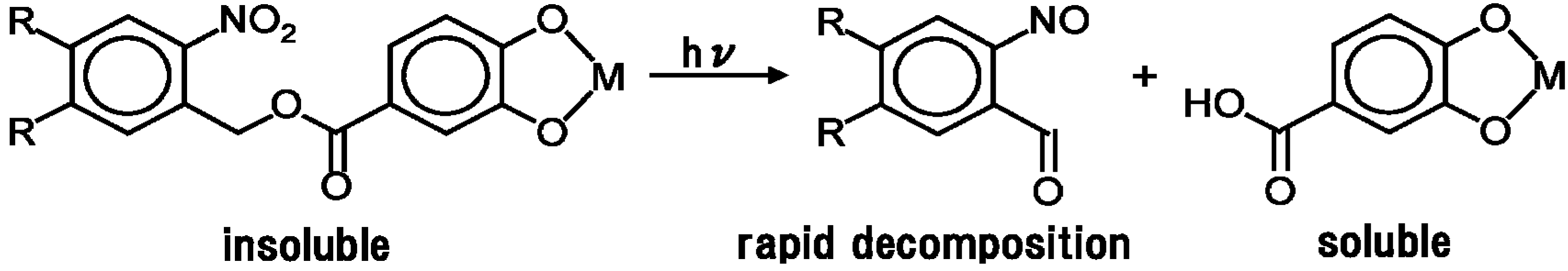
2.1. Photosolvolysis of Nitrobenzyloxycarbonylcatechol-Metal Solution Films
In addition to the metal elements reported so far, to explore the boundaries of the
1 (or
2) metal complex direct patterning technique, the photo-induced solubility and patterning of
1 (or
2) complex films of iron, copper and all the lanthanides (except for promethium) was examined. The photo-induced solubility dynamics of the
1 complex films of iron, copper and holmium as a representative of the lanthanides were investigated and the
2 complex film of holmium for comparison. The regions of the UV-Vis spectra defined in
Table 1 were used to monitor the photo-induced solubility.
Table 1.
Experimental parameters and observations of nitrobenzyl 3,4-dihydroxybenzoate metal complex film photo-solvolysis.
Table 1.
Experimental parameters and observations of nitrobenzyl 3,4-dihydroxybenzoate metal complex film photo-solvolysis.
| Compound | Thickness Complex Film (nm) | Thickness Metal Oxide Film (nm) | te for Pattern (min) | Unexposed Film Area (TMAH 30 s) | Exposed Film Area (TMAH 30 s) | Monitored (nm) | Line Pattern Resolution (μm) | Trench Pattern Resolution (μm) |
|---|
| 1-Fe | 634 ± 1 | 50.5 ± 2.3 | 25 | 94% | 0% | 290–300 | 2 | 2 a |
| 1-Cu | 574 ± 13 | 58.8 ± 5.5 | 30 | 85% | 0% | 280–310 | 2 b,c | 6 a,c,d |
| 1-La | – | – | 20 | 100% | 72% | 250–320 | – | – |
| 1-Ce | 541 ± 7 | 53.6 ± 1.2 | 30 | 100% | 3% | 250–320 | 2 e | 2 |
| 1-Pr | 451 ± 8 | 49.9 ± 2.0 | 20 | 100% | 19% | 250–320 | – | – |
| 1-Nd | – | – | 30 | 100% | 100% | 250–320 | – | – |
| 1-Sm | 419 ± 4 | 50.2 ± 2.5 | 20 | 96% | 1% | 250–320 | 2 b,e | 2 |
| 1-Eu | 479 ± 2 | 50.7 ± 2.0 | 30 | 100% | 1% | 250–320 | 2 e | 2 |
| 1-Gd | 452 ± 6 | 56.6 ± 6.3 | 30 | 100% | 49% | 250–320 | – | – |
| 1-Tb | 474 ± 4 | 50.3 ± 3.9 | 30 | 100% | 1% | 250–320 | 2 b | 2 |
| 1-Dy | 373 ± 19 | 50.2 ± 4.6 | 20 | 99% | 8% | 250–320 | 2 b,e | 2 b |
| 1-Ho | 457 ± 5 | 60.6 ± 2.4 | 15 | 100% | 0% | 250–320 | 2 b,e | 2 |
| 2-Ho | 508 ± 7 | 59.5 ± 3.6 | 10 | 99% | 1% | 250–320 | | |
| 1-Er | 464 ± 2 | 51.6 ± 3.9 | 30 | 100% | 0% | 250–320 | 2 | 2 |
| 1-Tm | – | – | 20 | 1% | 0% | 250–320 | – | – |
| 1-Yb | – | – | 20 | 0% | 0% | 250–320 | – | – |
| 1-Lu | 428 ± 5 | 46.5 ± 1.6 | 20 | 99% | 0% | 250–320 | 2 d | 2 |
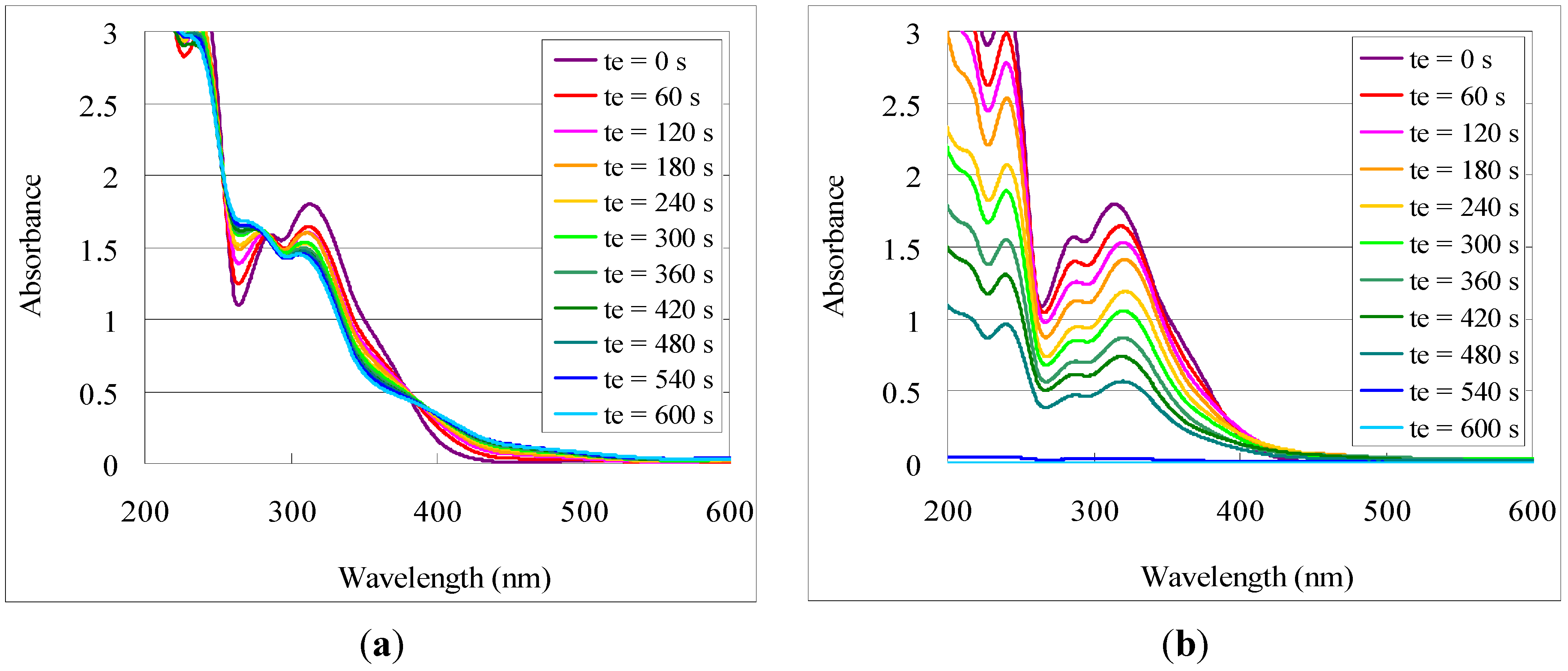
Figure 3 shows the overlaid UV-Vis spectra of
