Responses of Vascular Endothelial Cells to Photoembossed Topographies on Poly(Methyl Methacrylate) Films
Abstract
:1. Introduction
2. Materials and Methods
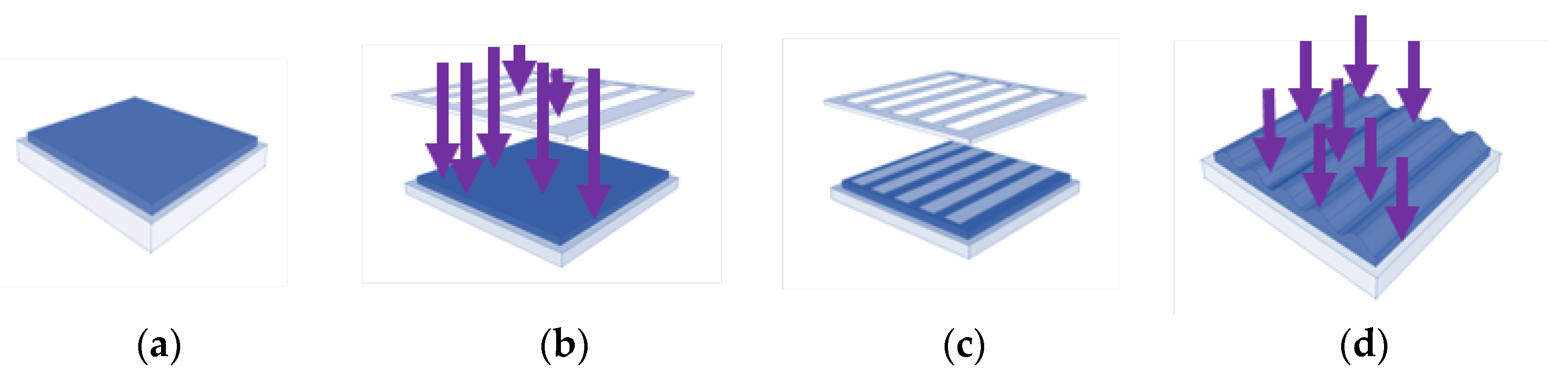
2.1. Grooved PMMA Films by Photoembossing
2.2. Cell Culture
2.3. Cell Proliferation Assay
