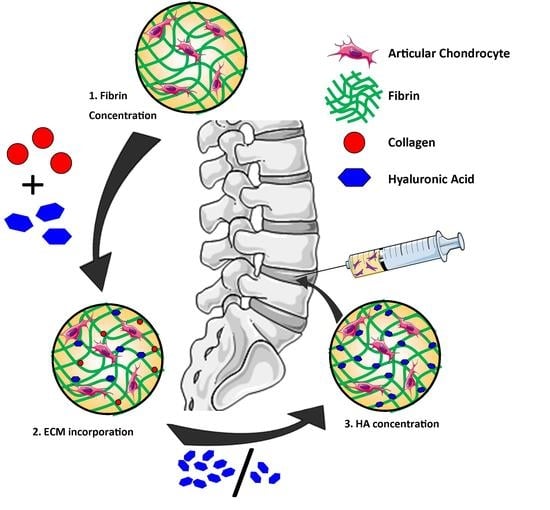
Incorporation of Collagen and Hyaluronic Acid to Enhance the Bioactivity of Fibrin-Based Hydrogels for Nucleus Pulposus Regeneration
Abstract
:1. Introduction
2. Results
2.1. Stage 1—Effect of Increasing Fibrinogen Concentration
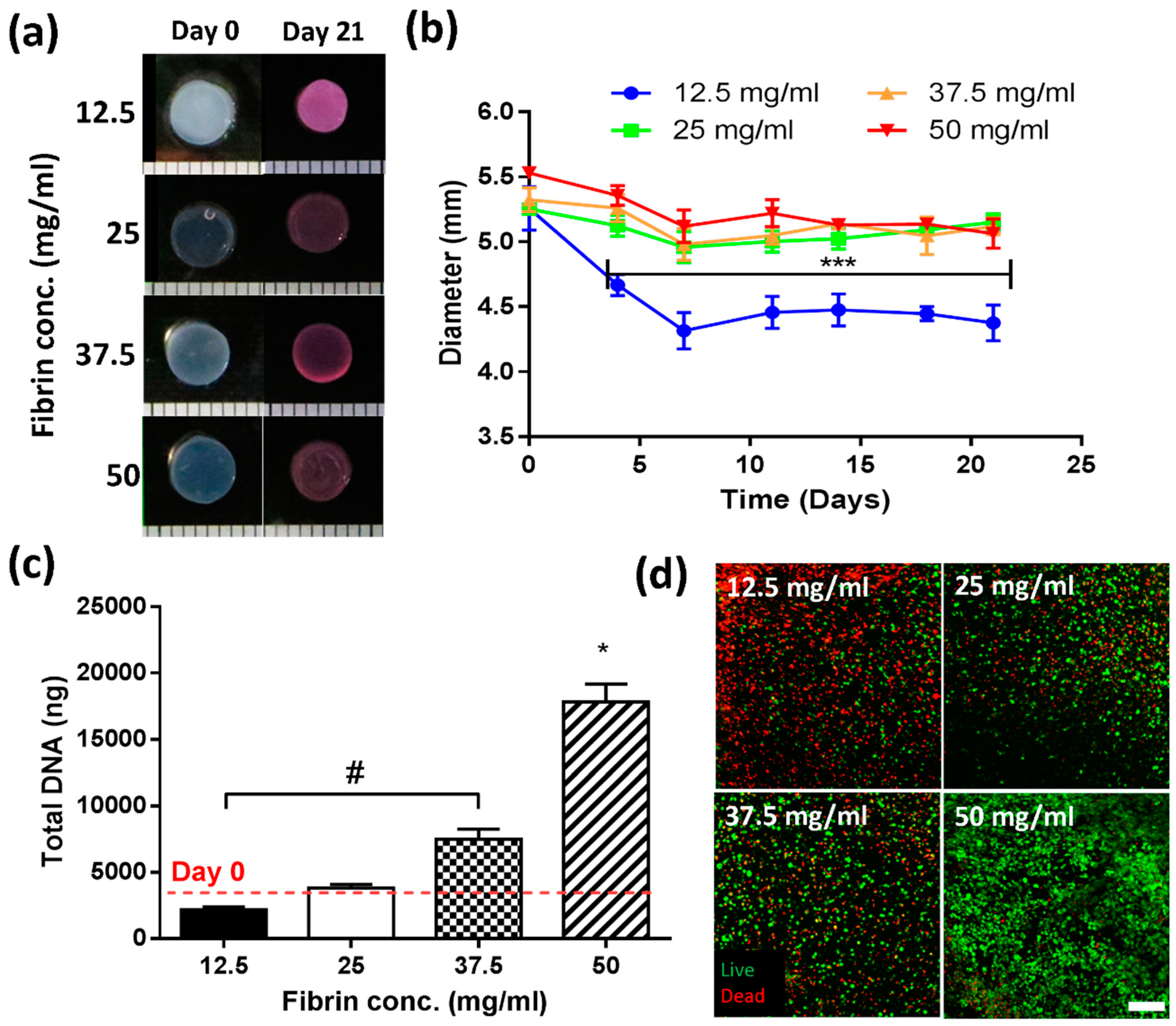
2.1.1. A Minimum Concentration of 25 mg/mL Fibrinogen Is Required to Maintain Construct Stability over 21 Days in Culture
2.1.2. Increasing Fibrinogen Concentration Enhances Cell Proliferation
2.2. Stage 2—Incorporation of ECM into Fibrin-Based Hydrogels
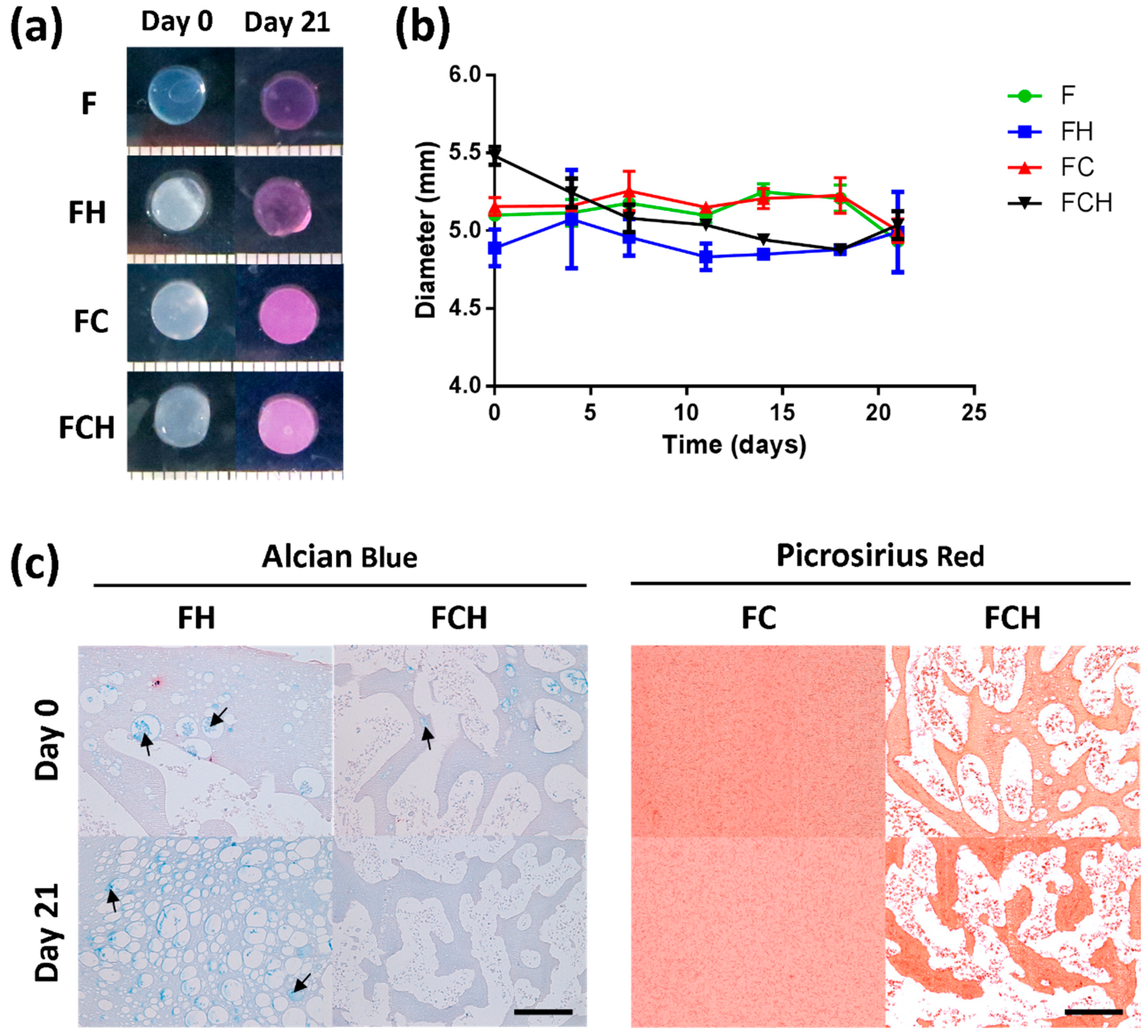
2.2.1. Fibrin-ECM Acellular Hydrogels Remain Stable in Culture over 21 Days
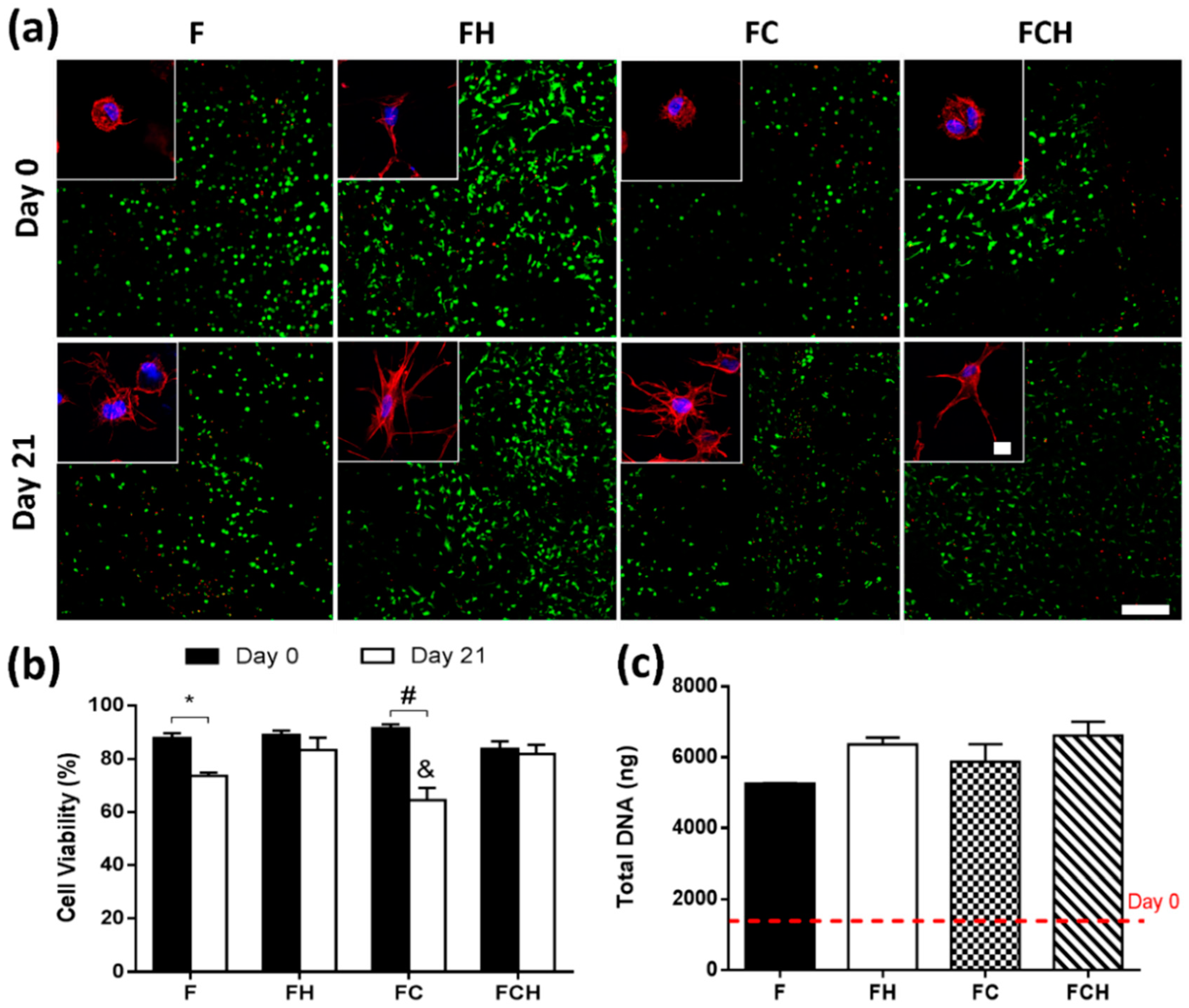
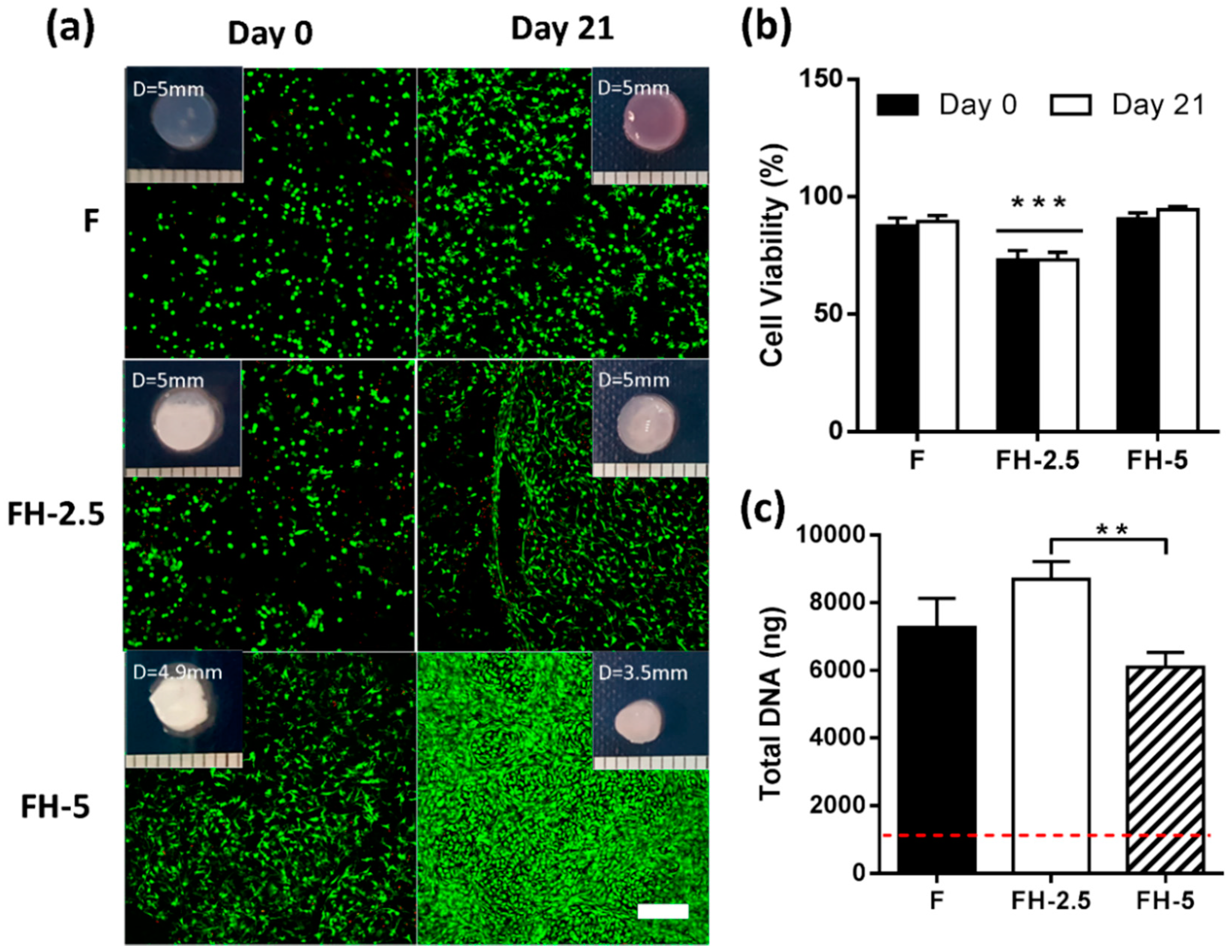
2.2.2. Fibrin-Based Hydrogels Incorporating Hyaluronic Acid Promote Chondrocyte Proliferation with Enhanced Cell Viability and Increased Cell Spreading
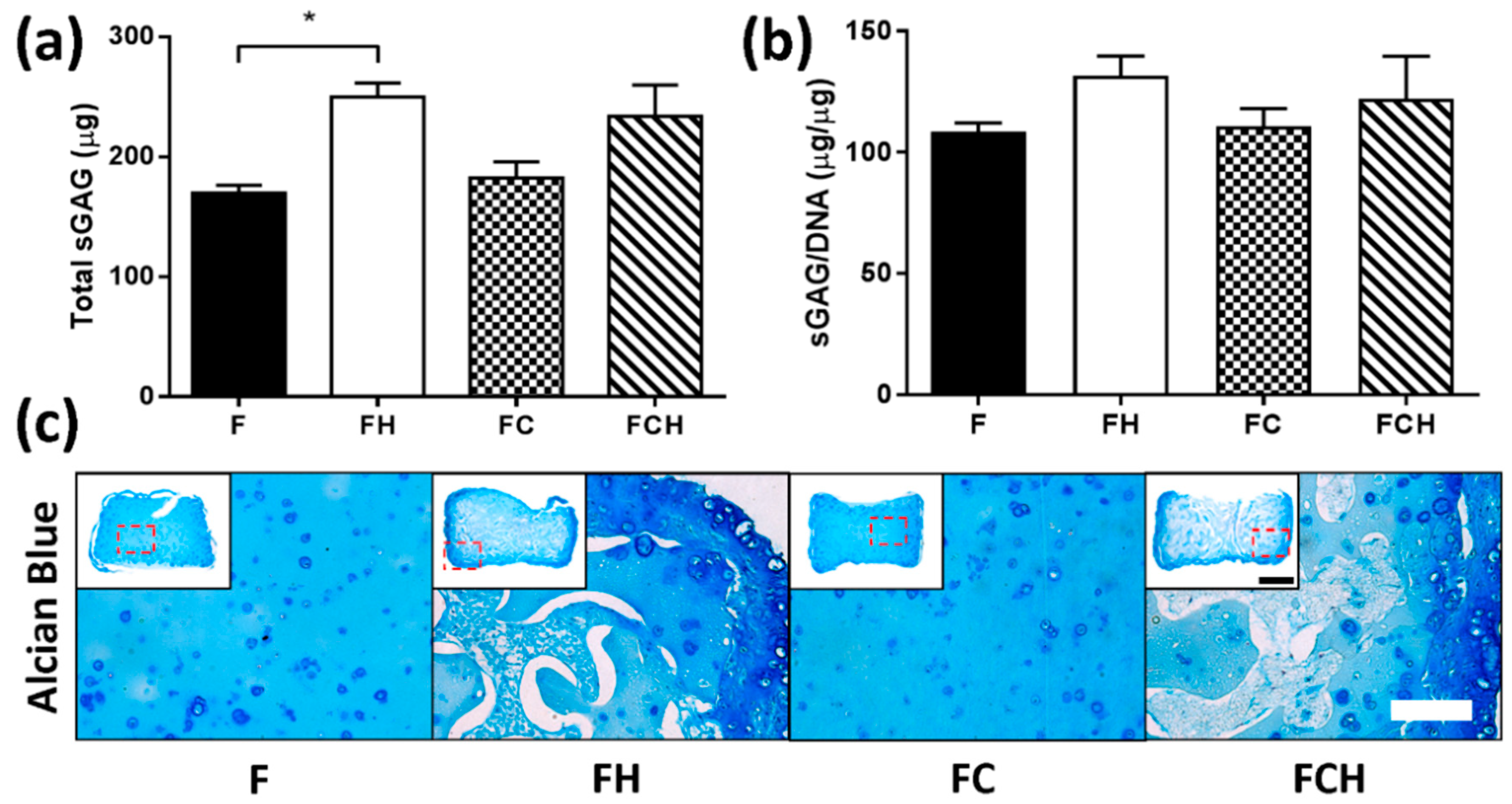
2.2.3. Hyaluronic Acid Enhances sGAG Accumulation and Increases sGAG/Collagen Ratio
2.3. Stage 3—Effect of Increasing Hyaluronic Acid Concentration in Fibrin-Based Hydrogels
2.3.1. Increasing Concentrations of Hyaluronic Acid Do Not Appear to Enhance Cell Viability or Proliferation
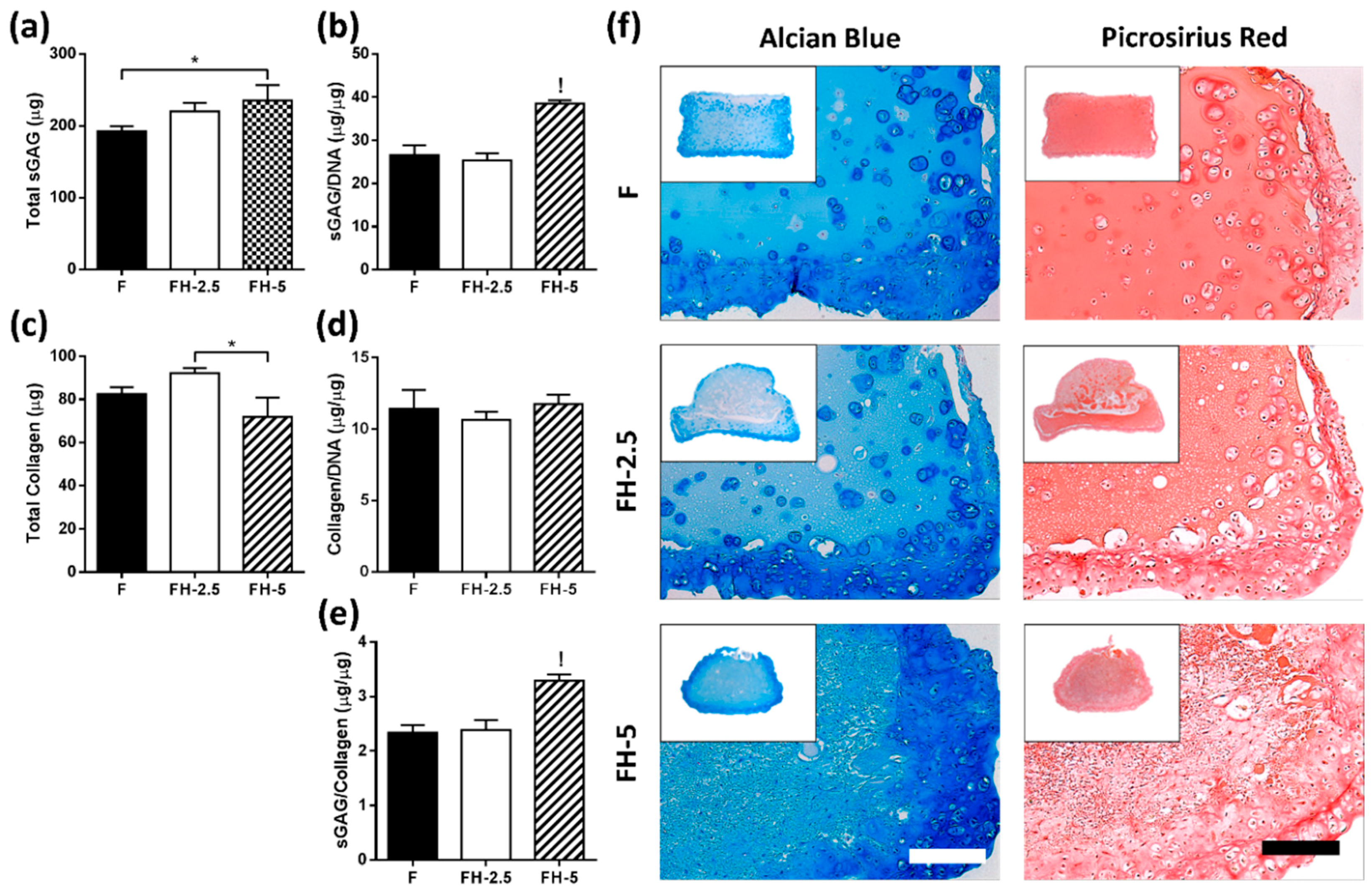
2.3.2. Hyaluronic Acid Enhances sGAG and Collagen Deposition in the Periphery of Constructs
3. Discussion
3.1. Lower Fibrinogen Concentration Results in Hydrogel Contraction
3.2. Higher Fibrin Concentrations Enhance Proliferation
3.3. Hyaluronic Acid Supports Cell Spreading, Proliferation, and the Accumulation of Proteoglycan-Rich Matrix
3.4. Collagen Accumulation Is Enhanced in Fibrin–Collagen Hydrogels
3.5. Hyaluronic Acid Appears to Supress Collagen ECM Accumulation
3.6. Incorporation of 5 mg/mL HMW Hyaluronic Acid into Fibrin Matrices Enhances sGAG/Collagen Ratio
4. Materials and Methods
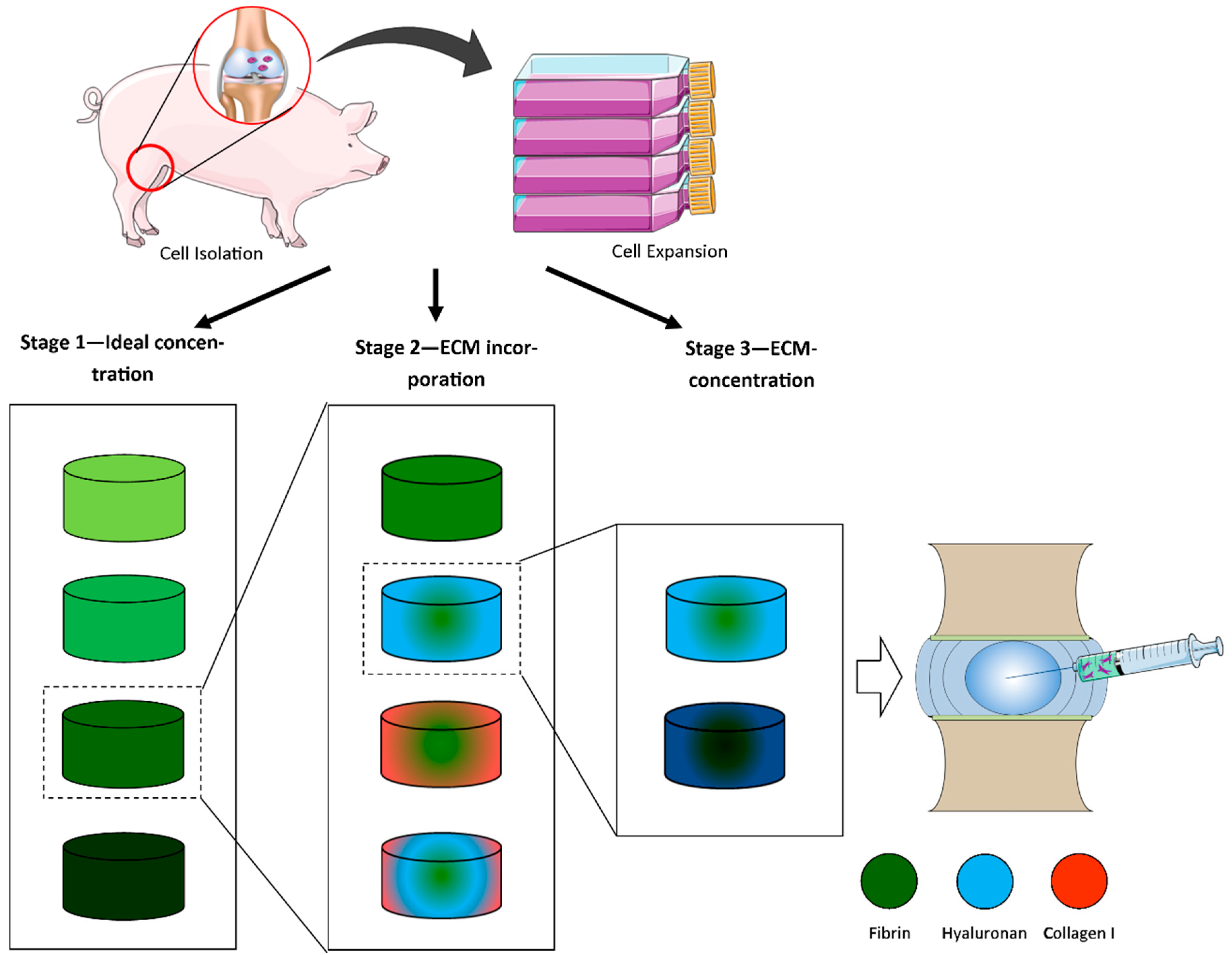
4.1. Study Design
4.2. Hydrogel Fabrication
4.2.1. Preparation of Fibrin Hydrogels with Various Concentrations of Fibrinogen
4.2.2. Fibrin Hydrogels Containing ECM Components (Collagen, Hyaluronic Acid)
4.2.3. Fibrin-Based Hydrogels with Increasing Hyaluronic Acid Concentrations
4.3. Chondrocyte Isolation and Monolayer Expansion
4.4. Cell Encapsulation
4.4.1. Varied Fibrin Concentration Hydrogels
4.4.2. Fibrin–Collagen and Fibrin–Hyaluronic Acid–Collagen Hydrogels
4.4.3. Fibrin–Hyaluronic Acid Hydrogels
4.5. Culture of Chondrocyte Laden Fibrin-Based Constructs
4.6. Determination of Hydrogel Contraction
4.7. Live/Dead Analysis