2-Ho films with UV exposure before and after developing as a representative example. In this case, change of the UV-Vis absorption profile with irradiation time (
te) was observed, reflecting the progression of the photochemical reaction. Upon developing, the absorption intensity decreased proportional to
te, leaving a profile similar to the unexposed film. This behavior coincides with the previously observed results for titanium and indium tin, suggesting a similar photo-reaction [
4,
5]. Deduced in a similar manner, the photo-induced solubility dynamics of
1-Fe,
1-Cu,
1-Ho, and
2-Ho are shown in
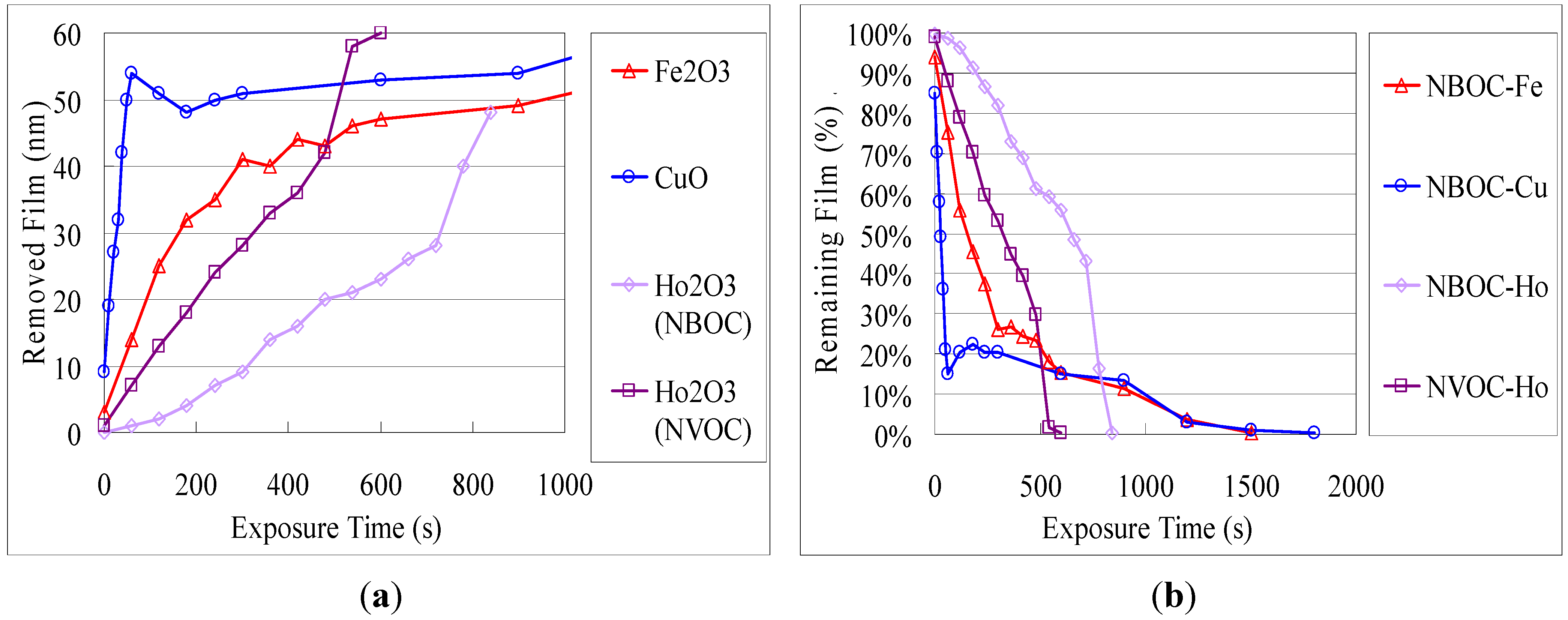
Figure 4 in terms of removed metal oxide film and percentage of remaining film after exposure and developing. Similar to the trend observed for the difference in photo-efficiency between titanium and indium tin, photo-efficiency of
1-Cu was highest followed by
1-Fe then
1-Ho. Similarly, the holmium complex ligand-metal charge transfer (LMCT) absorption overlap of the NBOC excitation was strongest followed by that of the iron complex and least for that of the copper complex. The LMCT of the iron and copper complexes were relatively broadened into the visible spectrum compared to that of holmium. The photo-efficiency of
2-Ho was significantly higher than that of
1-Ho. This finding may be explained as being due to the extended photoreaction profile of the NVOC system, which extends beyond the LMCT absorption, increasing the amount of effective irradiation on the NVOC moiety and therefore increasing the total system quantum yield. Although the initial photo-removal rate was faster for copper and iron, after 85% of the
1-Cu and 75% of the
1-Fe was removed the removal rate abruptly decreased. The reason for this phenomenon is unclear but it may be due to the influence of an intermediate species or a counteractive photo-induced polymerization or hydrolysis reaction.
Figure 3.
Photo-induced solubility of 2-Ho films, monitored by UV-Vis spectra of 2-Ho films (a) exposed to UVB irradiation for time te; and (b) after developing the irradiated films.
Figure 3.
Photo-induced solubility of 2-Ho films, monitored by UV-Vis spectra of 2-Ho films (a) exposed to UVB irradiation for time te; and (b) after developing the irradiated films.
Figure 4.
Photo-solvolysis of nitrobenzyl 3,4-dihydroxybenzoate metal complexes in terms of (a) the remaining fraction of the total complex film; and (b) the amount of removed film in terms of metal oxide film.
