Carbon Nano-Allotrope/Magnetic Nanoparticle Hybrid Nanomaterials as T2 Contrast Agents for Magnetic Resonance Imaging Applications
Abstract
:1. Introduction
2. CNT-MNP Hybrid MRI Contrast Agents
2.1. CNTs with Inherent Magnetic Catalyst Residues
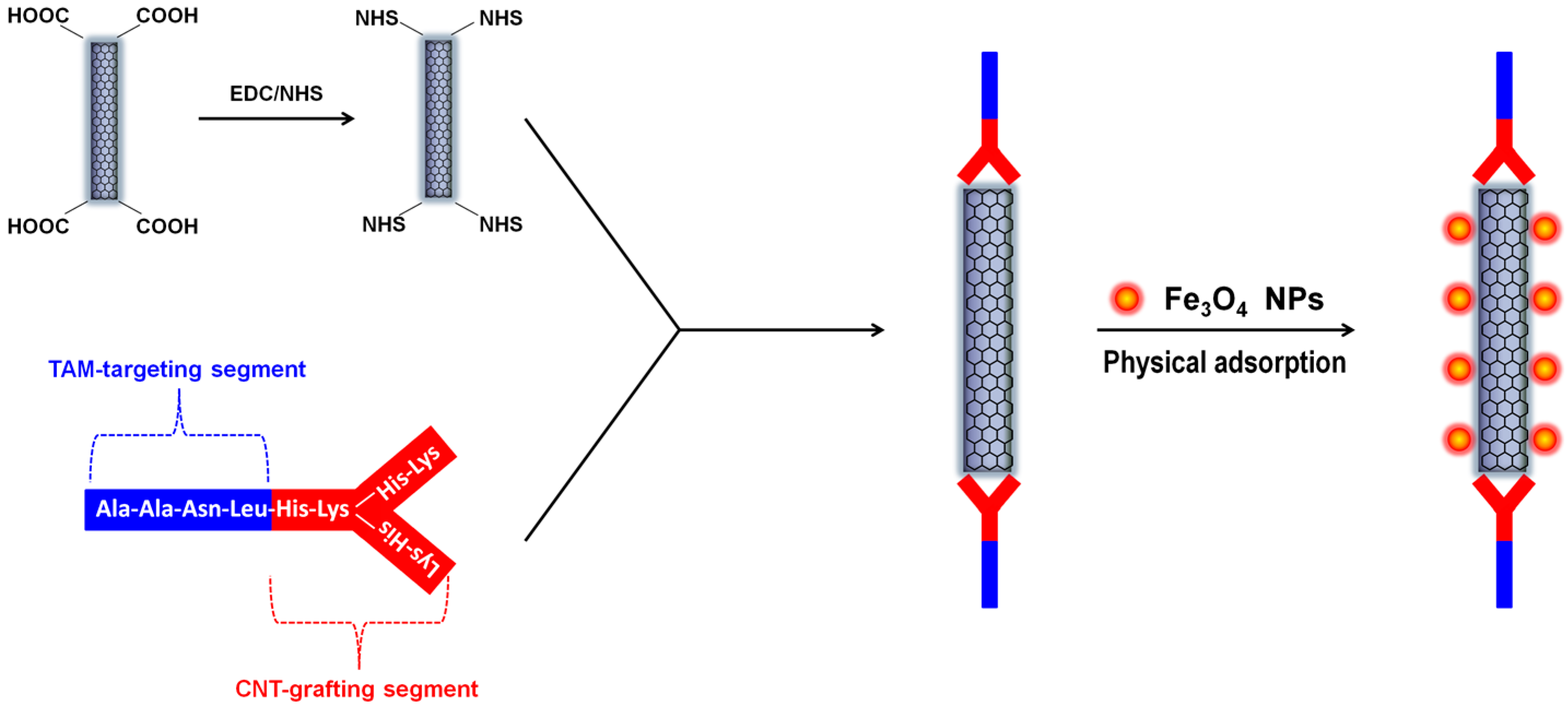
2.2. CNTs with Postsynthesis-Loaded Iron Oxide Nanoparticles
2.3. CNTs with Postsynthesis-Loaded Metal Ions, Metal Alloy, and Mixed-Metal Oxide Nanoparticles
3. Graphene-MNP Hybrid MRI Contrast Agents
4. MNPs Loaded on Other Carbon Nano-Allotropes
5. Carbon-MNP Hybrid MRI Contrast Agents Integrated with Multiple Functions
5.1. Carbon-MNP Hybrid MRI Agents with both T1 and T2 Contrast
5.2. Carbon-MNP Hybrid MRI Contrast Agents with Multimodal Imaging Capability
5.3. Carbon-MNP Hybrid MRI Contrast Agents with Drug Delivery Capability
5.4. Carbon-MNP Hybrid MRI Contrast Agents with Hypothermia Capability
6. Conclusions and Future Perspectives
Conflicts of Interest
Abbreviation
| Abbreviation | Label |
| CA | Contrast agent |
| CNF | Carbon nanofibre |
| CNT | Carbon nanotube |
| CT | Computed tomography |
| DAPI | Diamidino-2-phenylindole |
| DOX | Doxorubicin |
| DWNT | Double-walled carbon nanotube |
| FC | Fluorescent carbon |
| FITC | Fluorescein isothiocyanate |
| GC | Graphitic carbon |
| GNT | Gadolinium-loaded CNT |
| GO | Graphene oxide |
| GQD | Graphene quantum dot |
| hTERT | Human telomerase reverse transcriptase |
| LH | Lidocaine hydrochloride |
| MCF-7 | Michigan Cancer Foundation-7 |
| MDC | Monodansylcadaverine |
| MGQD | Magnetic graphene quantum dot |
| MNP | Magnetic nanoparticle |
| MR | Magnetic resonance |
| MRI | Magnetic resonance imaging |
| MWNT | Multi-walled carbon nanotube |
| NIR | Near-infrared |
| NP | Nanoparticle |
| OCNT | Oxidized carbon nanotube |
| PEG | Polyethylene glycol |
| PEI | Polyethyleneimine |
| PET | Positron emission tomography |
| PTT | Photothermal therapy |
| QD | Quantum dot |
| RF | Radio frequency |
| RGO | Reduced graphene oxide |
| RNA | Ribonucleic acid |
| si-RNA | Small interfering RNA |
| SPECT | Single-photon emission computed tomography |
| SPION | Superparamagnetic iron oxide nanoparticle |
| SWNT | Single-walled carbon nanotube |
| SWNT-COOH | SWNTs grafted with carboxylic acid groups |
| TAI | Thermoacoustic imaging |
| TAM | Tumor-associated macrophage |
References
- Sitharaman, B.; Kissell, K.R.; Hartman, K.B.; Tran, L.A.; Baikalov, A.; Rusakova, I.; Sun, Y.; Khant, H.A.; Ludtke, S.J.; Chiu, W.; et al. Superparamagnetic gadonanotubes are high-performance MRI contrast agents. Chem. Commun. 2005, 3915–3917. [Google Scholar] [CrossRef] [PubMed]
- Azria, D.; Blanquer, S.; Verdier, J.M.; Belamie, E. Nanoparticles as contrast agents for brain nuclear magnetic resonance imaging in alzheimer’s disease diagnosis. J. Mater. Chem. B 2017, 5, 7216–7237. [Google Scholar] [CrossRef]
- Xia, Y.; Matham, M.V.; Su, H.B.; Padmanabhan, P.; Gulyas, B. Nanoparticulate contrast agents for multimodality molecular imaging. J. Biomed. Nanotechnol. 2016, 12, 1553–1584. [Google Scholar] [CrossRef] [PubMed]
- Gitanjali, A.; Brahmkhatri, V.P.; Atreya, H.S. Nanomaterial based magnetic resonance imaging of cancer. J. Indian Inst. Sci. 2014, 94, 423–453. [Google Scholar]
- Ahmad, R.S.; Ali, Z.S.; Mou, X.B.; Wang, J.H.; Yi, H.; He, N.Y. Recent advances in magnetic nanoparticle design for cancer therapy. J. Nanosci. Nanotechnol. 2016, 16, 9393–9403. [Google Scholar] [CrossRef]
