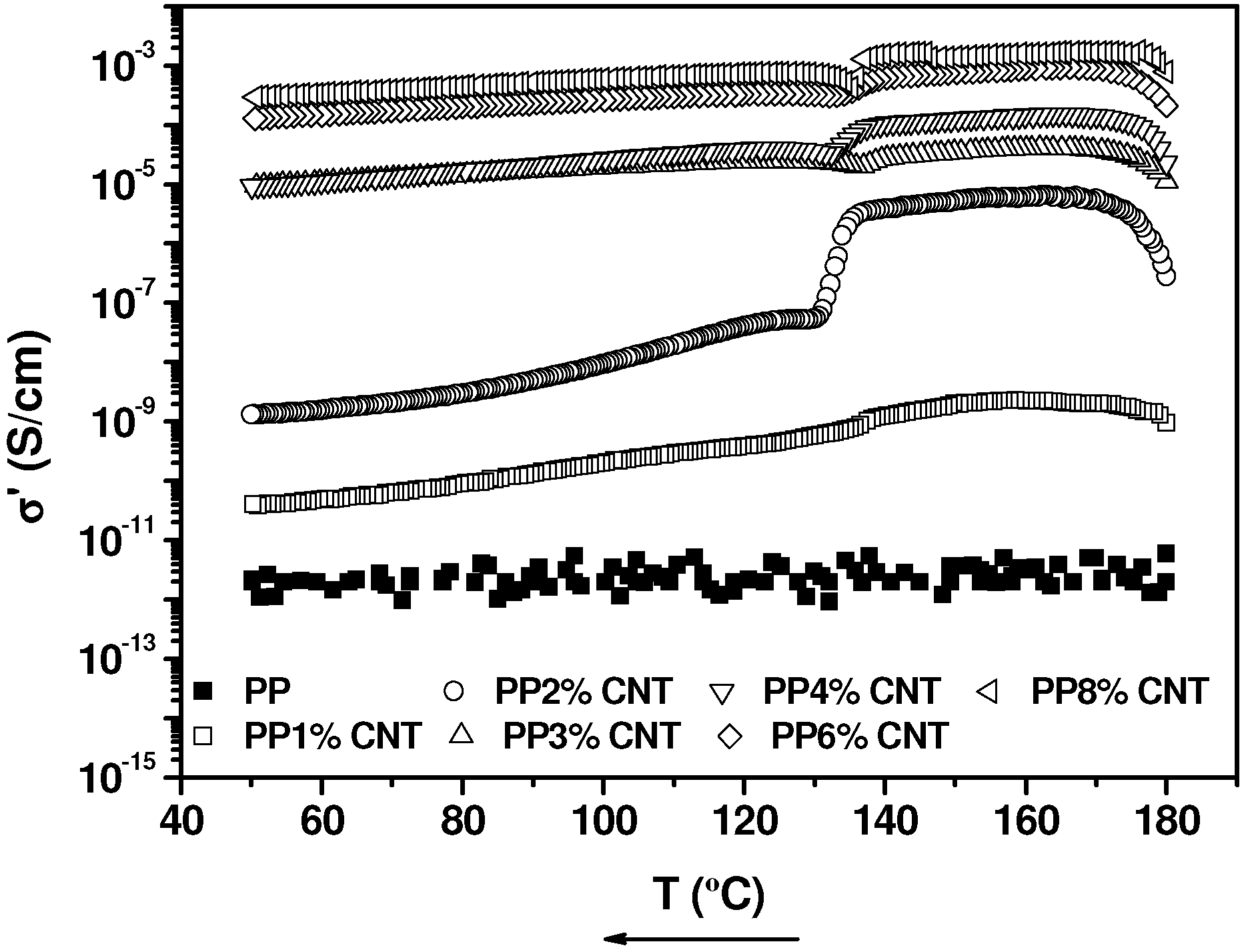
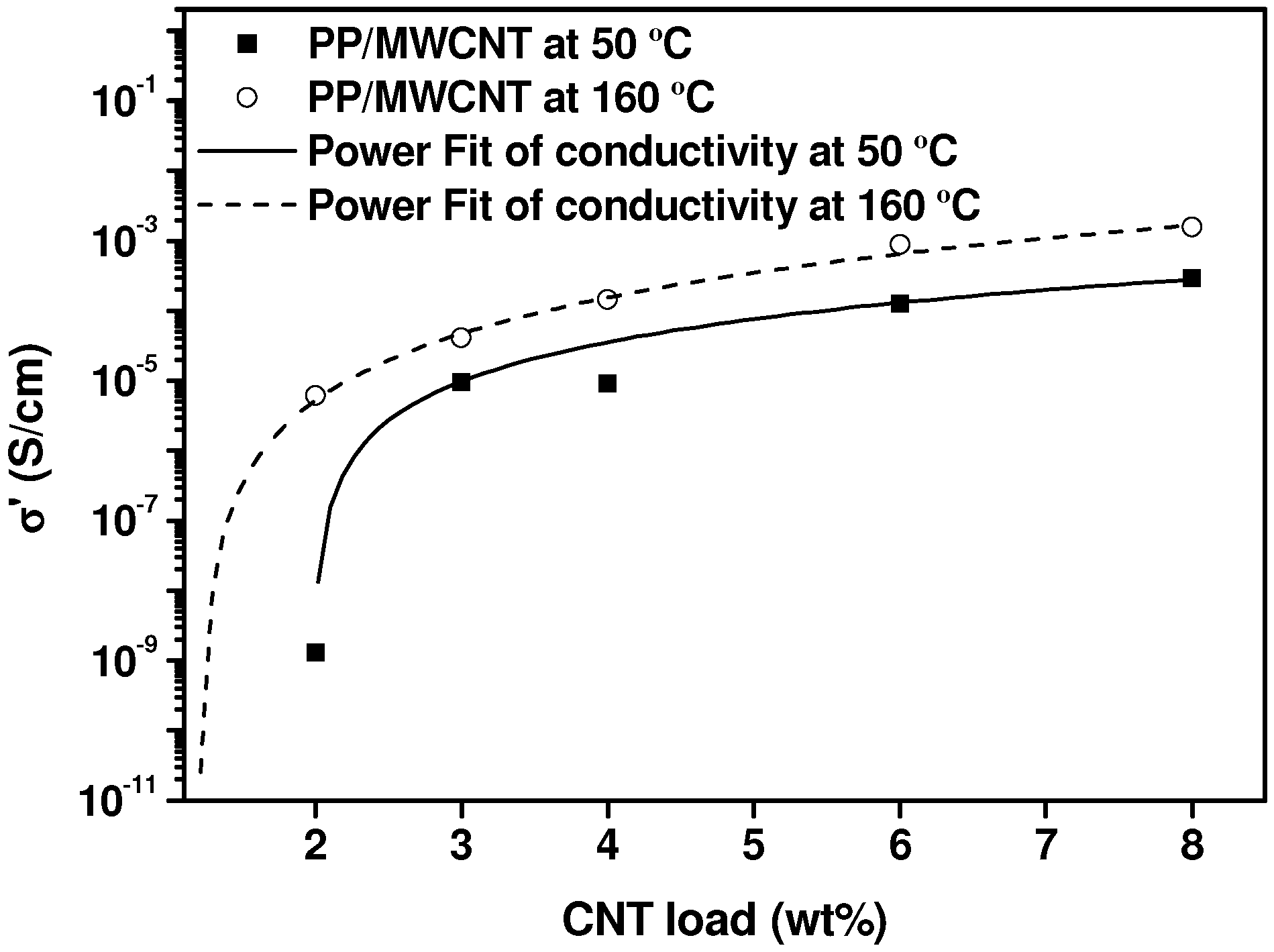
3. Experimental Section
3.1. Materials
3.2. Preparation of PP/MWCNT Nanocomposites
3.3. Characterization
3.3.1. Transmission Electron Microscopy (TEM)

3.3.2. Differential Scanning Calorimetry (DSC)
3.3.3. Rheological Characterization
3.3.4. Pressure-Volume-Temperature (PVT) Measurements
3.3.5. Conductivity Measurements
4. Conclusions
Acknowledgments
References
- Moniruzzaman, M.; Winey, K.I. Polymer nanocomposites containing carbon nanotubes. Macromolecules 2006, 39, 5194–5205. [Google Scholar] [CrossRef]
- Grady, B.P. Carbon Nanotube-Polymer Composites: Manufacture, Properties and Applications; Wiley: New York, NY, USA, 2011. [Google Scholar]
- Bose, S.; Khare, R.A.; Moldenaers, P. Assessing the strengths and weaknesses of various types of pre-treatments of carbon nanotubes on the properties of polymer/carbon nanotubes composites: A critical review. Polymer 2010, 51, 975–993. [Google Scholar] [CrossRef]
- Micusik, M.; Omastova, M.; Pionteck, J.; Pandis, C.; Logakis, E.; Pissis, P. Influence of surface treatment of multiwall carbon nanotubes on the properties of polypropylene/carbon nanotubes nanocomposites. Polym. Adv. Technol. 2011, 22, 38–47. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, M.W.; Kim, S.H.; Youn, J.R. Rheological and electrical properties of polypropylene/MWCNT composites prepared with MWCNT masterbatch chips. Eur. Polym. J. 2008, 44, 1620–1630. [Google Scholar] [CrossRef]
- Andrews, R.; Weisenberger, M.C. Carbon nanotube polymer composites. Curr. Opin. Solid State Mater. Sci. 2004, 8, 31–37. [Google Scholar] [CrossRef]
- Eitan, A.; Jiang, K.; Dukes, D.; Andrews, R.; Schadler, L.S. Surface modification of multiwalled carbon nanotubes: Toward the tailoring of the interface in polymer composites. Chem. Mater. 2003, 15, 3198–3201. [Google Scholar] [CrossRef]
- Bellayer, S.; Gilman, J.W.; Eidelman, N.; Bourbigot, S.; Flambard, X.; Fox, D.M.; de Long, H.C.; Trulove, P.C. Preparation of homogeneously dispersed multiwalled carbon nanotube/polystyrene nanocomposites via melt extrusion using trialkyl imidazolium compatibilizer. Adv. Funct. Mater. 2005, 15, 910–916. [Google Scholar] [CrossRef]
- Hwan Lee, S.; Cho, E.; Ryoun Youn, J. Rheological behavior of polypropylene/layered silicate nanocomposites prepared by melt compounding in shear and elongational flows. J. Appl. Polym. Sci. 2007, 103, 3506–3515. [Google Scholar] [CrossRef]