Figure 4.
Photo-solvolysis of nitrobenzyl 3,4-dihydroxybenzoate metal complexes in terms of (a) the remaining fraction of the total complex film; and (b) the amount of removed film in terms of metal oxide film.
Next, the photo-patterning ability of the iron, copper and lanthanide complexes of
1 and the
2-Ho complex was evaluated with the L-line photomask, using the known procedure [
4,
5]. The
te used for patterning, as listed in
Table 1, was determined as the minimum
te to completely remove a film for the four measured films. To evaluate the lanthanides other than holmium and promethium, typically 20 min. was used and 30 min. in some cases when dissolution was incomplete. The overlaid UV-Vis spectra of the film before and after exposure and developing in
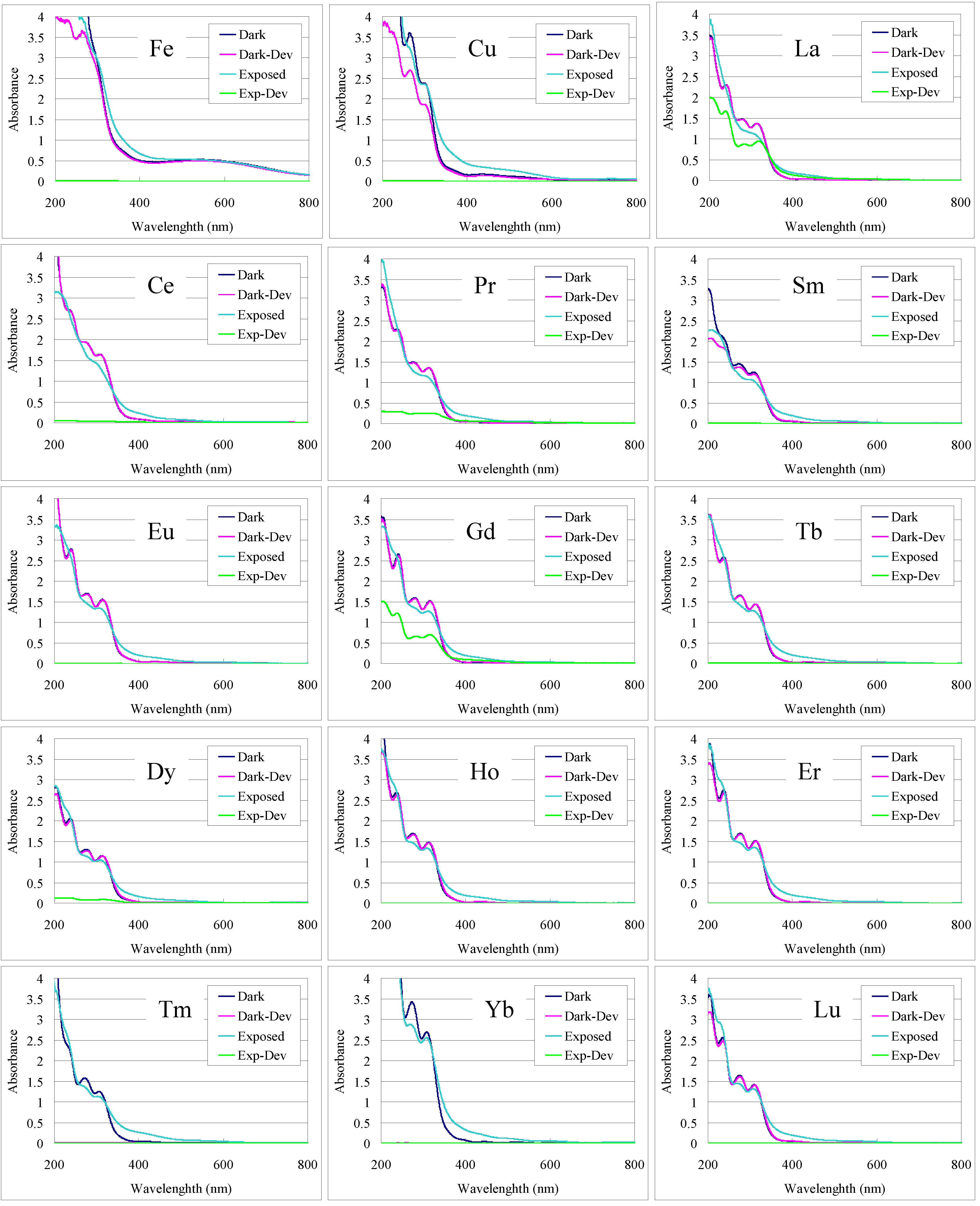
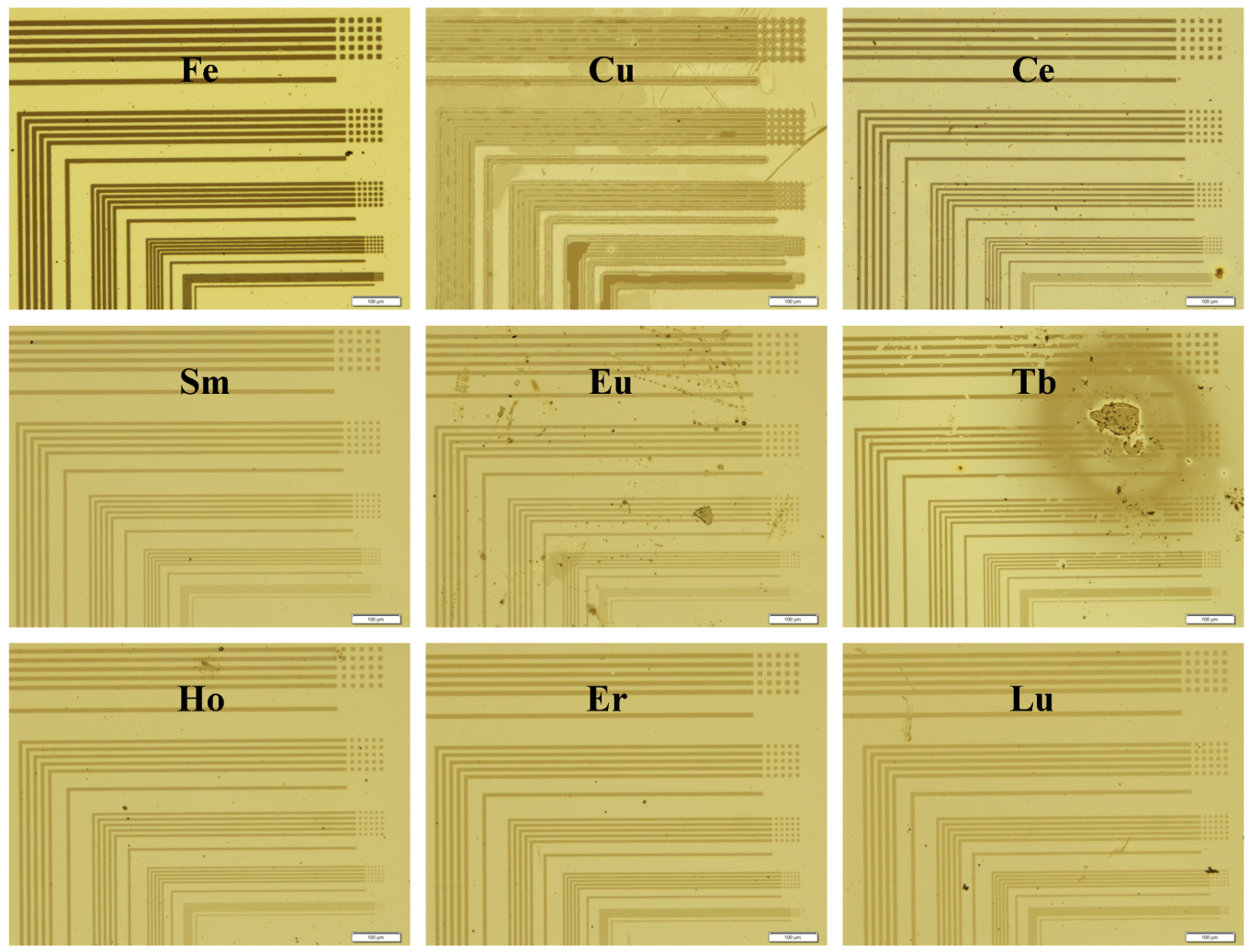
Figure 5 depict the contrast or potential for pattern formation. Microscope images of the L-trench and hole patterns of selected annealed films (
Figure 6) illustrate the patterning ability of the complex films and the continuity of the resulting pyrolyzed films.