- Hajba, L.; Guttman, A. The use of magnetic nanoparticles in cancer theranostics: Toward handheld diagnostic devices. Biotechnol. Adv. 2016, 34, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.; Yildirimer, L.; Rajadas, J.; De La Pena, H.; Pastorin, G.; Seifalian, A. Quantum dots and carbon nanotubes in oncology: A review on emerging theranostic applications in nanomedicine. Nanomedicine 2011, 6, 1101–1114. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.; Huh, M.S.; Sun, I.C.; Yuk, S.H.; Choi, K.; Kim, K.; Kwon, I.C. In vivo targeted delivery of nanoparticles for theranosis. Acc. Chem. Res. 2011, 44, 1018–1028. [Google Scholar] [CrossRef] [PubMed]
- Song, X.R.; Wang, X.Y.; Yu, S.X.; Cao, J.B.; Li, S.H.; Li, J.; Liu, G.; Yang, H.H.; Chen, X.Y. Co9Se8 nanoplates as a new theranostic platform for photoacoustic/magnetic resonance dual-modal-imaging-guided chemo-photothermal combination therapy. Adv. Mater. 2015, 27, 3285–3291. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, B.; Aswathy, R.G.; Romero-Aburto, R.; Mitcham, T.; Mitchel, K.A.; Nagaoka, Y.; Bouchard, R.R.; Ajayan, P.M.; Maekawa, T.; Sakthikumar, D.N. Highly versatile SPION encapsulated PLGA nanoparticles as photothermal ablators of cancer cells and as multimodal imaging agents. Biomater. Sci. 2017, 5, 432–443. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, K.; Mukherjee, S.P.; Gallud, A.; Burkert, S.C.; Bistarelli, S.; Bellucci, S.; Bottini, M.; Star, A.; Fadeel, B. Biological interactions of carbon-based nanomaterials: From coronation to degradation. Nanomed. Nanotechnol. 2016, 12, 333–351. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Liu, Z. Carbon nanotubes in biology and medicine: An overview. Chin. Sci. Bull. 2012, 57, 167–180. [Google Scholar] [CrossRef]
- Liu, Z.A.; Yang, K.; Lee, S.T. Single-walled carbon nanotubes in biomedical imaging. J. Mater. Chem. 2011, 21, 586–598. [Google Scholar] [CrossRef]
- Chen, D.Q.; Dougherty, C.A.; Zhu, K.C.; Hong, H. Theranostic applications of carbon nanomaterials in cancer: Focus on imaging and cargo delivery. J. Control. Release 2015, 210, 230–245. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Peng, R.; Liu, Z. Carbon nanotubes for biomedical imaging: The recent advances. Adv. Drug Deliver. Rev. 2013, 65, 1951–1963. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.; Park, S.H.; Lee, J.W. Applications of functionalized carbon nanotubes for the therapy and diagnosis of cancer. Polymers 2017, 9, 13. [Google Scholar] [CrossRef]
- Passeri, D.; Rinaldi, F.; Ingallina, C.; Carafa, M.; Rossi, M.; Terranova, M.L.; Marianecci, C. Biomedical applications of nanodiamonds: An overview. J. Nanosci. Nanotechnol. 2015, 15, 972–988. [Google Scholar] [CrossRef] [PubMed]
- Boncel, S.; Herman, A.P.; Walczak, K.Z. Magnetic carbon nanostructures in medicine. J. Mater. Chem. 2012, 22, 31–37. [Google Scholar] [CrossRef]
- Yan, L.; Gao, Y.; Pierce, R.; Dai, L.; Kim, J.; Zhang, M. Development of Y-shaped peptide for constructing nanoparticle systems targeting tumor-associated macrophages in vitro and in vivo. Mater. Res. Express 2014, 1, 025007. [Google Scholar] [CrossRef]
- Kuznik, N.; Tomczyk, M.M. Multiwalled carbon nanotube hybrids as MRI contrast agents. Beilstein J. Nanotechnol. 2016, 7, 1086–1103. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.F.; Zhu, L.; Yang, K.; Zhuang, R.Q.; Xie, J.; Zhang, F. Diffusion-weighted magnetic resonance imaging for therapy response monitoring and early treatment prediction of photothermal therapy. ACS Appl. Mater. Interfaces 2016, 8, 5137–5147. [Google Scholar] [CrossRef] [PubMed]
