Histologic and Histomorphometric Analysis of Bone Regeneration with Bovine Grafting Material after 24 Months of Healing. A Case Report
Abstract
:1. Introduction
2. Materials and Methods
Histologic Analysis
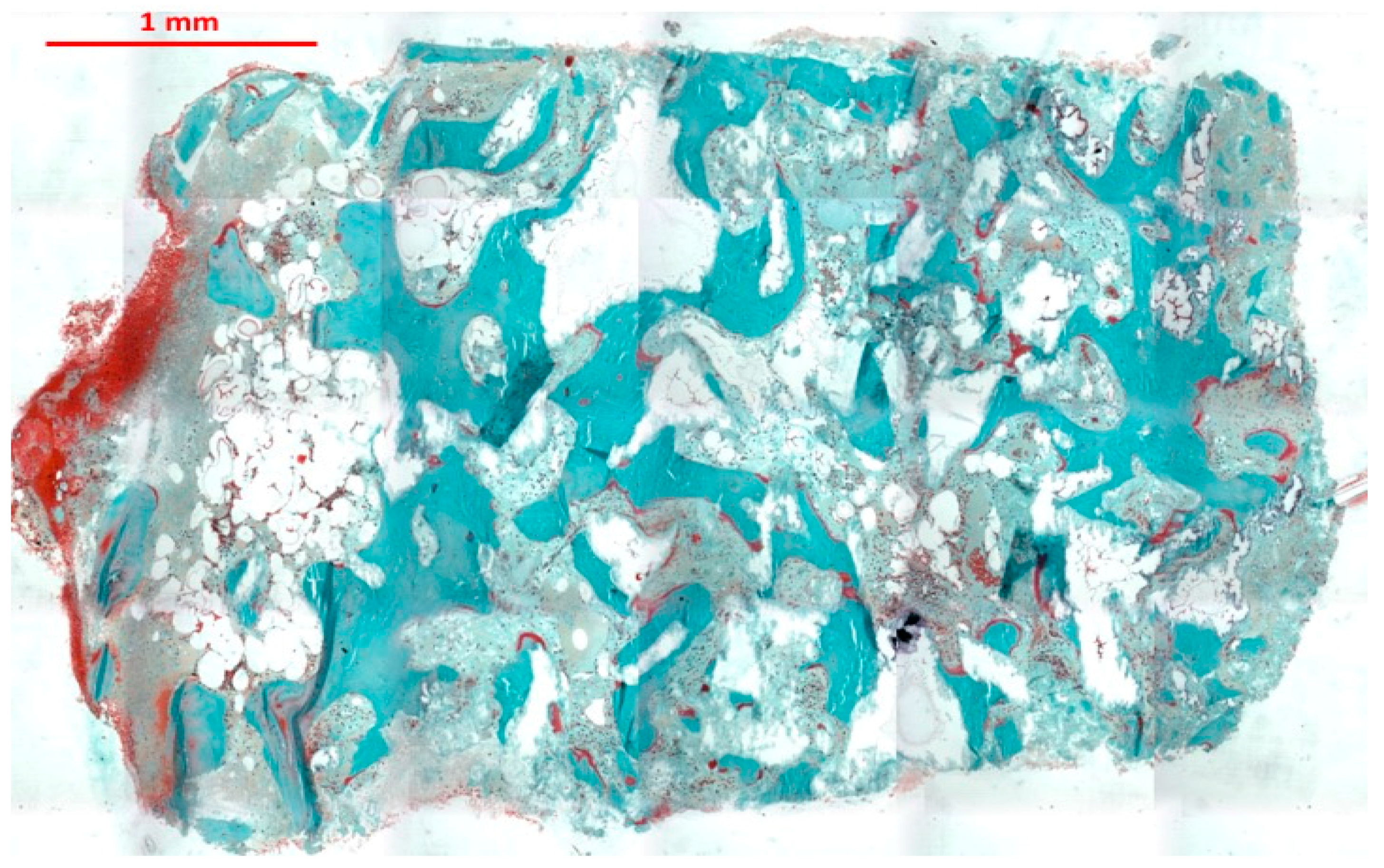
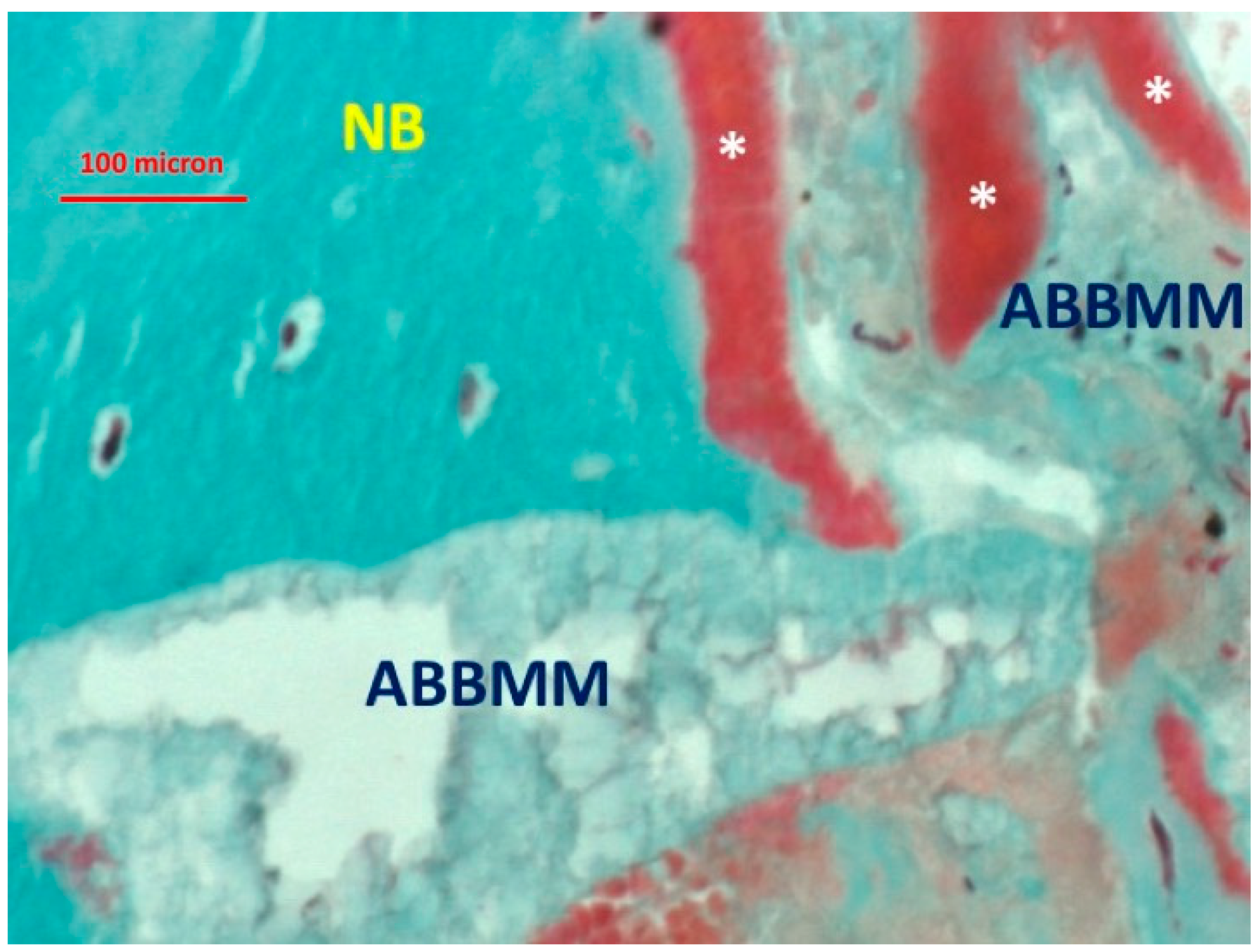
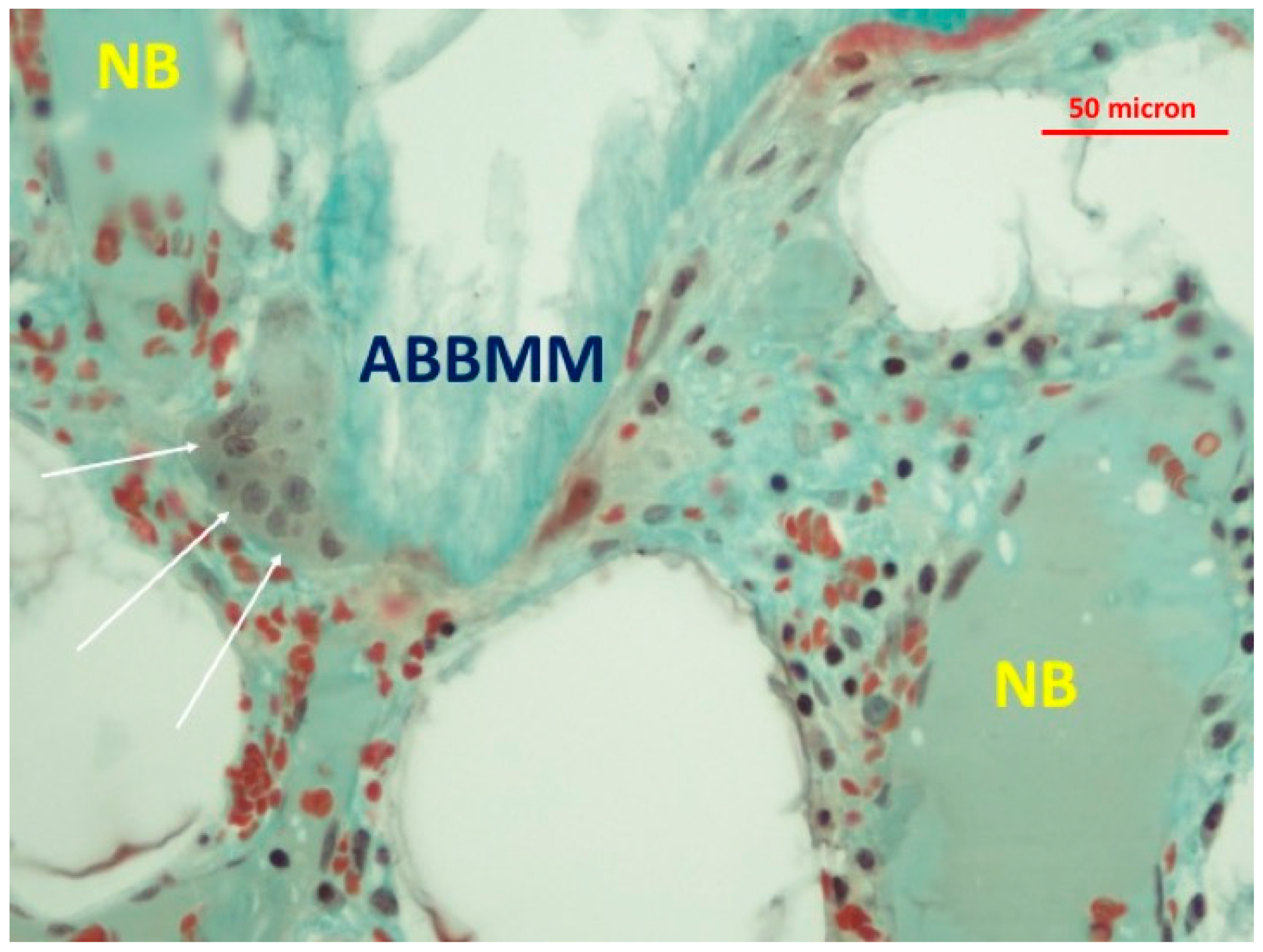
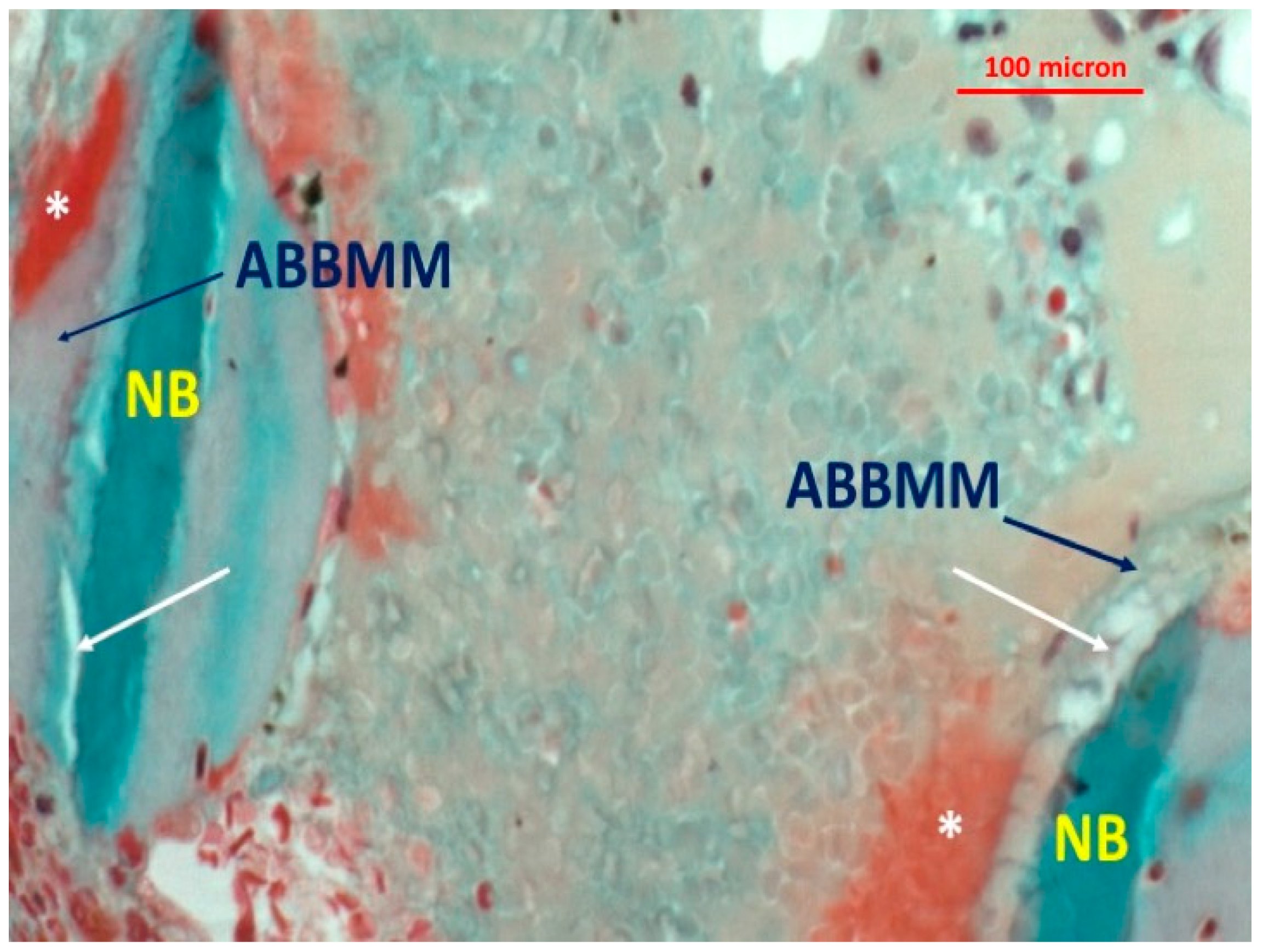
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jensen, O.T.; Shulman, L.B.; Block, M.S.; Iacono, V.J. Report of the Sinus Consensus Conference of 1996. Int. J. Oral Maxillofac. Implants 1998, 13, 11–45. [Google Scholar] [PubMed]
- Hallman, M.; Hedin, M.; Sennerby, L.; Lundgren, S. A prospective 1-year clinical and radiographic study of implants placed after maxillary sinus floor augmentation with bovine hydroxyapatite and autogenous bone. J. Oral Maxillofac. Surg. 2002, 60, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Misch, C.E.; Dietsh, F. Bone-grafting materials in implant dentistry. Implant Dent. 1993, 2, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Hallman, M.; Lederlund, A.; Linsskog, S.; Lundgren, S.; Sennerby, L. A clinical histologic study of bovine hydroxyapatite in combination with autogenous bone and fibrin glue for maxillary sinus floor augmentation. Results after 8 months of healing. Clin. Oral