Figure 5.
UV-Vis spectra of the masked (Dark) and exposed (Exposed) areas of 1 + metal films before and after developing in 0.25 wt % TMAH (Dev).
Figure 5.
UV-Vis spectra of the masked (Dark) and exposed (Exposed) areas of 1 + metal films before and after developing in 0.25 wt % TMAH (Dev).
Figure 6.
Microscope images of the 2, 4, 6, 8 and 10 μm trench and hole patterned 1 + metal films on Pyrex substrates after pyrolysis at 500 °C.
Figure 6.
Microscope images of the 2, 4, 6, 8 and 10 μm trench and hole patterned 1 + metal films on Pyrex substrates after pyrolysis at 500 °C.
The numerical values for the remaining films of the exposed and unexposed regions after developing listed in
Table 1 exhibit the contrast attained for each film. Contrast of the solubility in developer between the exposed and unexposed areas is required for patterning. Of the lanthanides, cerium, samarium, europium, terbium, dysprosium, holmium, erbium and lutetium could be patterned as a complex of
1. Some contrast was observed in lanthanum, praseodymium, and gadolinium films, although the photo-efficiency was comparatively lower and no contrast was observed in neodymium, thulium and ytterbium. In the case of neodymium, neither the exposed or unexposed areas of the film were soluble in developer. While for thulium and ytterbium, both the exposed and unexposed areas of the film were completely soluble. In the cases contrast was not sufficiently attained, the film thickness could not be measured by surface profile. Insufficient contrast under the conditions examined may be improved by use of alternate metal reagents, solvents or additives.
2.2. Two-Layer Film Patterning
A conventional method for depositing transparent electrodes on both sides of a transparent substrate may be long and energy consuming. For example: (i) Sputter ITO onto side-1 of the substrate; (ii) sputter ITO onto side-2 of the substrate; (iii) coat side-1 of the substrate with photoresist; (iv) expose with pattern-1; (v) develop, (vi) hard bake; (vii) coat side-2 of the substrate with photoresist; (viii) expose with pattern-2; (ix) develop; (x) hard bake; (xi) etch ITO; (xii) strip resist and remove scum (ashing). In this example, the films on both sides of the substrate consist of the same material and are of the same thickness. But in