- Gizzatov, A.; Keshishian, V.; Guven, A.; Dimiev, A.M.; Qu, F.F.; Muthupillai, R.; Decuzzi, P.; Bryant, R.G.; Tour, J.M.; Wilson, L.J. Enhanced MRI relaxivity of aquated Gd3+ ions by carboxyphenylated water-dispersed graphene nanoribbons. Nanoscale 2014, 6, 3059–3063. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Yang, X.M.; Ren, J.X.; Wang, Y.C.; Zhang, H.J.; Feng, Q.H.; Shi, Y.Y.; Shan, X.N.; Yuan, Y.J.; Zhang, Z.Z. A novel redox-sensitive system based on single-walled carbon nanotubes for chemo-photothermal therapy and magnetic resonance imaging. Int. J. Nanomed. 2016, 11, 607–624. [Google Scholar]
- Hou, L.; Zhang, H.J.; Wang, Y.T.; Wang, L.L.; Yang, X.M.; Zhang, Z.Z. Hyaluronic acid-functionalized single-walled carbon nanotubes as tumor-targeting MRI contrast agent. Int. J. Nanomed. 2015, 10, 4507–4520. [Google Scholar]
- Kobayashi, S.; Tsuruoka, S.; Usui, Y.; Haniu, H.; Aoki, K.; Takanashi, S.; Okamoto, M.; Nomura, H.; Tanaka, M.; Aiso, S.; et al. An advanced in situ imaging method using heavy metal-doped hollow tubes to evaluate the biokinetics of carbon nanotubes in vivo. NPG Asia Mater. 2015, 7, e203. [Google Scholar] [CrossRef]
- Lalwani, G.; Sundararaj, J.L.; Schaefer, K.; Button, T.; Sitharaman, B. Synthesis, characterization, in vitro phantom imaging, and cytotoxicity of a novel graphene-based multimodal magnetic resonance imaging-X-ray computed tomography contrast agent. J. Mater. Chem. B 2014, 2, 3519–3530. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Liu, X.W.; Yao, Y.J.; Zhou, J.; Zhu, J.; Sun, G.; He, D.N. One-step synthesis of surface passivated carbon microspheres for enhanced photoluminescence and their application in multifunctional magnetic-fluorescent imaging. RSC Adv. 2015, 5, 24049–24055. [Google Scholar] [CrossRef]
- Liu, W.J.; Liu, G.X.; Dong, X.T.; Wang, J.X.; Yu, W.S. Multifunctional MWCNTs-NaGdF4:Yb3+,Er3+,Eu3+ hybrid nanocomposites with potential dual-mode luminescence, magnetism and photothermal properties. Phys. Chem. Chem. Phys. 2015, 17, 22659–22667. [Google Scholar] [CrossRef] [PubMed]
- Marangon, I.; Menard-Moyon, C.; Kolosnjaj-Tabi, J.; Beoutis, M.L.; Lartigue, L.; Alloyeau, D.; Pach, E.; Ballesteros, B.; Autret, G.; Ninjbadgar, T.; et al. Covalent functionalization of multi-walled carbon nanotubes with a gadolinium chelate for efficient T1-weighted magnetic resonance imaging. Adv. Funct. Mater. 2014, 24, 7173–7186. [Google Scholar]
- Wen, S.H.; Zhao, Q.H.; An, X.; Zhu, J.Y.; Hou, W.X.; Li, K.; Huang, Y.P.; Shen, M.W.; Zhu, W.; Shi, X.Y. Multifunctional PEGylated multiwalled carbon nanotubes for enhanced blood pool and tumor MR imaging. Adv. Healthc. Mater. 2014, 3, 1568–1577. [Google Scholar] [CrossRef] [PubMed]
- Cisneros, B.T.; Law, J.J.; Matson, M.L.; Azhdarinia, A.; Sevick-Muraca, E.M.; Wilson, L.J. Stable confinement of positron emission tomography and magnetic resonance agents within carbon nanotubes for bimodal imaging. Nanomedicine 2014, 9, 2499–2509. [Google Scholar] [CrossRef] [PubMed]
- Cui, R.L.; Li, J.; Huang, H.; Zhang, M.Y.; Guo, X.H.; Chang, Y.A.; Li, M.; Dong, J.Q.; Sun, B.Y.; Xing, G.M. Novel carbon nanohybrids as highly efficient magnetic resonance imaging contrast agents. Nano Res. 2015, 8, 1259–1268. [Google Scholar] [CrossRef]