Implants Res. 2001, 12, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Block, J.E.; Poser, J. Does xenogeneic demineralized bone matrix have clinical utility as a bone graft substitute? Med. Hypotheses 1995, 45, 27–32. [Google Scholar] [CrossRef]
- Jensen, S.S.; Aaboe, M.; Pinholt, E.M.; Hjørting-Hansen, E.; Melsen, F.; Ruyter, I.E. Tissue reaction and material characteristics of four bone substitutes. Int. J. Oral Maxillofac. Implants 1996, 11, 55–66. [Google Scholar] [PubMed]
- Tadjoedin, E.S.; de Lange, G.L.; Bronckers, A.L.; Lyaruu, D.M.; Burger, E.H. Deproteinized cancellous bovine bone (Bio-Oss®) as bone substitute for sinus floor elevation. A retrospective, histomorphometrical study of five cases. J. Clin. Periodontol. 2003, 30, 261–270. [Google Scholar] [CrossRef] [PubMed]
- De Misquita, M.R.; Bentini, R.; Goncalves, F. The performance of bone tissue engineering scaffolds in in vivo animal models: A systematic review. J. Biomater. Appl. 2016, 31, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Hallman, M.; Thor, A. Bone substitutes and growth factors as an alternative/complement to autogenous bone for grafting in implant dentistry. Periodontology 2000, 47, 172–192. [Google Scholar] [CrossRef] [PubMed]