- Chen, G.; Chen, X.; Wang, H.; Wu, D. Dispersion of graphite nanosheets in polymer resins via masterbatch technique. J. Appl. Polym. Sci. 2007, 103, 3470–3475. [Google Scholar] [CrossRef]
- Wang, K.; Tang, C.; Zhao, P.; Yang, H.; Zhang, Q.; Du, R.; Fu, Q. Rheological investigations in understanding shear-enhanced crystallization of isotactic poly(propylene)/multi-walled carbon nanotube composites. Macromol. Rapid Commun. 2007, 28, 1257–1264. [Google Scholar] [CrossRef]
- Lee, S.H.; Cho, E.; Jeon, S.H.; Youn, J.R. Rheological and electrical properties of polypropylene composites containing functionalized multi-walled carbon nanotubes and compatibilizers. Carbon 2007, 45, 2810–2822. [Google Scholar] [CrossRef]
- Ahangari, M.G.; Fereidoon, A.; Saedodin, S. Mechanical and thermal properties of PP/compatibilized PP/Acid treated SWCNTs nanocomposites: Effect of different acid treatment times. E-Polymers 2008, 153, 1–15. [Google Scholar]
- Wang, Z.; Fan, X.; Wang, K.; Deng, H.; Chen, F.; Fu, Q. Fabrication of polypropylene/carbon nanotubes composites via a sequential process of (Rotating Solid-State Mixing)-Plus-(Melt Extrusion). Compos. Sci. Technol. 2011, 71, 1397–1403. [Google Scholar] [CrossRef]
- Alig, I.; Pötschke, P.; Lellinger, D.; Skipa, T.; Pegel, S.; Kasaliwal, G.R.; Villmow, T. Establishment, morphology and properties of carbon nanotube networks in polymer melts. Polymer 2012, 53, 4–28. [Google Scholar] [CrossRef]
- Alig, I.; Skipa, T.; Engel, M.; Lellinger, D.; Pegel, S.; Pötschke, P. Electrical conductivity recovery in carbon nanotube-polymer composites after transient shear. Phys. Status Solidi B 2007, 244, 4223–4226. [Google Scholar] [CrossRef]
- Alig, I.; Skipa, T.; Lellinger, D.; Pötschke, P. Destruction and formation of a carbon nanotube network in polymer melts: Rheology and conductivity spectroscopy. Polymer 2008, 49, 3524–3532. [Google Scholar] [CrossRef]
- Alig, I.; Skipa, T.; Lellinger, D.; Bierdel, M.; Meyer, H. dynamic percolation of carbon nanotube agglomerates in a polymer matrix: Comparison of different model approaches. Phys. Status Solidi B 2008, 245, 2264–2267. [Google Scholar] [CrossRef]
- Alig, I.; Lellinger, D.; Engel, M.; Skipa, T.; Pötschke, P. Destruction and formation of a conductive carbon nanotube network in polymer melts: In-line experiments. Polymer 2008, 49, 1902–1909. [Google Scholar] [CrossRef]