- Guo, L.L.; Shi, H.L.; Wu, H.X.; Zhang, Y.X.; Wang, X.; Wu, D.M.; An, L.; Yang, S.P. Prostate cancer targeted multifunctionalized graphene oxide for magnetic resonance imaging and drug delivery. Carbon 2016, 107, 87–99. [Google Scholar] [CrossRef]
- Sitharaman, B.; Wilson, L.J. Gadofullerenes and gadonanotubes: A new paradigm for high-performance magnetic resonance imaging contrast agent probes. J. Biomed. Nanotechnol. 2007, 3, 342–352. [Google Scholar] [CrossRef]
- Wang, S.; Lin, Q.J.; Chen, J.T.; Gao, H.L.; Fu, D.L.; Shen, S. Biocompatible polydopamine-encapsulated gadolinium-loaded carbon nanotubes for MRI and color mapping guided photothermal dissection of tumor metastasis. Carbon 2017, 112, 53–62. [Google Scholar] [CrossRef]
- Xu, Y.; Jia, X.H.; Yin, X.B.; He, X.W.; Zhang, Y.K. Carbon quantum dot stabilized gadolinium nanoprobe prepared via a one-pot hydrothermal approach for magnetic resonance and fluorescence dual-modality bioimaging. Anal. Chem. 2014, 86, 12122–12129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wu, H.X.; Wang, J.; Yang, Y.; Wu, D.M.; Zhang, Y.J.; Zhang, Y.; Zhou, Z.G.; Yang, S.P. Graphene oxide-BaGdF5 nanocomposites for multi-modal imaging and photothermal therapy. Biomaterials 2015, 42, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Nikolaev, P.; Bronikowski, M.J.; Bradley, R.K.; Rohmund, F.; Colbert, D.T.; Smith, K.A.; Smalley, R.E. Gas-phase catalytic growth of single-walled carbon nanotubes from carbon monoxide. Chem. Phys. Lett. 1999, 313, 91–97. [Google Scholar] [CrossRef]
- Choi, J.H.; Nguyen, F.T.; Barone, P.W.; Heller, D.A.; Moll, A.E.; Patel, D.; Boppart, S.A.; Strano, M.S. Multimodal biomedical imaging with asymmetric single-walled carbon nanotube/iron oxide nanoparticle complexes. Nano Lett. 2007, 7, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Doan, B.T.; Seguin, J.; Breton, M.; Le Beherec, R.; Bessodes, M.; Rodriguez-Manzo, J.A.; Banhart, F.; Beloeil, J.C.; Scherman, D.; Richard, C. Functionalized single-walled carbon nanotubes containing traces of iron as new negative MRI contrast agents for in vivo imaging. Contrast Med. Mol. Imaging 2012, 7, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Al Faraj, A.; Shaik, A.S.; Al Sayed, B. Preferential magnetic targeting of carbon nanotubes to cancer sites: Noninvasive tracking using MRI in a murine breast cancer model. Nanomedicine 2015, 10, 931–948. [Google Scholar] [CrossRef] [PubMed]
- Baranowska-Korczyc, A.; Jasiurkowska-Delaporte, M.; Maciejewska, B.M.; Warowicka, A.; Coy, L.E.; Zalewski, T.; Koziol, K.K.; Jurga, S. PEG-MWCNT/Fe hybrids as multi-modal contrast agents for MRI and optical imaging. RSC Adv. 2016, 6, 49891–49902. [Google Scholar] [CrossRef]
- Syed, F.; Riggio, C.; Masini, M.; Bugliani, M.; Battaglia, V.; Novelli, M.; Suleiman, M.; Vittorio, O.; Boggi, U.; Filipponi, F.; et al. Labeling and tracking of human pancreatic islets using carbon nanotubes. J. Biomed. Nanotechnol. 2015, 11, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Vittorio, O.; Duce, S.L.; Pietrabissa, A.; Cuschieri, A. Multiwall carbon nanotubes as MRI contrast agents for tracking stem cells. Nanotechnology 2011, 22, 095706. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.Z.; Lou, C.G.; Qiu, J.S.; Zhao, Z.B.; Zhou, Q.; Liang, M.J.; Ji, Z.; Yang, S.H.; Xing, D. Targeted Fe-filled carbon nanotube as a multifunctional contrast agent for thermoacoustic and magnetic resonance imaging of tumor in living mice. Nanomed. Nanotechnol. 2016, 12, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.F.; Singh, R.; Burke, A.; Hatcher, H.; Olson, J.; Kraft, R.A.; Schmid, M.; Carroll, D.; Bourland, J.D.; Akman, S.; et al. Development of iron-containing multiwalled carbon nanotubes for MR-guided laser-induced thermotherapy. Nanomedicine 2011, 6, 1341–1352. [Google Scholar] [CrossRef] [PubMed]