4.8. Cell Shape Analysis
4.9. Quantitative Biochemical Analysis
4.10. Histology
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hoy, D.; Brooks, P.; Blyth, F.; Buchbinder, R. The epidemiology of low back pain. Best Pract. Res. Clin. Rheumatol. 2010, 24, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Deyo, R.A.; Weinstein, J.N. Low back pain. N. Engl. J. Med. 2001, 344, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Meisel, H.J.; Siodla, V.; Ganey, T.; Minkus, Y.; Hutton, W.C.; Alasevic, O.J. Clinical experience in cell-based therapeutics: Disc chondrocyte transplantation a treatment for degenerated or damaged intervertebral disc. Biomol. Eng. 2007, 24, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Hegewald, A.A.; Endres, M.; Abbushi, A.; Cabraja, M.; Woiciechowsky, C.; Schmieder, K.; Kaps, C.; Thome, C. Adequacy of herniated disc tissue as a cell source for nucleus pulposus regeneration. J. Neurosurg. Spine 2011, 14, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Masuda, K. Biological repair of the degenerated intervertebral disc by the injection of growth factors. Eur. Spine J. 2008, 17 (Suppl. 4), 441–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coric, D.; Kim, P.K.; Clemente, J.D.; Boltes, M.O.; Nussbaum, M.; James, S. Prospective randomized study of cervical arthroplasty and anterior cervical discectomy and fusion with long-term follow-up: Results in 74 patients from a single site. J. Neurosurg. Spine 2013, 18, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Acosta, F.L., Jr.; Metz, L.; Adkisson, H.D.; Liu, J.; Carruthers-Liebenberg, E.; Milliman, C.; Maloney, M.; Lotz, J.C. Porcine intervertebral disc repair using allogeneic juvenile articular chondrocytes or mesenchymal stem cells. Tissue Eng. Part A 2011, 17, 3045–3055. [Google Scholar] [CrossRef] [PubMed]
- Hohaus, C.; Ganey, T.M.; Minkus, Y.; Meisel, H.J. Cell transplantation in lumbar spine disc degeneration disease. Eur. Spine J. 2008, 17 (Suppl. 4), 492–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allon, A.A.; Butcher, K.; Schneider, R.A.; Lotz, J.C. Structured coculture of mesenchymal stem cells and disc cells enhances differentiation and proliferation. Cells Tissues Organs 2012, 196, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Zhao, X.; Liu, H.; Zhang, H.; Chen, X.; Shi, R.; Liu, X.; Zhao, X.; Zhang, W.; Wang, B. Transplantation of mesenchymal stem cells and nucleus pulposus cells in a degenerative disc model in rabbits: A comparison of 2 cell types as potential candidates for disc