instances where the material on both sides of the substrate are not similar in etching characteristics or do not bear the same etching requirements, each side must also be etched independently, further lengthening and complicating the fabrication procedure. In situations where etching rates or film thickness vary, prolonged exposure of one side may result in undercut.
As shown in
Section 2.1. and elsewhere [
4,
5,
6,
7], many metal complexes can be patterned by developing under the same conditions in diluted TMAH for 30 s, completely independent of the element and film thickness. Similarly, the various patterned metal oxide films have been formed by pyrolysis under the same conditions. Based on the material independence of the
1 or
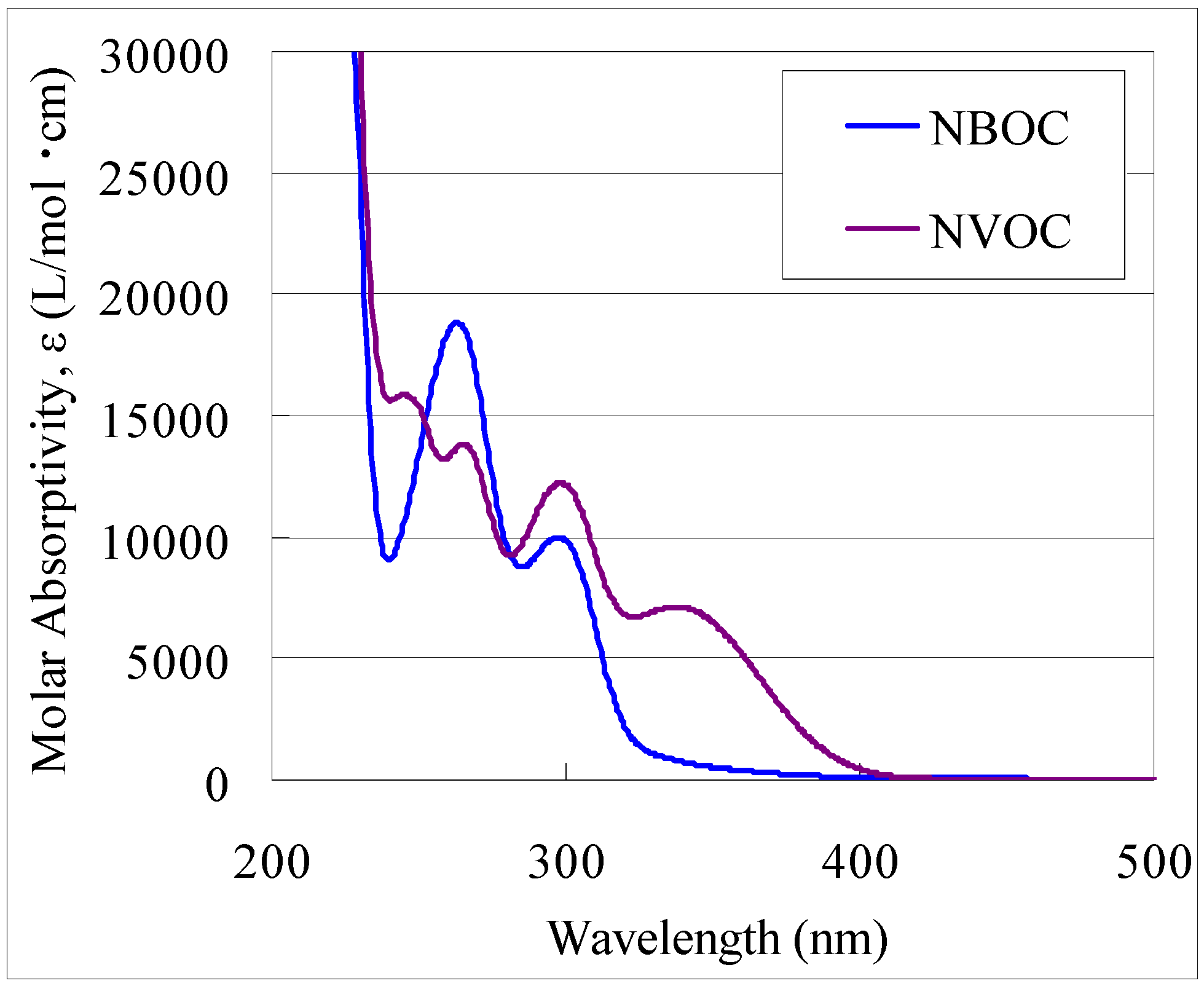
2 metal complex patterning process and on the photoreaction selectivity between
1 and
2 with 370–400 nm irradiation (
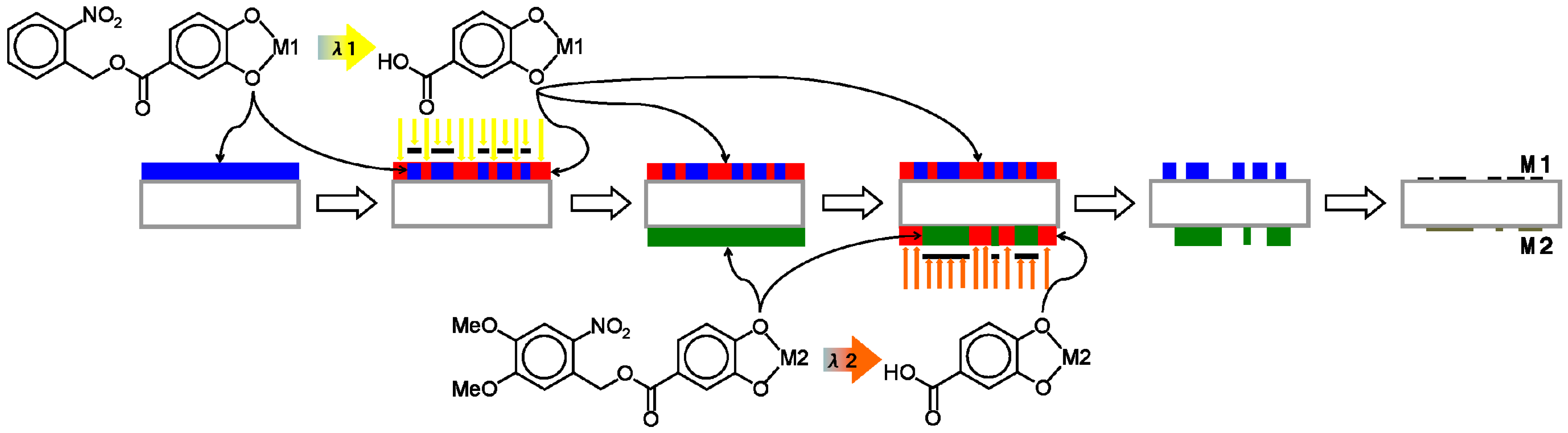
Figure 2), two methods for simultaneously patterning two independent metal oxide films on a transparent substrate were devised. One method (Method A) is as illustrated in
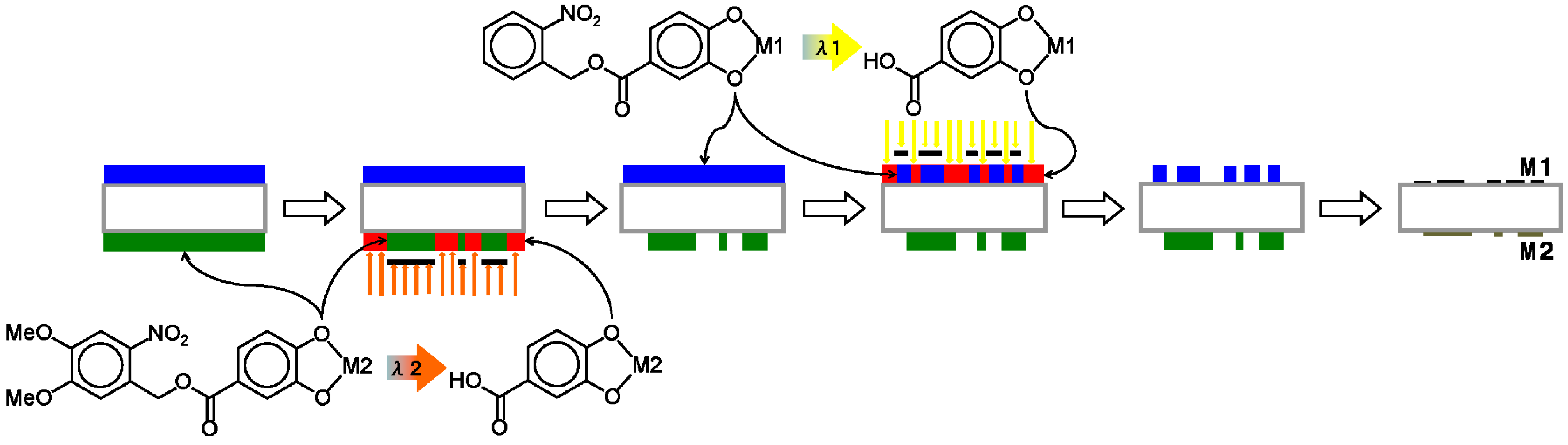
Figure 7: (i) Coat side-1 of the substrate with the NBOC metal-1 complex; (ii) expose side-1 to irradiation between 200 and 370 nm with pattern-1; (iii) coat side-2 of the substrate with the NVOC metal-2 complex; (iv) expose side-2 to irradiation between 370 and 400 nm with pattern-2; (v) submerse in developer; (vi) oxidize to give patern-1 of metal oxide-1 on side-1 and patern-2 of metal oxide-2 on side-2. The other method (Method B) is as illustrated in
Figure 8: (i) Coat side-1 of the substrate with the NBOC metal-1 complex; (ii) coat side-2 of the substrate with the NVOC metal-2 complex; (iii) expose side-2 to irradiation between 370 and 400 nm with pattern-2; (iv) develop side-2; (v) expose side-1 to irradiation between 200 and 370 nm with pattern-1; (vi) develop side-1; (vii) oxidize to give patern-1 of metal oxide-1 on side-1 and patern-2 of metal oxide-2 on side-2. Each method has tradeoffs. Method A only requires one developing step although each complex coat must be dried individually; in Method B, both coatings can be dried simultaneously. Depending on production equipment and conditions one method may sanction advantages over the other.