- Maciejewska, B.M.; Warowicka, A.; Baranowska-Korczyc, A.; Zaleski, K.; Zalewski, T.; Koziol, K.K.; Jurga, S. Magnetic and hydrophilic MWCNT/Fe composites as potential T2-weighted MRI contrast agents. Carbon 2015, 94, 1012–1020. [Google Scholar] [CrossRef]
- Liu, Y.; Hughes, T.C.; Muir, B.W.; Waddington, L.J.; Gengenbach, T.R.; Easton, C.D.; Hinton, T.M.; Moffat, B.A.; Hao, X.J.; Qiu, J.S. Water-dispersible magnetic carbon nanotubes as T2-weighted MRI contrast agents. Biomaterials 2014, 35, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Lamanna, G.; Garofalo, A.; Popa, G.; Wilhelm, C.; Begin-Colin, S.; Felder-Flesch, D.; Bianco, A.; Gazeau, F.; Menard-Moyon, C. Endowing carbon nanotubes with superparamagnetic properties: Applications for cell labeling, MRI cell tracking and magnetic manipulations. Nanoscale 2013, 5, 4412–4421. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.J.; Marangon, I.; Melinte, G.; Wilhelm, C.; Menard-Moyon, C.; Pichon, B.P.; Ersen, O.; Aubertin, K.; Baaziz, W.; Pham-Huu, C.; et al. Design of covalently functionalized carbon nanotubes filled with metal oxide nanoparticles for imaging, therapy, and magnetic manipulation. ACS Nano 2014, 8, 11290–11304. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Wang, M.L.; Miao, F.; Ji, Y.X.; Tian, Z.; Shen, H.B.; Jia, N.Q. Water-dispersible multiwalled carbon nanotube/iron oxide hybrids as contrast agents for cellular magnetic resonance imaging. Carbon 2012, 50, 2162–2170. [Google Scholar] [CrossRef]
- Cabana, L.; Bourgognon, M.; Wang, J.T.W.; Protti, A.; Klippstein, R.; de Rosales, R.T.M.; Shah, A.M.; Fontcuberta, J.; Tobias-Rossell, E.; Sosabowski, J.K.; et al. The shortening of MWNT-SPION hybrids by steam treatment improves their magnetic resonance imaging properties in vitro and in vivo. Small 2016, 12, 2893–2905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuznik, N.; Tomczyk, M.M.; Wyskocka, M.; Przypis, L.; Herman, A.P.; Jedrysiak, R.; Koziol, K.K.; Boncel, S. Amalgamation of complex iron(III) ions and iron nanoclusters with MWCNTs as a route to potential T2 MRI contrast agents. Int. J. Nanomed. 2015, 10, 3581–3591. [Google Scholar]
- Chou, S.W.; Shau, Y.H.; Wu, P.C.; Yang, Y.S.; Shieh, D.B.; Chen, C.C. In vitro and in vivo studies of FePt nanoparticles for dual modal CT/MRI molecular imaging. J. Am. Chem. Soc. 2010, 132, 13270–13278. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.H.; Zheng, X.W.; Li, S.L.; Zhang, W.Z.; Wen, X.; Yue, L.D.; Wang, J.L. One-pot synthesis of FePt/CNTs nanocomposites for efficient cellular imaging and cancer therapy. J. Nanopart. Res. 2015, 17, 444. [Google Scholar] [CrossRef]
- Wu, H.X.; Liu, G.; Wang, X.; Zhang, J.M.; Chen, Y.; Shi, J.L.; Yang, H.; Hu, H.; Yang, S.P. Solvothermal synthesis of cobalt ferrite nanoparticles loaded on multiwalled carbon nanotubes for magnetic resonance imaging and drug delivery. Acta Biomater. 2011, 7, 3496–3504. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.D.; Hussain, S.T.; Gilani, S.R. Synthesis, characterization and magnetic properties of carbon nanotubes decorated with magnetic MIIFe2O4 nanoparticles. Appl. Surf. Sci. 2013, 271, 118–124. [Google Scholar] [CrossRef]