- Pjetursson, B.E.; Tan, W.C.; Zwahlen, M.; Lang, N.P. A systematic review of the success of sinus floor elevation and survival of implants inserted in combination with sinus floor elevation. J. Clin. Periodontol. 2008, 35 (Suppl. 8), 216–240. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.; Linder, E.; Wennström, J.; Lindhe, J. The influence of Bio-Oss Collagen on healing of an extraction socket: An experimental study in the dog. Int. J. Periodontics Restor. Dent. 2008, 28, 123–135. [Google Scholar]
- Rosenlicht, J.L.; Tarnow, D.P. Human histologic evidence of integration of functionally loaded implants placed simultaneously with sinus augmentation: A case report 2.5 years postplacement. J. Oral Implantol. 1999, 25, 7–10. [Google Scholar] [CrossRef]
- Piattelli, M.; Favero, G.A.; Scarano, A.; Orsini, G.; Piattelli, A. Bone reactions to anorganic bovine bone (Bio-Oss®) used in sinus lifting procedure: A histologic long-term report of 20 cases in man. Int. J. Oral Maxillofac. Implants 1999, 14, 835–840. [Google Scholar] [PubMed]
- Ewers, R.; Goriwoda, W.S.; Chopper, C.; Moser, D.; Spassova, E. Histologic findings at augmented bone areas supplied with two different bone substitute materials combined with sinus floor lifting. Report of one case. Clin. Oral Implants Res. 2004, 15, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Pecora, G.; Piattelli, M.; Piattelli, A. Osseointegration in a sinus augmented with bovine porous mineral: Histological results in an implant retrieved 4 years after insertion. A case report. J. Periodontol. 