- Skipa, T.; Lellinger, D.; Saphiannikova, M.; Alig, I. Shear-stimulated formation of multi-wall carbon nanotube networks in polymer melts. Phys. Status Solidi B 2009, 246, 2453–2456. [Google Scholar] [CrossRef]
- Skipa, T.; Lellinger, D.; Böhm, W.; Saphiannikova, M.; Alig, I. Influence of shear deformation on carbon nanotube networks in polycarbonate melts: Interplay between build-up and destruction of agglomerates. Polymer 2010, 51, 201–210. [Google Scholar] [CrossRef]
- Lellinger, D.; Skipa, T.; Böhm, W.; Alig, I. Spatial decorrelation of the conductive nanotube network in a polymer melt. Phys. Status Solidi B 2009, 246, 2667–2670. [Google Scholar] [CrossRef]
- Cipriano, B.H.; Kota, A.K.; Gershon, A.L.; Laskowski, C.J.; Kashiwagi, T.; Bruck, H.A.; Raghavan, S.R. Conductivity enhancement of carbon nanotube and nanofiber-based polymer nanocomposites by melt annealing. Polymer 2008, 49, 4846–4851. [Google Scholar] [CrossRef]
- Palza, H.; Garzón, C.; Arias, O. Modifying the electrical behaviour of polypropylene/carbon nanotube composites by adding a second nanoparticle and by annealing processes. Express Polym. Lett. 2012, 6, 639–646. [Google Scholar] [CrossRef]
- Alig, I.; Pötschke, P.; Pegel, S.; Dubkin, S.; Lellinger, D. Plastic composites containing carbon nanotubes: Optimisation of processing conditions and properties. Rubber Fiber Plast. 2008, 3, 92–95. [Google Scholar] [CrossRef]
- Alig, I.; Lellinger, D.; Dudkin, S.M.; Pötschke, P. Conductivity spectroscopy on melt processed polypropylene-multiwalled carbon nanotube composites: Recovery after shear and crystallization. Polymer 2007, 48, 1020–1029. [Google Scholar]
- Xu, D.; Wang, Z. Role of multi-wall carbon nanotube network in composites to crystallization of isotactic polypropylene matrix. Polymer 2008, 49, 330–338. [Google Scholar] [CrossRef]
- Logakis, E.; Pollatos, E.; Pandis, C.; Peoglos, V.; Zuburtikudis, I.; Delides, C.G.; Vatalis, A.; Gjoka, M.; Syskakis, E.; Viras, K.; et al. Structure-property relationships in isotactic polypropylene/multi-walled carbon nanotubes nanocomposites. Compos. Sci. Technol. 2010, 70, 328–335. [Google Scholar] [CrossRef]
- Andrews, R.; Jacques, D.; Minot, M.; Rantell, T. Fabrication of carbon multiwall nanotube/polymer composites by shear mixing. Macromol. Mater. Eng. 2002, 287, 395–403. [Google Scholar] [CrossRef]
- Seo, M.K.; Park, S.J. Electrical resistivity and rheological behaviors of carbon nanotubes-filled polypropylene composites. Chem. Phys. Lett. 2004, 395, 44–48. [Google Scholar] [CrossRef]