regeneration. J. Neurosurg. Spine 2011, 14, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Omlor, G.W.; Bertram, H.; Kleinschmidt, K.; Fischer, J.; Brohm, K.; Guehring, T.; Anton, M.; Richter, W. Methods to monitor distribution and metabolic activity of mesenchymal stem cells following in vivo injection into nucleotomized porcine intervertebral discs. Eur. Spine J. 2010, 19, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Bertram, H.; Kroeber, M.; Wang, H.; Unglaub, F.; Guehring, T.; Carstens, C.; Richter, W. Matrix-assisted cell transfer for intervertebral disc cell therapy. Biochem. Biophys. Res. Commun. 2005, 331, 1185–1192. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Chen, C.; Liu, W.; Fu, Q.; Han, Z.; Li, Y.; Feng, S.; Li, X.; Qi, C.; Wu, J.; et al. Injectable microcryogels reinforced alginate encapsulation of mesenchymal stromal cells for leak-proof delivery and alleviation of canine disc degeneration. Biomaterials 2015, 59, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Murua, A.; Portero, A.; Orive, G.; Hernandez, R.M.; de Castro, M.; Pedraz, J.L. Cell microencapsulation technology: Towards clinical application. J. Control. Release 2008, 132, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.J.; Smith, M.K.; Mooney, D.J. Designing alginate hydrogels to maintain viability of immobilized cells. Biomaterials 2003, 24, 4023–4029. [Google Scholar] [CrossRef]
- Naqvi, S.M.; Buckley, C.T. Differential response of encapsulated nucleus pulposus and bone marrow stem cells in isolation and coculture in alginate and chitosan hydrogels. Tissue Eng. Part A 2015, 21, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Parmar, P.A.; Chow, L.W.; St-Pierre, J.P.; Horejs, C.M.; Peng, Y.Y.; Werkmeister, J.A.; Ramshaw, J.A.; Stevens, M.M. Collagen-mimetic peptide-modifiable hydrogels for articular cartilage regeneration. Biomaterials 2015, 54, 213–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rafat, M.; Xeroudaki, M.; Koulikovska, M.; Sherrell, P.; Groth, F.; Fagerholm, P.; Lagali, N. Composite core-and-skirt collagen hydrogels with differential degradation for corneal therapeutic applications. Biomaterials 2016, 83, 142–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eyrich, D.; Brandl, F.; Appel, B.; Wiese, H.; Maier, G.; Wenzel, M.; Staudenmaier, R.; Goepferich, A.; Blunk, T. Long-term stable fibrin gels for cartilage engineering. Biomaterials 2007, 28, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Colombini, A.; Lopa, S.; Ceriani, C.; Lovati, A.B.; Croiset, S.J.; Di Giancamillo, A.; Lombardi, G.; Banfi, G.; Moretti, M. In vitro characterization and in vivo behavior of human nucleus pulposus and annulus fibrosus cells in clinical-grade fibrin and collagen-enriched fibrin gels. Tissue Eng. Part A 2015, 21, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Rowley, J.A.; Madlambayan, G.; Mooney, D.J. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials 1999, 20, 45–53. [Google Scholar] [CrossRef]
- Bae, K.H.; Lee, F.; Xu, K.; Keng, C.T.; Tan, S.Y.; Tan, Y.J.; Chen, Q.; Kurisawa, M. Microstructured dextran hydrogels for burst-free sustained release of pegylated protein drugs. Biomaterials 2015, 63, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, F.Y.; Lin, H.H.; Hsu, S.H. 3d bioprinting of neural stem cell-laden thermoresponsive biodegradable polyurethane hydrogel and potential in central nervous system repair. Biomaterials 2015, 71, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.; Fabry, B.; Boccaccini, A.R. Fibrous protein-based hydrogels for cell encapsulation. Biomaterials 2014, 35, 6727–6738. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Dewi, R.E.; Heilshorn, S.C. Injectable hydrogels with in situ double network formation enhance retention of transplanted stem cells. Adv. Funct. Mater. 2015, 25, 1344–1351. [Google Scholar] [CrossRef] [PubMed]
- Eyrich, D.; Göpferich, A.; Blunk, T. Fibrin in tissue engineering. In Tissue Engineering; Springer: Boston, MA, USA, 2006; pp. 379–392. [Google Scholar]
- MacGillivray, T.E. Fibrin sealants and glues. J. Card. Surg. 2003, 18, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Fattahi, T.; Mohan, M.; Caldwell, G.T. Clinical applications of fibrin sealants. J. Oral Maxillofac. Surg. 2004, 62, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Bensaid, W.; Triffitt, J.T.; Blanchat, C.; Oudina, K.; Sedel, L.; Petite, H. A biodegradable fibrin scaffold for mesenchymal stem cell transplantation. Biomaterials 2003, 24, 2497–2502. [Google Scholar] [CrossRef]
- Cox, S.; Cole, M.; Tawil, B. Behavior of human dermal fibroblasts in three-dimensional fibrin clots: Dependence on fibrinogen and thrombin concentration. Tissue Eng. 2004, 10, 942–954. [Google Scholar] [CrossRef] [PubMed]
- Gille, J.; Meisner, U.; Ehlers, E.; Müller, A.; Russlies, M.; Behrens, P. Migration pattern, morphology and viability of cells suspended in or sealed with fibrin glue: A histomorphologic study. Tissue Cell 2005, 37, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Sporn, L.A.; Bunce, L.A.; Francis, C.W. Cell proliferation on fibrin: Modulation by fibrinopeptide cleavage. Blood 1995, 86, 1802–1810. [Google Scholar] [PubMed]