- Wang, G.S.; Chen, G.Y.; Wei, Z.Y.; Dong, X.F.; Qi, M. Multifunctional Fe3O4/graphene oxide nanocomposites for magnetic resonance imaging and drug delivery. Mater. Chem. Phys. 2013, 141, 997–1004. [Google Scholar] [CrossRef]
- Cong, H.P.; He, J.J.; Lu, Y.; Yu, S.H. Water-soluble magnetic-functionalized reduced graphene oxide sheets: In situ synthesis and magnetic resonance imaging applications. Small 2010, 6, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Guo, F.; Qiu, Y.; Hu, H.R.; Kulaots, I.; Walsh, E.; Hurt, R.H. Encapsulation of particle ensembles in graphene nanosacks as a new route to multifunctional materials. ACS Nano 2013, 7, 3744–3753. [Google Scholar] [CrossRef] [PubMed]
- Justin, R.; Tao, K.; Roman, S.; Chen, D.X.; Xu, Y.W.; Geng, X.S.; Ross, I.M.; Grant, R.T.; Pearson, A.; Zhou, G.D.; et al. Photoluminescent and superparamagnetic reduced graphene oxide-iron oxide quantum dots for dual-modality imaging, drug delivery and photothermal therapy. Carbon 2016, 97, 54–70. [Google Scholar] [CrossRef]
- Yang, K.; Hu, L.L.; Ma, X.X.; Ye, S.Q.; Cheng, L.; Shi, X.Z.; Li, C.H.; Li, Y.G.; Liu, Z. Multimodal imaging guided photothermal therapy using functionalized graphene nanosheets anchored with magnetic nanoparticles. Adv. Mater. 2012, 24, 1868–1872. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Shi, S.X.; Feng, L.Z.; Chen, F.; Graves, S.A.; Ehlerding, E.B.; Goel, S.; Sun, H.Y.; England, C.G.; Nickles, R.J.; et al. Long circulating reduced graphene oxide-iron oxide nanoparticles for efficient tumor targeting and multimodality imaging. Nanoscale 2016, 8, 12683–12692. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.S.; Ma, Y.Y.; Wei, Z.Y.; Qi, M. Development of multifunctional cobalt ferrite/graphene oxide nanocomposites for magnetic resonance imaging and controlled drug delivery. Chem. Eng. J. 2016, 289, 150–160. [Google Scholar] [CrossRef]
- Chaudhary, P.R.; Kangasniemi, K.; Takahashi, M.; Mohanty, K.S.; Koymen, R.A. Fe core–carbon shell nanoparticles as advanced MRI contrast enhancer. J. Funct. Biomater. 2017, 8, 46. [Google Scholar] [CrossRef] [PubMed]
- Seo, W.S.; Lee, J.H.; Sun, X.M.; Suzuki, Y.; Mann, D.; Liu, Z.; Terashima, M.; Yang, P.C.; McConnell, M.V.; Nishimura, D.G.; et al. FeCo/graphitic-shell nanocrystals as advanced magnetic-resonance-imaging and near-infrared agents. Nat. Mater. 2006, 5, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Mararenko, A.; Cao, G.X.; Gai, Z.; Hong, K.L.; Banerjee, P.; Zhou, S.Q. Multifunctional 1D magnetic and fluorescent nanoparticle chains for enhanced MRI, fluorescent cell imaging, and combined photothermal/chemotherapy. ACS Appl. Mater. Interfaces 2014, 6, 15309–15317. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.Y.; Sandoval, S.; Puig, T.; Obradors, X.; Tobias, G.; Ros, J.; Ricart, S. Novel Fe3O4@GNF@SiO2 nanocapsules fabricated through the combination of an in situ formation method and SiO2 coating process for magnetic resonance imaging. RSC Adv. 2017, 7, 24690–24697. [Google Scholar] [CrossRef]
- Metelkina, O.N.; Lodge, R.W.; Rudakovskaya, P.G.; Gerasimov, V.M.; Lucas, C.H.; Grebennikov, I.S.; Shchetinin, I.V.; Savchenko, A.G.; Pavlovskaya, G.E.; Rance, G.A.; et al. Nanoscale engineering of hybrid magnetite-carbon nanofibre materials for magnetic resonance imaging contrast agents. J. Mater. Chem. C 2017, 5, 2167–2174. [Google Scholar] [CrossRef]