In the event M1 and M2 differ, there are several factors to consider as to which element to use as M1 and which to use as M2. Two important factors are the photo-induced solubility selectivity and necessary exposure time. As illustrated, the complex LMCT absorption affects the photoreaction efficiency and use of the cut-filter significantly reduces the amount of incident reaction inducing irradiation. In order to maximize photo-selectivity and minimize exposure time, the less reactive element should be applied as M1 and the faster reacting element as M2. For the example of TiO2 on one side and ITO on the other of quartz glass, more slowly reacting titanium was used as M1 and faster reacting indium tin as M2. With this selection the total exposure time, te1 + te2 = 25 min. If indium tin were used as M1 and titanium as M2, the total exposure time (te1 + te2) would require 62 min. and prolonged exposure of the M2 film may increase the probability of influence to the M1 film.
Figure 7.
Procedure (Method A) for simultaneous photo patterning of independent metal/metal oxide bi-layer films using two-tone photoacid generating nitrobenzyl ester derived complexes.
Figure 7.
Procedure (Method A) for simultaneous photo patterning of independent metal/metal oxide bi-layer films using two-tone photoacid generating nitrobenzyl ester derived complexes.
Figure 8.
Procedure (Method B) for simultaneous photo patterning of independent metal/metal oxide bi-layer films using two-tone photoacid generating nitrobenzyl ester derived complexes.
Figure 8.
Procedure (Method B) for simultaneous photo patterning of independent metal/metal oxide bi-layer films using two-tone photoacid generating nitrobenzyl ester derived complexes.
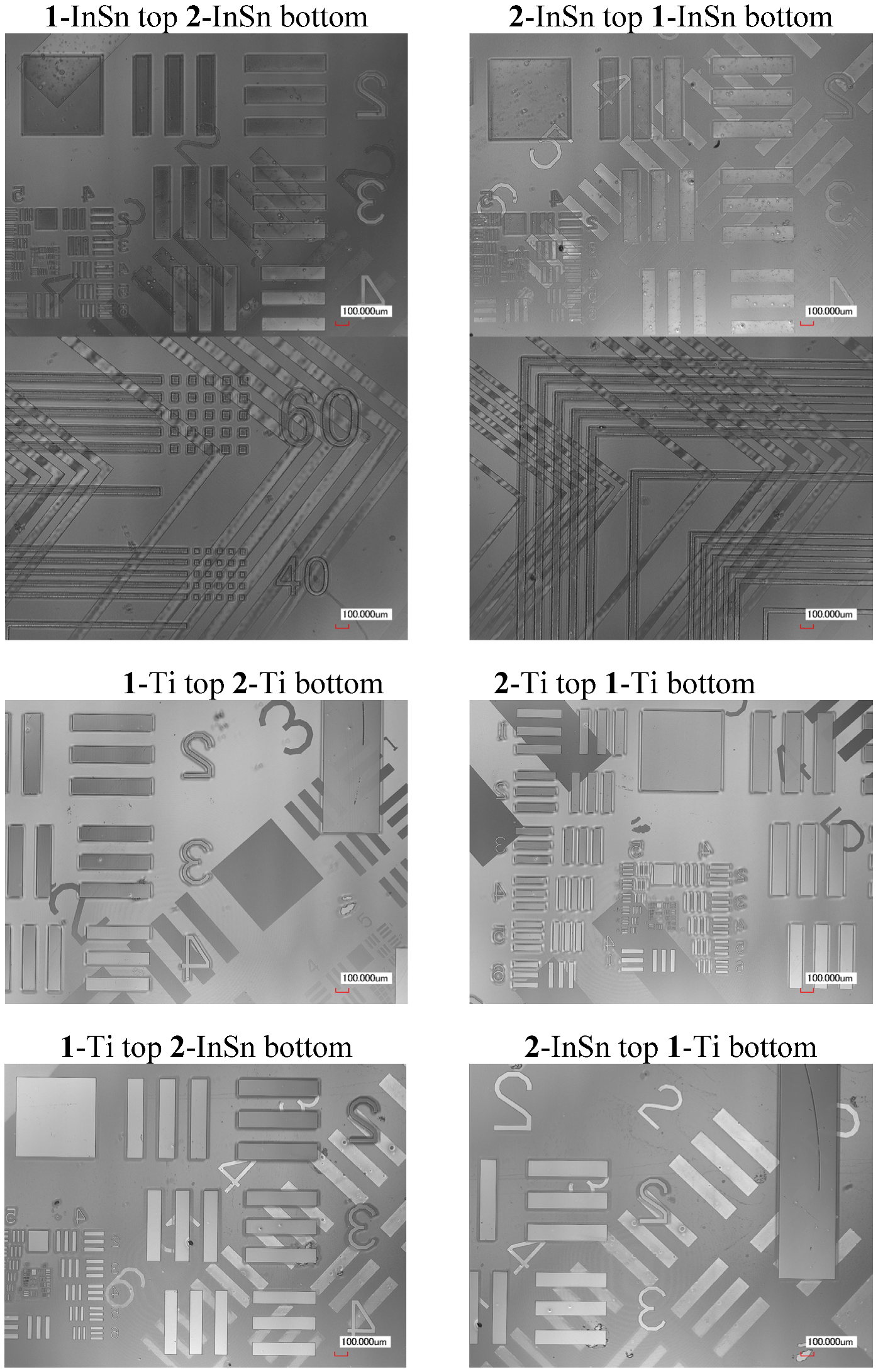
The dual-side patterned films in
Table 2 were produced by Method A and B. Selected laser microscope images of samples produced using Method A are displayed in
Figure 9.