- Du, F.; Scogna, R.C.; Zhou, W.; Brand, S.; Fischer, J.E.; Winey, K.I. Nanotube networks in polymer nanocomposites: Rheology and electrical conductivity. Macromolecules 2004, 37, 9048–9055. [Google Scholar] [CrossRef]
- Abbasi, S.; Derdouri, A.; Carreau, P.J. Properties of microinjection molding of polymer multiwalled carbon nanotube conducting composites. Polym. Eng. Sci. 2011, 51, 992–1003. [Google Scholar] [CrossRef]
- Grady, B.P. Effects of carbon nanotubes on polymer physics. J. Polym. Sci. B 2012, 50, 591–623. [Google Scholar] [CrossRef]
- Bhattacharyya, A.R.; Sreekumar, T.V.; Liu, T.; Kumar, S.; Ericson, L.M.; Hauge, R.H.; Smalley, R.E. Crystallization and orientation studies in polypropylene/single wall carbon nanotube composite. Polymer 2003, 44, 2373–2377. [Google Scholar]
- Jana, R.; Im, C.; Bhunia, H. Effect of multiwalled carbon nanotubes on crystallization behavior of poly(epsilon-caprolactone)diol. J. Thermoplast. Compos. Mater. 2009, 22, 531–546. [Google Scholar] [CrossRef]
- Bikiaris, D. Microstructure and properties of polypropylene/carbon nanotube nanocomposites. Materials 2010, 3, 2884–2946. [Google Scholar] [CrossRef]
- Lu, K.; Grossiord, N.; Koning, C.E.; Miltner, H.E.; Mele, B.V.; Loos, J. Carbon nanotube/isotactic polypropylene composites prepared by latex technology: Morphology analysis of cnt-induced nucleation. Macromolecules 2008, 41, 8081–8085. [Google Scholar] [CrossRef]
- Bikiaris, D.; Vassiliou, A.; Chrissafis, K.; Paraskevopoulos, K.M.; Jannakoudakis, A.; Docoslis, A. Effect of acid treated multi-walled carbon nanotubes on the mechanical, permeability, thermal properties and thermo-oxidative stability of isotactic polypropylene. Polym. Degrad. Stab. 2008, 93, 952–967. [Google Scholar] [CrossRef]
- Manchado, M.A.L.; Valentini, L.; Biagiotti, J.; Kenny, J.M. Thermal and mechanical properties of single-walled carbon nanotubes-polypropylene composites prepared by melt processing. Carbon 2005, 43, 1499–1505. [Google Scholar] [CrossRef]
- Zeng, Y.; Liu, P.; Du, J.; Zhao, L.; Ajayan, P.M.; Cheng, H.M. Increasing the electrical conductivity of carbon nanotube/polymer composites by using weak nanotube-polymer interactions. Carbon 2010, 48, 3551–3558. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.; Wei, F. A treatment method to give separated multi-walled carbon nanotubes with high purity, high crystallization and a large aspect ratio. Carbon 2003, 41, 2939–2948. [Google Scholar] [CrossRef]