- Homminga, G.N.; Buma, P.; Koot, H.W.; van der Kraan, P.M.; van den Berg, W.B. Chondrocyte behavior in fibrin glue in vitro. Acta Orthop. Scand. 1993, 64, 441–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Kaplan, K.M.; Wertzel, A.; Peroglio, M.; Amit, B.; Alini, M.; Grad, S.; Yayon, A. Biomimetic fibrin-hyaluronan hydrogels for nucleus pulposus regeneration. Regen. Med. 2014, 9, 309–326. [Google Scholar] [CrossRef] [PubMed]
- Stern, S.; Lindenhayn, K.; Perka, C. Human intervertebral disc cell culture for disc disorders. Clin. Orthop. Relat. Res. 2004, 419, 238–244. [Google Scholar] [CrossRef]
- Hegewald, A.A.; Enz, A.; Endres, M.; Sittinger, M.; Woiciechowsky, C.; Thome, C.; Kaps, C. Engineering of polymer-based grafts with cells derived from human nucleus pulposus tissue of the lumbar spine. J. Tissue Eng. Regen. Med. 2011, 5, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Stern, S.; Lindenhayn, K.; Schultz, O.; Perka, C. Cultivation of porcine cells from the nucleus pulposus in a fibrin/hyaluronic acid matrix. Acta Orthop. Scand. 2000, 71, 496–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buser, Z.; Liu, J.; Thorne, K.J.; Coughlin, D.; Lotz, J.C. Inflammatory response of intervertebral disc cells is reduced by fibrin sealant scaffold in vitro. J. Tissue Eng. Regen. Med. 2014, 8, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Hunter, C.J.; Mouw, J.K.; Levenston, M.E. Dynamic compression of chondrocyte-seeded fibrin gels: Effects on matrix accumulation and mechanical stiffness. Osteoarthr. Cartil./OARS Osteoarthr. Res. Soc. 2004, 12, 117–130. [Google Scholar] [CrossRef]
- Ho, W.; Tawil, B.; Dunn, J.C.; Wu, B.M. The behavior of human mesenchymal stem cells in 3d fibrin clots: Dependence on fibrinogen concentration and clot structure. Tissue Eng. 2006, 12, 1587–1595. [Google Scholar] [CrossRef] [PubMed]
- Rowe, S.L.; Lee, S.; Stegemann, J.P. Influence of thrombin concentration on the mechanical and morphological properties of cell-seeded fibrin hydrogels. Acta Biomater. 2007, 3, 59–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorodetsky, R.; Levdansky, L.; Gaberman, E.; Gurevitch, O.; Lubzens, E.; McBride, W.H. Fibrin microbeads loaded with mesenchymal cells support their long-term survival while sealed at room temperature. Tissue Eng. Part C Methods 2011, 17, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Bertolo, A.; Mehr, M.; Aebli, N.; Baur, M.; Ferguson, S.J.; Stoyanov, J.V. Influence of different commercial scaffolds on the in vitro differentiation of human mesenchymal stem cells to nucleus pulposus-like cells. Eur. Spine J. 2012, 21 (Suppl. 