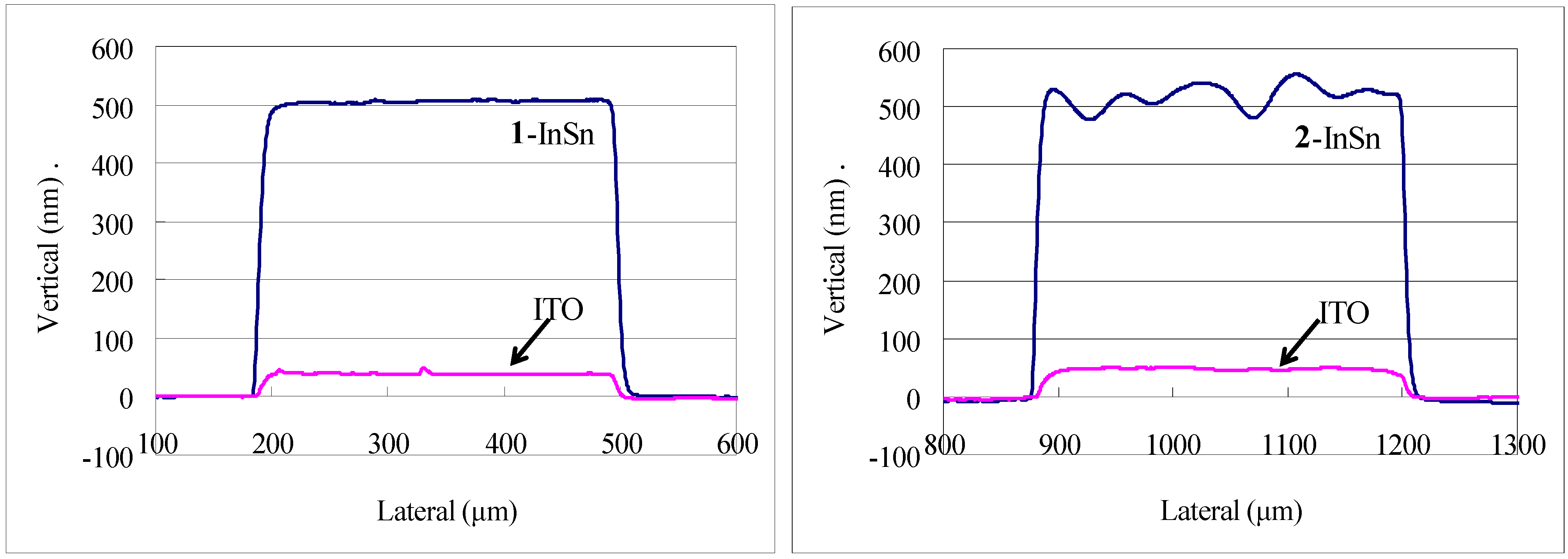
Figure 10 shows the profile of a 0.3 mm line of
1-InSn and
2-InSn films on each side of a quartz substrate before and after pyrolysis at 500 °C. The final results of either method for production of films in
Table 2 were found to be equivalent. In Method A, after side-1 was exposed, side-2 was coated and dried at 100–120 °C. Despite the exposure to elevated temperature, the irradiated material on side-1 was completely removed upon developing in all cases examined. This exhibits the thermally inert nature of the photoproduct and compatibility of these compounds to this method. The electrical and optical properties and analysis of the respective ITO or TiO
2 films formed by pyrolysis of the InSn and Ti complexes under these conditions have been reported [
4].
Table 2.
Dual-sided films.
Table 2.
Dual-sided films.
| Sample Side-1/Side-2 | Compound Side-1/Side-2 | Photomask | Dry Film-1 (nm) | Pyrolyzed Film-1 (nm) | Dry Film-2 (nm) | Pyrolyzed Film-2 (nm) |
|---|
| ITO/ITO | 1-InSn/2-InSn | 1951 USAF | 506 ± 2 | 39.2 ± 1.6 | 518 ± 20 | 48.4 ± 1.5 |
| ITO/ITO | 1-InSn/2-InSn | L-line/trench | 592 ± 16 | 49.9 ± 1.2 | 714 ± 68 | 67.5 ± 1.4 |
| TiO2/TiO2 | 1-Ti/2-Ti | 1951 USAF | 324 ± 4 | 24.9 ± 2.8 | 400 ± 8 | 38.1 ± 3.5 |
| TiO2/ITO | 1-Ti/2-InSn | 1951 USAF | 334 ± 3 | 24.1 ± 2.3 | 617 ± 20 | 53.2 ± 2.1 |
Figure 9.
Laser microscope images of quartz substrates with patterned metal oxide films on both sides deposited by two-tone patterning Method A.
Figure 9.
Laser microscope images of quartz substrates with patterned metal oxide films on both sides deposited by two-tone patterning Method A.
Figure 10.
Surface profiles of patterned 1-InSn and 2-InSn films on each side of a quartz substrate deposited by two-tone patterning Method A and the resulting indium tin oxide (ITO) films formed by pyrolysis at 500 °C.
Figure 10.
Surface profiles of patterned 1-InSn and 2-InSn films on each side of a quartz substrate deposited by two-tone patterning Method A and the resulting indium tin oxide (ITO) films formed by pyrolysis at 500 °C.