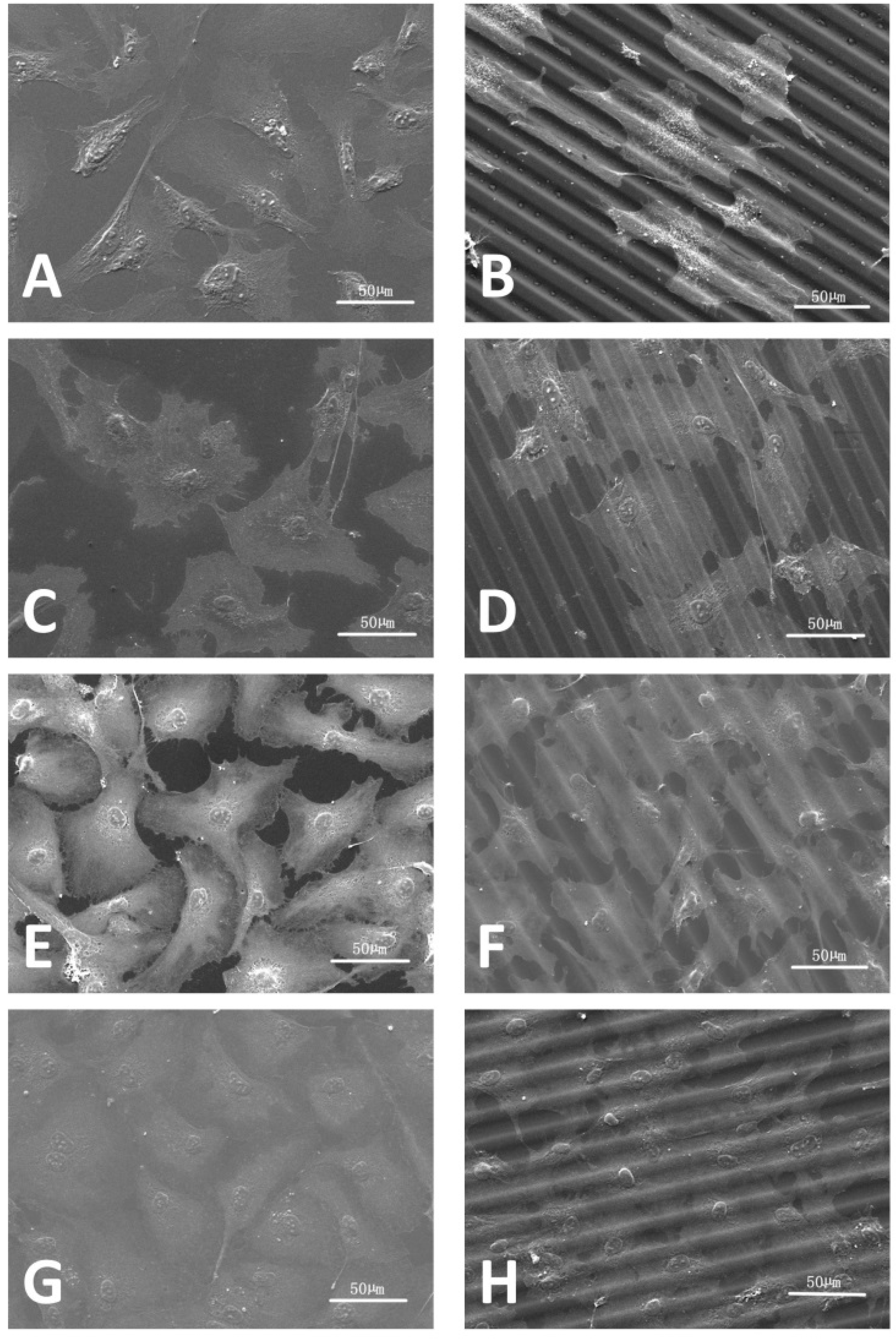
2.4. Analysis of Films and Cells Morphology
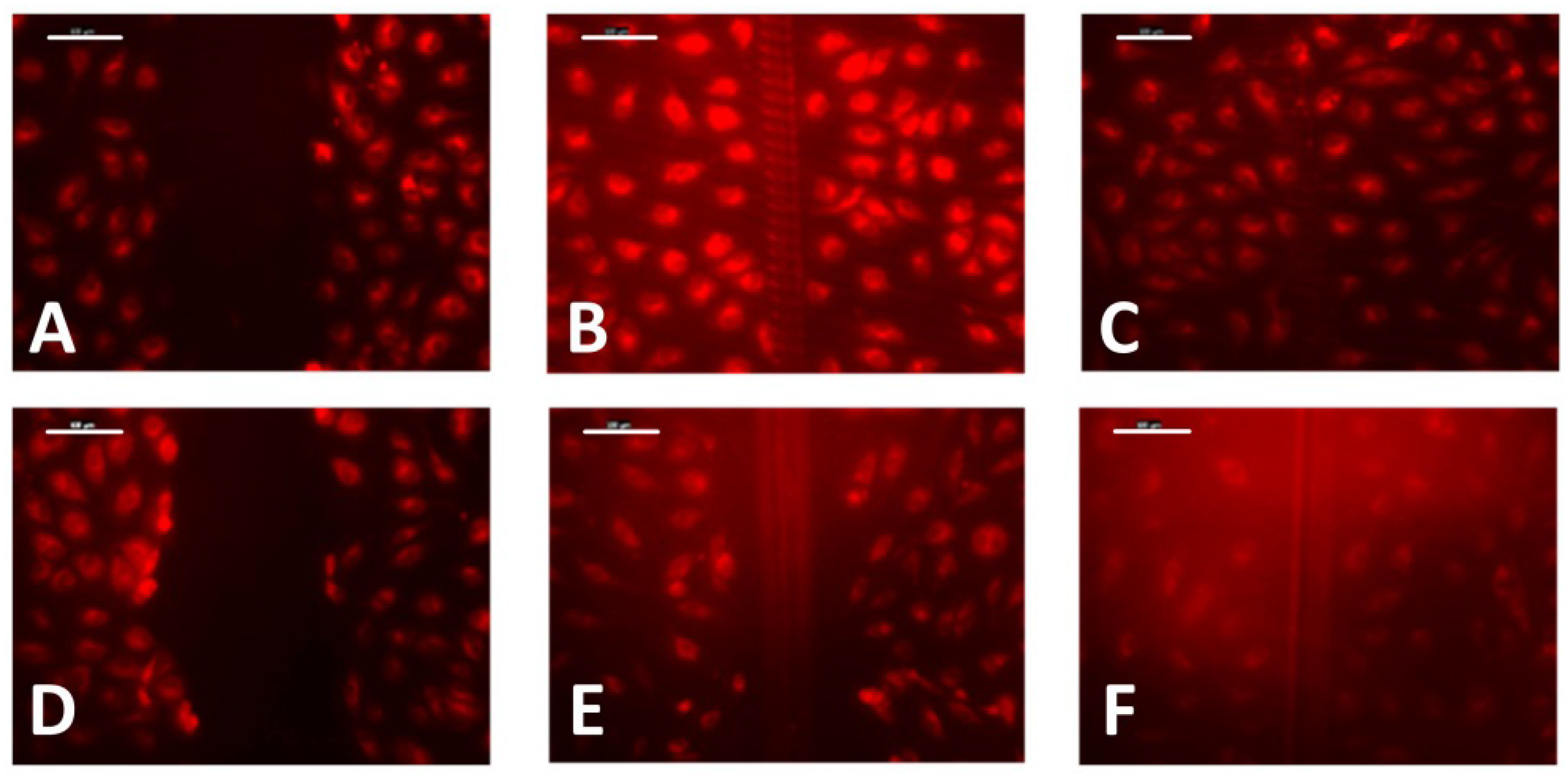
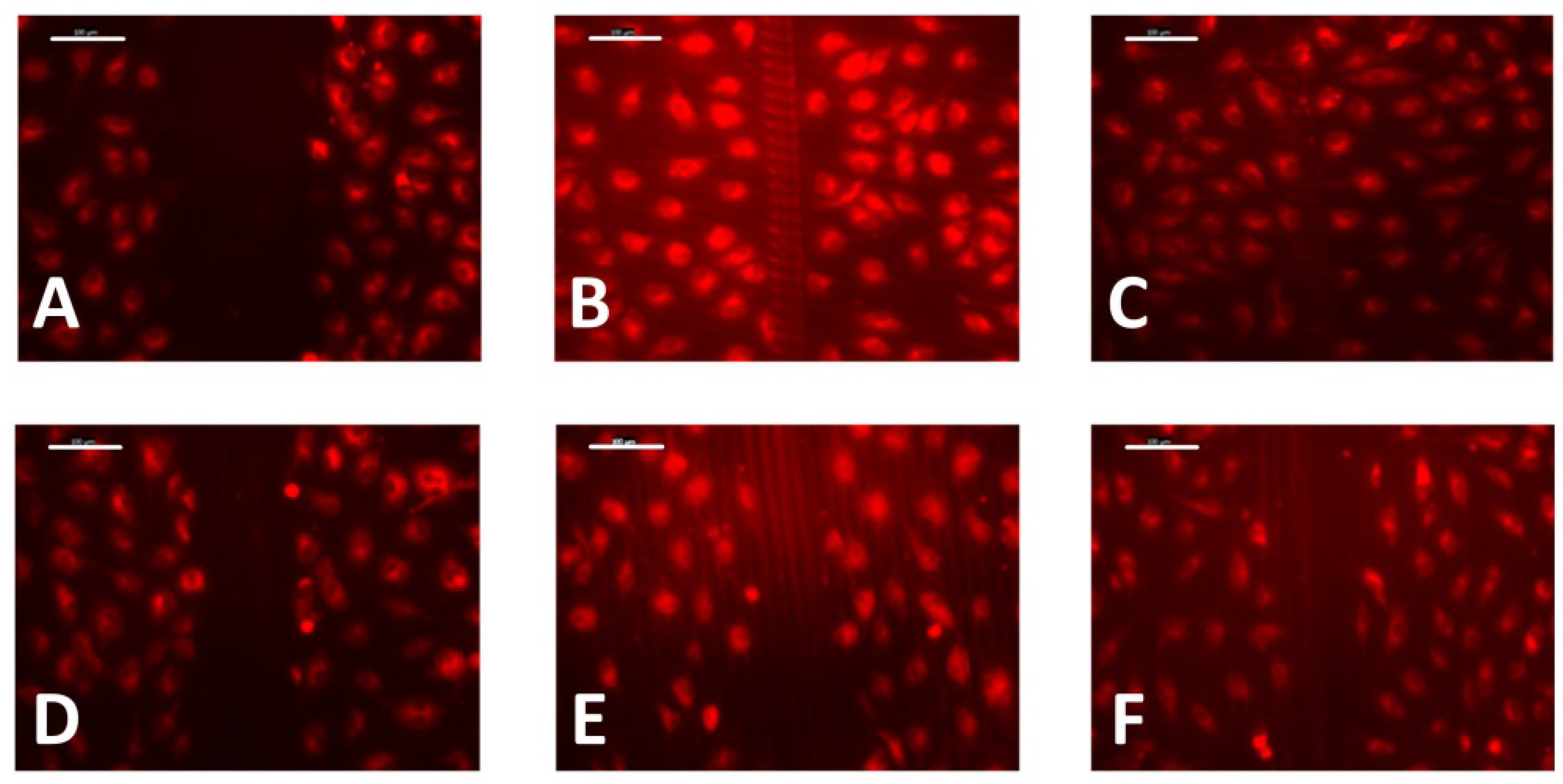