- Sitharaman, B.; Van Der Zande, M.; Ananta, J.S.; Shi, X.F.; Veltien, A.; Walboomers, X.F.; Wilson, L.J.; Mikos, A.G.; Heerschap, A.; Jansen, J.A. Magnetic resonance imaging studies on gadonanotube-reinforced biodegradable polymer nanocomposites. J. Biomed. Mater. Res. A 2010, 93A, 1454–1462. [Google Scholar] [CrossRef] [PubMed]
- Sitharaman, B.; Jacobson, B.D.; Wadghiri, Y.Z.; Bryant, H.; Frank, J. The magnetic, relaxometric, and optical properties of gadolinium-catalyzed single walled carbon nanotubes. J. Appl. Phys. 2013, 113, 134308. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.M.; Ananta, J.S.; Zhao, H.; Cisneros, B.T.; Lam, E.Y.; Wong, S.T.; Wilson, L.J.; Wong, K.K. Cellular uptake and imaging studies of gadolinium-loaded single-walled carbon nanotubes as MRI contrast agents. Contrast Med. Mol. Imaging 2011, 6, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Rammohan, N.; MacRenaris, K.W.; Moore, L.K.; Parigi, G.; Mastarone, D.J.; Manus, L.M.; Lilley, L.M.; Preslar, A.T.; Waters, E.A.; Filicko, A.; et al. Nanodiamond-gadolinium(III) aggregates for tracking cancer growth in vivo at high field. Nano Lett. 2016, 16, 7551–7564. [Google Scholar] [CrossRef] [PubMed]
- Richard, C.; Doan, B.T.; Beloeil, J.C.; Bessodes, M.; Toth, E.; Scherman, D. Noncovalent functionalization of carbon nanotubes with amphiphilic Gd3+ chelates: Toward powerful T1 and T2 MRI contrast agents. Nano Lett. 2008, 8, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Chao, Y.; Liu, J.J.; Huang, J.; Pan, J.; Guo, W.L.; Wu, J.Z.; Sheng, M.; Yang, K.; Wang, J.; et al. Polydopamine coated single-walled carbon nanotubes as a versatile platform with radionuclide labeling for multimodal tumor imaging and therapy. Theranostics 2016, 6, 1833–1843. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.D.; Zhang, H.; Du, N.; Zhang, B.; Wu, Y.L.; Shi, D.L.; Yang, D.R. Magnetic-fluorescent nanohybrids of carbon nanotubes coated with Eu, Gd co-doped LaF3 as a multimodal imaging probe. J. Colloid Interface Sci. 2012, 367, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.L.; Li, J.; Sui, J.H.; Li, Z.G.; Cai, W. Synthesis and characterization of one-dimensional bifunctional carbon nanotubes/Fe3O4@SiO2 (FITC) nanohybrids. J. Nanosci. Nanotechnol. 2013, 13, 3928–3935. [Google Scholar] [CrossRef] [PubMed]
- Su, X.Q.; Chan, C.Y.; Shi, J.Y.; Tsang, M.K.; Pan, Y.; Cheng, C.M.; Gerile, O.; Yang, M. A graphene quantum dot@Fe3O4@SiO2 based nanoprobe for drug delivery sensing and dual-modal fluorescence and MRI imaging in cancer cells. Biosens. Bioelectron. 2017, 92, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ma, X.X.; Ye, S.Q.; Cheng, L.; Yang, K.; Guo, L.; Li, C.H.; Li, Y.G.; Liu, Z. Protamine functionalized single-walled carbon nanotubes for stem cell labeling and in vivo Raman/magnetic resonance/photoacoustic triple-modal imaging. Adv. Funct. Mater. 2012, 22, 2363–2375. [Google Scholar] [CrossRef]
- Liu, J.J.; Wang, C.; Wang, X.J.; Wang, X.; Cheng, L.; Li, Y.G.; Liu, Z. Mesoporous silica coated single-walled carbon nanotubes as a multifunctional light-responsive platform for cancer combination therapy. Adv. Funct. Mater. 2015, 25, 384–392. [Google Scholar] [CrossRef]