6), S826–S838. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.N.; Birkinshaw, C. Hyaluronic acid based scaffolds for tissue engineering—A review. Carbohydr. Polym. 2013, 92, 1262–1279. [Google Scholar] [CrossRef] [PubMed]
- Kotlarchyk, M.A.; Shreim, S.G.; Alvarez-Elizondo, M.B.; Estrada, L.C.; Singh, R.; Valdevit, L.; Kniazeva, E.; Gratton, E.; Putnam, A.J.; Botvinick, E.L. Concentration independent modulation of local micromechanics in a fibrin gel. PLoS ONE 2011, 6, e20201. [Google Scholar] [CrossRef] [PubMed]
- Duong, H.; Wu, B.; Tawil, B. Modulation of 3d fibrin matrix stiffness by intrinsic fibrinogen-thrombin compositions and by extrinsic cellular activity. Tissue Eng. Part A 2009, 15, 1865–1876. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.L.; Hecht, V.; Duong, H.; Wu, B.; Tawil, B. Permeability of three-dimensional fibrin constructs corresponds to fibrinogen and thrombin concentrations. BioRes. Open Access 2012, 1, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Alves, C.S.; Yakovlev, S.; Medved, L.; Konstantopoulos, K. Biomolecular characterization of cd44-fibrin(ogen) binding: Distinct molecular requirements mediate binding of standard and variant isoforms of cd44 to immobilized fibrin(ogen). J. Biol. Chem. 2009, 284, 1177–1189. [Google Scholar] [CrossRef] [PubMed]
- Alves, C.S.; Burdick, M.M.; Thomas, S.N.; Pawar, P.; Konstantopoulos, K. The dual role of cd44 as a functional p-selectin ligand and fibrin receptor in colon carcinoma cell adhesion. Am. J. Physiol. Cell Physiol. 2008, 294, C907–C916. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, D.J.; Knudson, W.; Knudson, C.B. Internalization of the hyaluronan receptor cd44 by chondrocytes. Exp. Cell Res. 1999, 252, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Chow, G.; Knudson, C.B.; Homandberg, G.; Knudson, W. Increased expression of cd44 in bovine articular chondrocytes by catabolic cellular mediators. J. Biol. Chem. 1995, 270, 27734–27741. [Google Scholar] [CrossRef] [PubMed]
- Ramos, T.L.; Sanchez-Abarca, L.I.; Muntion, S.; Preciado, S.; Puig, N.; Lopez-Ruano, G.; Hernandez-Hernandez, A.; Redondo, A.; Ortega, R.; Rodriguez, C.; et al. Msc surface markers (cd44, cd73, and cd90) can identify human msc-derived extracellular vesicles by conventional flow cytometry. Cell Commun. Signal. CCS 2016, 14, 2. [Google Scholar] [CrossRef] [PubMed]