2004, 75, 1161–1166. [Google Scholar] [CrossRef] [PubMed]
- Traini, T.; Valentini, P.; Iezzi, G.; Piattelli, A. A Histologic and Histomorphometric Evaluation of Anorganic Bovine Bone Retrieved 9 Years after a Sinus Augmentation Procedure. J. Periodontol. 2007, 78, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Zaffe, D.; Leghissa, G.C.; Pradelli, J.; Botticelli, A.R. Histological study on sinus lift grafting by Fisiograft and Bio-Oss. J. Mater. Sci. Mater. Med. 2005, 16, 789–793. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, A.K.; Donath, K. Bio-Oss—A resorbable bone substitute? J. Long-Term Eff. Med. Implants 1998, 8, 201–209. [Google Scholar] [PubMed]
- Artzi, Z.; Nemcovsky, C.E.; Dayan, D. Bovine-HA spongiosa blocks and immediate implant placement in sinus augmentation procedures. Histopathological and histomorphometric observations on different histological stainings in 10 consecutive patients. Clin. Oral Implants Res. 2002, 13, 420–442. [Google Scholar] [CrossRef] [PubMed]
- Galindo-Moreno, P.; Hernández-Cortés, P.; Mesa, F.; Carranza, N.; Juodzbalys, G.; Aguilar, M.; O’Valle, F. Slow resorption of anorganic bovine bone by osteoclasts in maxillary sinus augmentation. Clin. Implant Dent. Relat. Res. 2013, 15, 858–866. [Google Scholar] [CrossRef] [PubMed]
- Corbella, S.; Taschieri, S.; Weinstein, R.; Del Fabbro, M. Histomorphometric outcomes after lateral sinus floor elevation procedure: A systematic review of the literature and meta-analysis. Clin. Oral Implants Res. 2016, 27, 1106–1122. [Google Scholar] [CrossRef] [PubMed]
- Barone, A.; Aldini, N.N.; Fini, M.; Giardino, R.; Calvo Guirado, J.L.; Covani, U. Xenograft versus extraction alone for ridge preservation after tooth removal: A clinical and histomorphometric study. J. Periodontol. 2008, 79, 1370–1377. [Google Scholar] [CrossRef] [PubMed]
- Barone, A.; Toti, P.; Quaranta, A.; Alfonsi, F.; Cucchi, A.; Negri, B.; Di Felice, R.; Marchionni, S.; Calvo-Guirado, J.L.; Covani, U.; et al. Clinical and Histological changes after ridge preservation with two xenografts: Preliminary results from a multicentre randomized controlled clinical trial. J. Clin. Periodontol. 2017, 44, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.S.; Broggini, N.; Hjørting-Hansen, E.; Schenk, R.; Buser, D. Bone healing and graft resorption of autograft, anorganic bovine bone and beta-tricalcium phosphate. A histologic and histomorphometric study in the mandibles