2.3. Pyrolysis of Patterned Complex Films
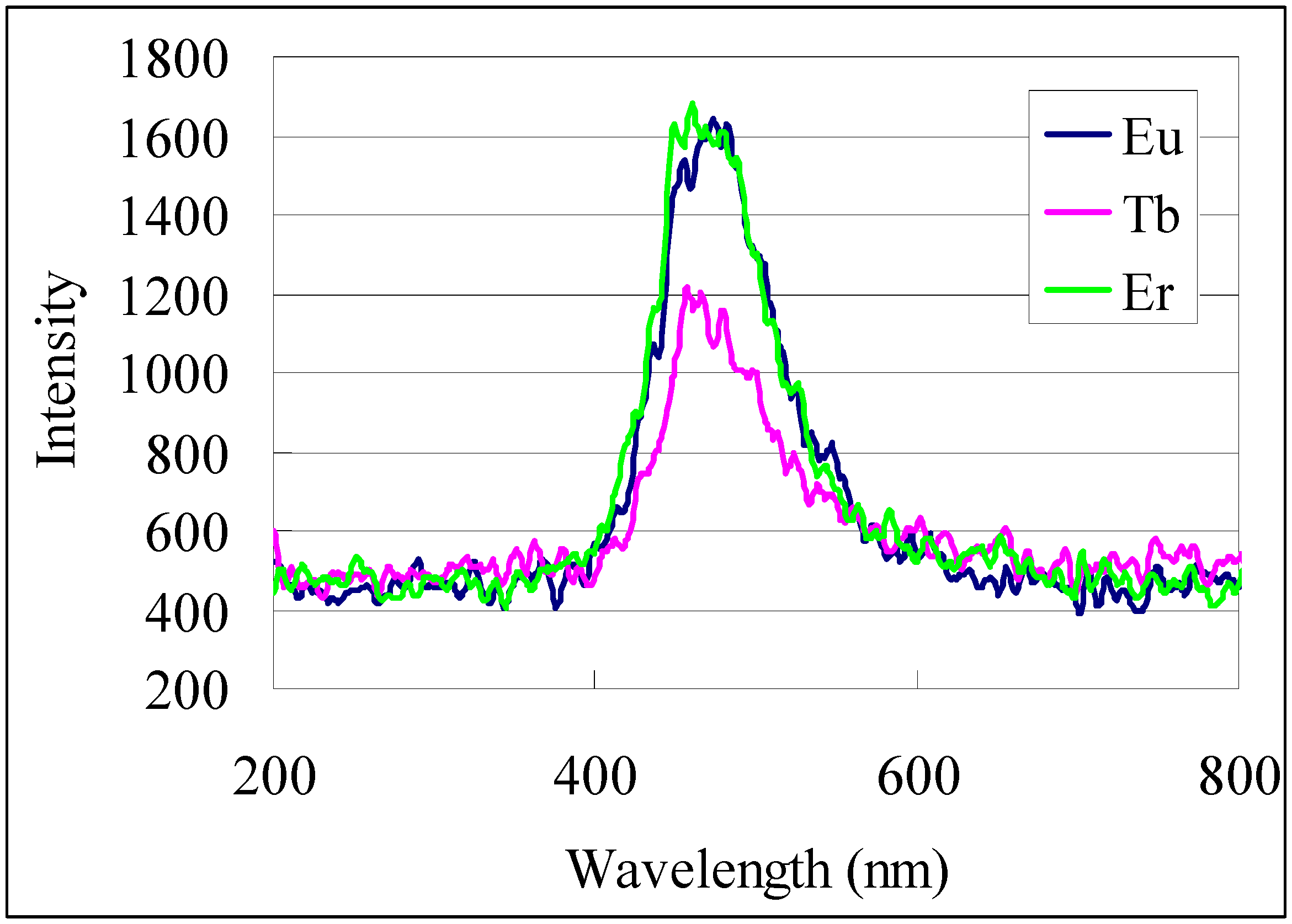
The transformation of organometallic precursors to various materials can be accomplished by many methods, but in this investigation we only performed a simple thermal treatment of the complex films at 500 °C in air. Metal oxide films formed by pyrolysis of the respective complex films showed reasonable optical characteristics compared with known materials. Except for copper oxide, the XRD patterns from the metal oxides formed here were to feint for characterization. The copper oxide films however showed appropriate diffraction patterns for the CuO monoclinic crystal phase. The cathode luminescence of the patterned europium oxide, terbium oxide, and erbium oxide films selected as representatives of the lanthanide oxide films was investigated. All three oxide films had an emission peak centered at about 470 nm as shown in the cathode luminescence spectra (
Figure 11).
Figure 11.
Cathode luminescence spectra of patterned metal oxides formed from pyrolysis of 1-Eu, 1-Tb and 1-Er films at 500 °C.
Figure 11.
Cathode luminescence spectra of patterned metal oxides formed from pyrolysis of 1-Eu, 1-Tb and 1-Er films at 500 °C.
Formation of the appropriate metal oxide from the respective metal complex was confirmed by EPMA.
Table 3 lists the elemental analysis of the patterned metal oxide films. The precursor complexes are composed of carbon, nitrogen, oxygen, and the chelated metal. Theoretically, the pyrolyzed films could contain any of these elements and the glass substrate could contain silicon. For each sample, the oxide, nitride, carbide, and silicide formation affinity and the glass infusion rate may vary depending on the metal. In addition, carburization may leave carbon residue in or on the film, carbon monoxide or carbon dioxide may be absorbed from the atmosphere, and carbon based fouling may be transferred to the sample during handling. Nitrogen was not detected in any of the samples, and as shown in
Table 3, the carbon content varied among the samples. Given the detection of carbon on the bare substrate, carbon presence in other samples may have been transferred during handling. The respective film metals, silicon from the glass and oxygen from both the pyrolyzed films and the glass were appropriately detected. In the case of ITO formation, an appropriate 5–10 atom % tin content with respect to indium and tin was observed in all three cases.
Table 3.
EPMA elemental analysis of films formed by pyrolysis of metal complexes at 500 °C in an air atmosphere.
Table 3.
EPMA elemental analysis of films formed by pyrolysis of metal complexes at 500 °C in an air atmosphere.
| Compound | Metal | [Metal] (atom %) | [O] (atom %) | [C] (atom %) | [Si] (atom %) | Sample Current (nA) |
|---|
| 1-Fe | Fe | 4.0 | 28.7 | 2.4 | 64.9 | 26.3 |
| 1-Cu | Cu | 3.0 | 24.3 | N.D. * | 72.2 | 26.5 |
| 1-Ce | Ce | 2.5 | 23.3 | N.D. | 74.2 | 25.2 |
| 1-Sm | Sm | 1.1 | 45.8 | 1.8 | 51.3 | 16.5 |
| 1-Eu | Eu | 2.0 | 33.0 | N.D. | 65.0 | 27.6 |
| 1-Tb | Tb | 3.2 | 23.4 | 4.0 | 69.4 | 24.4 |
| 1-Dy | Dy | 1.3 | 44.2 | N.D. | 54.5 | 16.6 |
| 1-Ho | Ho | 3.7 | 16.4 | 4.1 | 75.8 | 13.9 |
| 1-Er | Er | 2.5 | 28.5 | N.D. | 69.0 | 14.1 |
| 1-Lu | Lu | 2.3 | 16.6 | 32.4 | 48.8 | 14.9 |
| 1-InSn/ | In + Sn | 1.1 + 0.1 | 28.8 | 3.9 | 66.1 | 16.0 |
| 2-InSn | In + Sn | 1.5 + 0.1 | 29.8 | 1.7 | 66.9 | 16.4 |
| 1-Ti/ | Ti | 1.2 | 35.0 | 4.8 | 59.0 | 16.0 |
| 2-InSn | In + Sn | 1.0 + 0.1 | 36.1 | 4.0 | 58.8 | 15.4 |
| 1-Ti/ | Ti | 1.0 | 42.0 | 2.5 | 54.5 | 16.2 |
| 2-Ti | Ti | 1.1 | 40.9 | 1.4 | 56.6 | 16.7 |
| Substrate | – | – | 28.1 | 6.1 | 65.8 | 15.6 |
Depending on the complex, coating solution solvent, drying conditions, heating rate, pyrolysis temperature and gas atmosphere during pyrolysis among other parameters, the film nano-structure, crystal structure, porosity, elemental composition/stoichiometry (carbon/oxygen/nitrogen/sulfur/hydrogen ratio to metal), uniformity, continuity, physical and chemical properties of the resulting inorganic material may be controlled. More detailed investigation of the complexes to inorganic material transformations, analysis of the formed materials and transformation condition-material property correlations will be presented elsewhere. Control of the film properties by the solution and transformation parameters may enable tailoring the formed material to specific requirements.