- Wang, J.T.W.; Cabana, L.; Bourgognon, M.; Kafa, H.; Protti, A.; Venner, K.; Shah, A.M.; Sosabowski, J.K.; Mather, S.J.; Roig, A.; et al. Magnetically decorated multiwalled carbon nanotubes as dual MRI and SPECT contrast agents. Adv. Funct. Mater. 2014, 24, 1880–1894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al Faraj, A.; Shaik, A.P.; Shaik, A.S. Magnetic single-walled carbon nanotubes as efficient drug delivery nanocarriers in breast cancer murine model: Noninvasive monitoring using diffusion-weighted magnetic resonance imaging as sensitive imaging biomarker. Int. J. Nanomed. 2015, 10, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Al Faraj, A.; Shaik, A.S.; Halwani, R.; Alfuraih, A. Magnetic targeting and delivery of drug-loaded SWCNTs theranostic nanoprobes to lung metastasis in breast cancer animal model: Noninvasive monitoring using magnetic resonance imaging. Mol. Imaging Biol. 2016, 18, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.X.; Tao, H.Q.; Yang, K.; Feng, L.Z.; Cheng, L.; Shi, X.Z.; Li, Y.G.; Guo, L.; Liu, Z. A functionalized graphene oxide-iron oxide nanocomposite for magnetically targeted drug delivery, photothermal therapy, and magnetic resonance imaging. Nano Res. 2012, 5, 199–212. [Google Scholar] [CrossRef]
- Lee, P.C.; Lin, C.Y.; Peng, C.L.; Shieh, M.J. Development of a controlled-release drug delivery system by encapsulating oxaliplatin into SPIO/MWNT nanoparticles for effective colon cancer therapy and magnetic resonance imaging. Biomater. Sci. 2016, 4, 1742–1753. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Gao, Y.; Caja, K.; Zhao, B.; Kim, J.A. Non-viral nanoparticle delivers small interfering RNA to macrophages in vitro and in vivo. PLoS ONE 2015, 10, e0118472. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Shi, J.J.; Hao, Y.W.; Zhang, P.P.; Zhao, Y.L.; Meng, D.H.; Li, D.; Chang, J.B.; Zhang, Z.Z. Magnetic multi-walled carbon nanotubes for tumor theranostics. J. Biomed. Nanotechnol. 2015, 11, 1653–1661. [Google Scholar] [CrossRef] [PubMed]
- Gollavelli, G.; Ling, Y.C. Magnetic and fluorescent graphene for dual modal imaging and single light induced photothermal and photodynamic therapy of cancer cells. Biomaterials 2014, 35, 4499–4507. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.Z.; Gong, H.; Li, Y.J.; Wang, C.; Cheng, L.; Liu, Z. Graphene-based magnetic plasmonic nanocomposite for dual bioimaging and photothermal therapy. Biomaterials 2013, 34, 4786–4793. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, Q.; Luo, X.F.; Li, J.; He, H.; Yang, F.; Di, Y.; Jin, C.; Jiang, X.G.; Shen, S.; et al. Magnetic graphene-based nanotheranostic agent for dual-modality mapping guided photothermal therapy in regional lymph nodal metastasis of pancreatic cancer. Biomaterials 2014, 35, 9473–9483. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, Q.; Yang, P.; Yu, X.R.; Huang, L.Y.; Shen, S.; Cai, S.J. Manganese oxide-coated carbon nanotubes as dual-modality lymph mapping agents for photothermal therapy of tumor metastasis. ACS Appl. Mater. Interfaces 2016, 8, 3736–3743. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y. Carbon Nano-Allotrope/Magnetic Nanoparticle Hybrid Nanomaterials as T2 Contrast Agents for Magnetic Resonance Imaging Applications. J. Funct. Biomater. 2018, 9, 16. https://doi.org/10.3390/jfb9010016
Gao Y. Carbon Nano-Allotrope/Magnetic Nanoparticle Hybrid Nanomaterials as T2 Contrast Agents for Magnetic Resonance Imaging Applications. Journal of Functional Biomaterials. 2018; 9(1):16. https://doi.org/10.3390/jfb9010016
Chicago/Turabian StyleGao, Yunxiang. 2018. "Carbon Nano-Allotrope/Magnetic Nanoparticle Hybrid Nanomaterials as T2 Contrast Agents for Magnetic Resonance Imaging Applications" Journal of Functional Biomaterials 9, no. 1: 16. https://doi.org/10.3390/jfb9010016