- Zoller, M. Cd44, hyaluronan, the hematopoietic stem cell, and leukemia-initiating cells. Front. Immunol. 2015, 6, 235. [Google Scholar] [PubMed]
- Knudson, C.B.; Knudson, W. Hyaluronan and cd44: Modulators of chondrocyte metabolism. Clin. Orthop. Relat. Res. 2004, 427, S152–S162. [Google Scholar] [CrossRef]
- Liu, D.; Liu, T.; Li, R.; Sy, M.S. Mechanisms regulating the binding activity of cd44 to hyaluronic acid. Front. Biosci. A J. Virtual Libr. 1998, 3, d631–d636. [Google Scholar]
- Underhill, C.B.; Toole, B.P. Physical characteristics of hyaluronate binding to the surface of simian virus 40-transformed 3t3 cells. J. Biol. Chem. 1980, 255, 4544–4549. [Google Scholar] [PubMed]
- Raja, R.H.; McGary, C.T.; Weigel, P.H. Affinity and distribution of surface and intracellular hyaluronic acid receptors in isolated rat liver endothelial cells. J. Biol. Chem. 1988, 263, 16661–16668. [Google Scholar] [PubMed]
- Lesley, J.; Hascall, V.C.; Tammi, M.; Hyman, R. Hyaluronan binding by cell surface cd44. J. Biol. Chem. 2000, 275, 26967–26975. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Choi, J.C.; Shim, J.H.; Lee, J.S.; Park, H.; Kim, S.W.; Doh, J.; Cho, D.W. A comparative study on collagen type i and hyaluronic acid dependent cell behavior for osteochondral tissue bioprinting. Biofabrication 2014, 6, 035004. [Google Scholar] [CrossRef] [PubMed]
- Kurtis, M.S.; Schmidt, T.A.; Bugbee, W.D.; Loeser, R.F.; Sah, R.L. Integrin-mediated adhesion of human articular chondrocytes to cartilage. Arthritis Rheumat. 2003, 48, 110–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reyhani, V.; Seddigh, P.; Guss, B.; Gustafsson, R.; Rask, L.; Rubin, K. Fibrin binds to collagen and provides a bridge for alphavbeta3 integrin-dependent contraction of collagen gels. Biochem. J. 2014, 462, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Rowe, S.L.; Institute, R.P. Mechanical Characterization of Collagen-Fibrin Composites for Vascular Tissue Engineering; Rensselaer Polytechnic Institute: Troy, NY, USA, 2007. [Google Scholar]
- Stegemann, J.P.; Cummings, C.L.; Gawlitta, D.; Nerem, R.M. Collagen, fibrin and collagen-fibrin mixtures as matrix materials for vascular tissue engineering. In Proceedings of the Second Joint 24th Annual Conference and the Annual Fall Meeting of the Biomedical Engineering Society] [Engineering in Medicine and Biology, Houston, TX, USA, 23–26 October 2002; Volume 811, pp. 817–818. [Google Scholar]