of minipigs. Clin. Oral Implants Res. 2006, 17, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Artzi, Z.; Weinreb, M.; Givol, N.; Rohrer, M.D.; Nemcovsky, C.E.; Prasad, H.S.; Tal, H. Biomaterial resorption rate and healing site morphology of inorganic bovine bone and beta-tricalcium phosphate in the canine: A 24 month longitudinal histologic study and morphometric analysis. Int. J. Oral Maxillofac. Implants 2004, 19, 357–368. [Google Scholar] [PubMed]
- Galindo-Moreno, P.; Moreno-Riestra, I.; Avila, G.; Fernández-Barbero, J.E.; Mesa, F.; Aguilar, M.; Wang, H.L.; O’Valle, F. Histomorphometric comparison of maxillary pristine bone and composite bone graft biopsies obtained after sinus augmentation. Clin. Oral Implants Res. 2010, 21, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, R.; DeVilliers, P.; Grande, M.; Stefanelli, L.V.; Di Carlo, S.; Pompa, G. Histologic evaluation of bone healing of adjacent alveolar sockets grafted with bovine and porcine-derived bone: A comparative case report in humans. Regener. Biomater. 2017, 4, 125–128. [Google Scholar] [CrossRef]
- Greenspan, D.C. Physical and Chemical Properties of Commercially Available Mineralized Bone Allograft. Available online: http://www.zimmerbiomet.co.il/images/zimmer_minisite_2608%201.pdf (accessed on 6 August 2018).