- Deng, H.; Bilotti, E.; Zhang, R.; Peijs, T. Effective reinforcement of carbon nanotubes in polypropylene matrices. J. Appl. Polym. Sci. 2010, 118, 30–41. [Google Scholar]
- Pötschke, P.; Dudkin, S.M.; Alig, I. Dielectric spectroscopy on melt processed polycarbonate-multiwalled carbon nanotube composites. Polymer 2003, 44, 5023–5030. [Google Scholar] [CrossRef]
- Pötschke, P.; Abdel-Goad, M.; Alig, I.; Dudkin, S.; Lellinger, D. Rheological and dielectrical characterization of melt mixed polycarbonate-multiwalled carbon nanotube composites. Polymer 2004, 45, 8863–8870. [Google Scholar] [CrossRef]
- Socher, R.; Krause, B.; Müller, M.T.; Boldt, R.; Pötschke, P. The influence of matrix viscosity on mwcnt dispersion and electrical properties in different thermoplastic nanocomposites. Polymer 2012, 53, 495–504. [Google Scholar] [CrossRef]
- Kasaliwal, G.R.; Göldel, A.; Pötschke, P.; Heinrich, G. Influences of polymer matrix melt viscosity and molecular weight on MWCNT agglomerate dispersion. Polymer 2011, 52, 1027–1036. [Google Scholar] [CrossRef]
- Fernandez, I.; Santamaría, A.; Muñoz, M.E.; Castell, P. A rheological analysis of interactions in phenoxy/organoclay nanocomposites. Eur. Polym. J. 2007, 43, 3171–3176. [Google Scholar] [CrossRef]
- Noll, A.; Burkhart, T. Morphological characterization and modelling of electrical conductivity of multi-walled carbon nanotube/poly(p-phenylene sulfide) nanocomposites obtained by Twin Screw Extrusion. Compos. Sci. Technol. 2011, 71, 499–505. [Google Scholar] [CrossRef]
- Stricker, F.; Maier, R.D.; Bruch, M.; Thomann, R.; Mülhaupt, R. Influence of glass bead fillers on phase transitions of syndiotactic polypropene. Polymer 1999, 40, 2077–2084. [Google Scholar] [CrossRef]
- Zoller, P. A Study of the pressure-volume-temperature relationships of four related amorphous polymers: Polycarbonate, polyarylate, phenoxy, and polysulfone. J. Polym. Sci. B 1982, 20, 1453–1464. [Google Scholar]
- Zoller, P. Analysis of the equations of state of polyolefin melts in terms of the simha-somcynsky hole theory. J. Polym. Sci. B 1978, 16, 1491–1498. [Google Scholar]
- Utracki, L.A.; Simha, R. Pressure-volume-temperature dependence of polypropylene/organoclay nanocomposites. Macromolecules 2004, 37, 10123–10133. [Google Scholar] [CrossRef]
- Utracki, L.A.; Simha, R.; Garcia-Rejon, A. Pressure-volume-temperature dependence of poly-e-caprolactam/clay nanocomposites. Macromolecules 2003, 36, 2114–2121. [Google Scholar] [CrossRef]