- Sarath Babu, N.; Krishnan, S.; Brahmendra Swamy, C.V.; Venkata Subbaiah, G.P.; Gurava Reddy, A.V.; Idris, M.M. Quantitative proteomic analysis of normal and degenerated human intervertebral disc. Spine J. 2016, 16, 989–1000. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, C.; Jin, G.-Z.; Kim, H.-W. Alginate-hyaluronic acid-collagen composite hydrogel favorable for the culture of chondrocytes and their phenotype maintenance. Tissue Eng. Regen. Med. 2016, 13, 538–546. [Google Scholar] [CrossRef]
- Akmal, M.; Singh, A.; Anand, A.; Kesani, A.; Aslam, N.; Goodship, A.; Bentley, G. The effects of hyaluronic acid on articular chondrocytes. J. Bone Jt. Surg. Br. Vol. 2005, 87, 1143–1149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindenhayn, K.; Perka, C.; Spitzer, R.; Heilmann, H.; Pommerening, K.; Mennicke, J.; Sittinger, M. Retention of hyaluronic acid in alginate beads: Aspects for in vitro cartilage engineering. J. Biomed. Mater. Res. 1999, 44, 149–155. [Google Scholar] [CrossRef]
- Kafienah, W.; Sims, T.J. Biochemical methods for the analysis of tissue-engineered cartilage. Methods Mol. Biol. 2004, 238, 217–230. [Google Scholar] [PubMed]
- Ignat’eva, N.Y.; Danilov, N.; Averkiev, S.; Obrezkova, M.; Lunin, V.; Sobol, E. Determination of hydroxyproline in tissues and the evaluation of the collagen content of the tissues. J. Anal. Chem. 2007, 62, 51–57. [Google Scholar] [CrossRef]

| Fibrinogen Concentration (mg/mL) | Fibrinogen: Thrombin Ratio | Final Fibrinogen Concentration (mg/mL) |
|---|---|---|
| 25 | 1:1 | 12.5 |
| 50 | 1:1 | 25 |
| 75 | 1:1 | 37.5 |
| 100 | 1:1 | 50 |
| Abbreviation | Final Fibrin Conc. (mg/mL) | Col Conc. (mg/mL) | Hyaluronic Acid Conc. (mg/mL) | |
|---|---|---|---|---|
| Fibrin | F | 50 | ||
| Fibrin–HA | FH | 50 | 1.5 | |
| Fibrin–COL | FC | 50 | 1.33 | |
| Fibrin–Col–HA | FCH | 50 | 1.33 | 1.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gansau, J.; Buckley, C.T. Incorporation of Collagen and Hyaluronic Acid to Enhance the Bioactivity of Fibrin-Based Hydrogels for Nucleus Pulposus Regeneration. J. Funct. Biomater. 2018, 9, 43. https://doi.org/10.3390/jfb9030043
Gansau J, Buckley CT. Incorporation of Collagen and Hyaluronic Acid to Enhance the Bioactivity of Fibrin-Based Hydrogels for Nucleus Pulposus Regeneration. Journal of Functional Biomaterials. 2018; 9(3):43. https://doi.org/10.3390/jfb9030043
Chicago/Turabian StyleGansau, Jennifer, and Conor Timothy Buckley. 2018. "Incorporation of Collagen and Hyaluronic Acid to Enhance the Bioactivity of Fibrin-Based Hydrogels for Nucleus Pulposus Regeneration" Journal of Functional Biomaterials 9, no. 3: 43. https://doi.org/10.3390/jfb9030043