- Danesh-Sani, S.A.; Engebretson, S.P.; Janal, M.N. Histomorphometric results of different grafting materials and effect of healing time on bonematuration after sinus floor augmentation: A systematic review and meta-analysis. J. Periodont. Res. 2017, 52, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Felice, P.; Scarano, A.; Pistilli, R.; Checchi, L.; Piattelli, M.; Pellegrino, G.; Esposito, M. A comparison of two techniques to augment maxillary sinuses using the lateral window approach: Rigid synthetic resorbable barriers versus anorganic bovine bone. Five-month post-loading clinical and histological results of a pilot randomised controlled clinical trial. Eur. J. Oral Implantol. 2009, 2, 293–306. [Google Scholar] [PubMed]
- Hallman, M.; Sennerby, L.; Lundgren, S. A clinical and histologic evaluation of implant integration in the posterior maxilla after sinus floor augmentation with au-togenous bone, bovine hydroxyapatite, or a 20:80 mixture. Int. J. Oral Maxillofac. Implants 2002, 17, 635–643. [Google Scholar] [PubMed]
- McAllister, B.S.; Margolin, M.D.; Cogan, A.G.; Buck, D.; Hollinger, J.O.; Lynch, S.E. Eighteen-month radiographic and histologic evaluation of sinus grafting with anorganic bovine bone in the chimpanzee. Int. J. Oral Maxillofac. Implants 1999, 14, 361–368. [Google Scholar] [PubMed]
- Carmagnola, D.; Berglundh, T.; Lindhe, J. The effect of a fibrin glue on the integration of Bio-Oss with bone tissue: A experimental study in labrador dogs. J. Clin. Periodontol. 2002, 29, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Perrotti, V.; Nicholls, B.M.; Horton, M.A.; Piattelli, A. Human osteoclast formation and activity on a xenogenous bone mineral. J. Biomed. Mater. Res. A 2009, 90, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Hammerle, C.H.F.; Chiantella, G.C.; Karring, T.; Lang, N.P. The effect of a deproteinized bovine bone mineral on bone regeneration around titanium dental implants. Clin. Oral Implants Res. 1998, 9, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Hürzeler, M.B.; Quiñones, C.R.; Kirsch, A.; Gloker, C.; Schüpbachs, P.; Strub, J.R.; Caffesse, R.G. Maxillary sinus augmentation using different grafting materials and dental implants in monkeys. Part I. Evaluation of anorganic bovine-derived bone matrix. Clin. Oral Implants Res. 1997, 8, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.G.; Liljenberg, B.; Lindhe, J. Dynamics of Bio-Oss Collagen incorporation in fresh extraction wounds: An experimental study in the dog. Clin. Oral Implants Res. 2010, 21, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Merkx, M.A.; Maltha, J.C.; Freihofer, H.P. Incorporation of composite bone implants in the facial skeleton. Clin. Oral Implants Res. 2000, 11, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Simion, M.; Fontana, F.; Rasperini, G.; Maiorana, C. Vertical ridge augmentation by expanded-polytetrafluoroethylene membrane and a combination of intraoral autogenous bone graft and deproteinized anorganic bovine bone (Bio Oss). Clin. Oral Implants Res. 2007, 18, 620–629. [Google Scholar] [CrossRef] [PubMed]
- Kadoya, Y.; Al-Saffar, N.; Kobayashi, A.; Revell, P.A. The expression of osteoclast markers on foreign body giant cells. Bone 1994, 27, 85–96. [Google Scholar] [CrossRef]
- Pogoda, P.; Priemel, M.; Rueger, J.M.; Amling, M. Bone remodeling: New aspects of a key process that controls skeletal maintenance and repair. Osteoporos. Int. 2005, 16, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, Y.; Haynes, D.R.; Berger, G.; Gildenhaar, R.; Lucas, H.; Holding, C.; Zreiqat, H. Bioceramics composition modulate resorption of human osteoclasts. J. Mater. Sci. Mater. Med. 2005, 16, 1199–1205. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Fernández, M.P.; Calvo-Guirado, J.L.; Delgado-Ruiz, R.A.; Maté-Sánchez del Val, J.E.; Negri, B.; Penarrocha Diago, M. Ultrastructural study by backscattered electron imaging and elemental microanalysis of biomaterial-to bone interface and mineral degradation of bovine xenografts in maxillary sinus floor elevation. Clin. Oral Implants Res. 2013, 24, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Trajkovski, B.; Jaunich, M.; Müller, W.D.; Beuer, F.; Zafiropoulos, G.G.; Houshmand, A. Hydrophilicity, Viscoelastic and Physicochemical Properties Variations in Dental Bone Grafting Substitutes. Materials 2018, 11, 215. [Google Scholar] [CrossRef] [PubMed]