- Utracki, L.A. PVT of amorphous and crystalline polymers and their nanocomposites. Polym. Degrad. Stab. 2010, 95, 411–421. [Google Scholar] [CrossRef] [Green Version]
- Utracki, L.A. Equations of state for polyamide-6 and its nanocomposites. II. Effects of clay. J. Polym. Sci. B 2009, 47, 966–980. [Google Scholar] [CrossRef]
- Utracki, L.A. Equations of state for polyamide-6 and its nanocomposites. 1. Fundamentals and the matrix. J. Polym. Sci. B 2009, 47, 299–313. [Google Scholar] [CrossRef] [Green Version]
- Utracki, L.A. Free volume of molten and glassy polystyrene and its nanocomposites. J. Polym. Sci. B 2008, 46, 2504–2518. [Google Scholar] [CrossRef] [Green Version]
- Utracki, L.A. Compressibility and thermal expansion coefficients of nanocomposites with amorphous and crystalline polymer matrix. Eur. Polym. J. 2009, 45, 1891–1903. [Google Scholar] [CrossRef]
- Fernández, M.; Landa, M.; Muñoz, M.E.; Santamaría, A. Electrical conductivity of pur/mwcnt nanocomposites in the molten state, during crystallization and in the solid state. Eur. Polym. J. 2011, 47, 2078–2086. [Google Scholar] [CrossRef]
- Pang, H.; Zhang, Y.C.; Chen, T.; Zeng, B.Q.; Li, Z.M. Tunable positive temperature coefficient of resistivity in an electrically conducting polymer/graphene composite. Appl. Phys. Lett. 2010, 96, 251907–251907-3. [Google Scholar]
- Grady, B.P. Recent developments concerning the dispersion of carbon nanotubes in polymers. Macromol. Rapid Commun. 2010, 31, 247–257. [Google Scholar] [CrossRef]
- Wunderlich, B. Macromolecular Physics; Academic Press: New York, NY, USA, 1976. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Huegun, A.; Fernández, M.; Peña, J.; Muñoz, M.E.; Santamaría, A. Liquid-State and Solid-State Properties of Nanotube/Polypropylene Nanocomposites Elaborated via a Simple Procedure. Nanomaterials 2013, 3, 173-191. https://doi.org/10.3390/nano3010173
Huegun A, Fernández M, Peña J, Muñoz ME, Santamaría A. Liquid-State and Solid-State Properties of Nanotube/Polypropylene Nanocomposites Elaborated via a Simple Procedure. Nanomaterials. 2013; 3(1):173-191. https://doi.org/10.3390/nano3010173
Chicago/Turabian StyleHuegun, Arrate, Mercedes Fernández, Juanjo Peña, María Eugenia Muñoz, and Antxon Santamaría. 2013. "Liquid-State and Solid-State Properties of Nanotube/Polypropylene Nanocomposites Elaborated via a Simple Procedure" Nanomaterials 3, no. 1: 173-191. https://doi.org/10.3390/nano3010173