- Riachi, F.; Naaman, N.; Tabarani, C.; Aboelsaad, N.; Aboushelib, M.N.; Berberi, A.; Salameh, Z. Influence of material properties on rate of resorption of two bone graft materials after sinus liftusing radiographic assessment. Int. J. Dent. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Lapczyna, H.; Galea, L.; Wüst, S.; Bohner, M.; Jerban, S.; Sweedy, A.; Doebelin, N.; Van Garderen, N.; Hofmann, S.; Baroud, G.; et al. Effect of grain size and microporosity on the in vivo behaviour of β tricalcium phosphate scaffolds. Eur. Cells Mater. 2014, 28, 299–319. [Google Scholar] [CrossRef]
- Yamada, Y.; Tamura, T.; Hariu, K.; Asano, Y.; Sato, S.; Ito, K. Angiogenesis in newly augmented bone observed in rabbit calvarium using a titanium cap. Clin. Oral Implants Res. 2008, 19, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Cullinane, D.M. The role of osteocytes in bone regulation: Mineral homeostasis versus mechanoreception. J. Musculoskelet. Neuronal Interact. 2002, 2, 242–244. [Google Scholar] [PubMed]
- Bonewald, L.F. The amazing osteocyte. J. Bone Miner. Res. 2011, 26, 229–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, R.B. Toward a unifying theory of bone remodeling. Bone 2000, 26, 1–6. [Google Scholar] [CrossRef]
- Gu, G.; Mulari, M.; Peng, Z.; Hentunen, T.A.; Väänänen, H.K. Death of osteocytes turns off the inhibition of osteoclasts and triggers local bone resorption. Biochem. Biophys. Res. Commun. 2005, 335, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, O.D.; Herman, B.C.; Laudier, D.M.; Majeska, R.J.; Sun, H.B.; Schaffler, M.B. Activation of resorption in fatigueloaded bone involves both apoptosis and active pro-osteoclastogenic signaling by distinct osteocyte populations. Bone 2012, 50, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Rao, D.S.; Fyrhie, D.P.; Palmitkar, S.; Parfitt, A.M. The morphological association between microcracks and osteocyte lacunae in human cortical bone. Bone 2005, 37, 10–12. [Google Scholar] [CrossRef] [PubMed]


| Column | Mean | SD |
|---|---|---|
| Tt. Tissue Area. | 7.205 | (1.8) |
| Tt. Area of Bone | 1.952 | (0.5) |
| Tt. Area of Bone Graft | 3.103 | (0.9) |
| Tt. Osteoid Area | 0.095 | (0.03) |
| Tt. Connective Tissue Area | 2.054 | (0.8) |
| %. Marrow spaces/Tt. Area | 23.84 | (4.6) |
| %. Bone/Tt. Tissue Area | 40.84 | (3.3) |
| %. Graft/Tt. Tissue Area | 33.59 | (2.8) |
| %. Osteoid/Tt. Tissue Area | 1.697 | (0.4) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guarnieri, R.; Belleggia, F.; DeVillier, P.; Testarelli, L. Histologic and Histomorphometric Analysis of Bone Regeneration with Bovine Grafting Material after 24 Months of Healing. A Case Report. J. Funct. Biomater. 2018, 9, 48. https://doi.org/10.3390/jfb9030048
Guarnieri R, Belleggia F, DeVillier P, Testarelli L. Histologic and Histomorphometric Analysis of Bone Regeneration with Bovine Grafting Material after 24 Months of Healing. A Case Report. Journal of Functional Biomaterials. 2018; 9(3):48. https://doi.org/10.3390/jfb9030048
Chicago/Turabian StyleGuarnieri, Renzo, Fabrizio Belleggia, Patricia DeVillier, and Luca Testarelli. 2018. "Histologic and Histomorphometric Analysis of Bone Regeneration with Bovine Grafting Material after 24 Months of Healing. A Case Report" Journal of Functional Biomaterials 9, no. 3: 48. https://doi.org/10.3390/jfb9030048