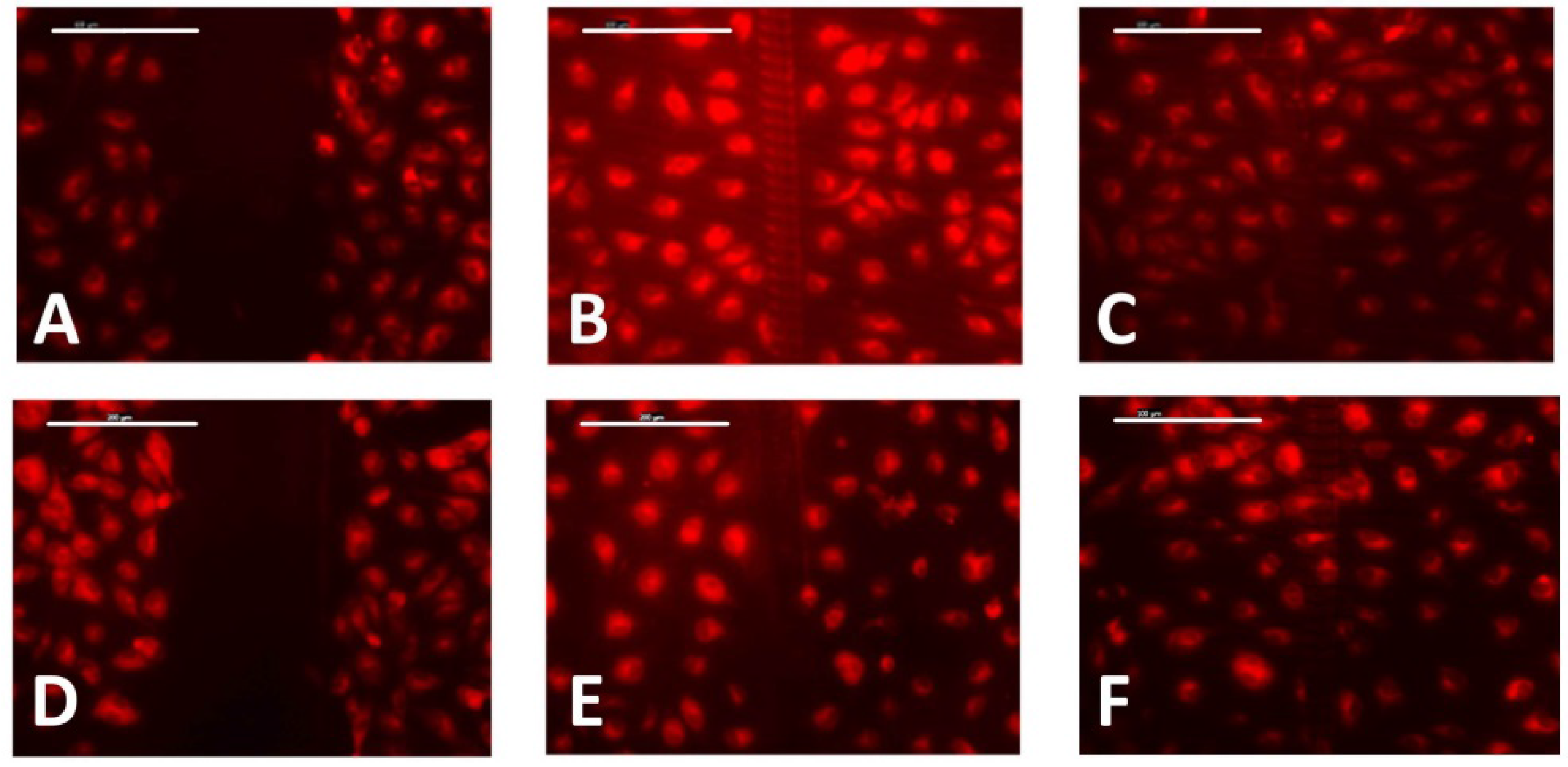
2.5. Wound-Healing Assay
2.6. Immunostaining
2.7. Data Analysis and Statistical Methods
3. Results
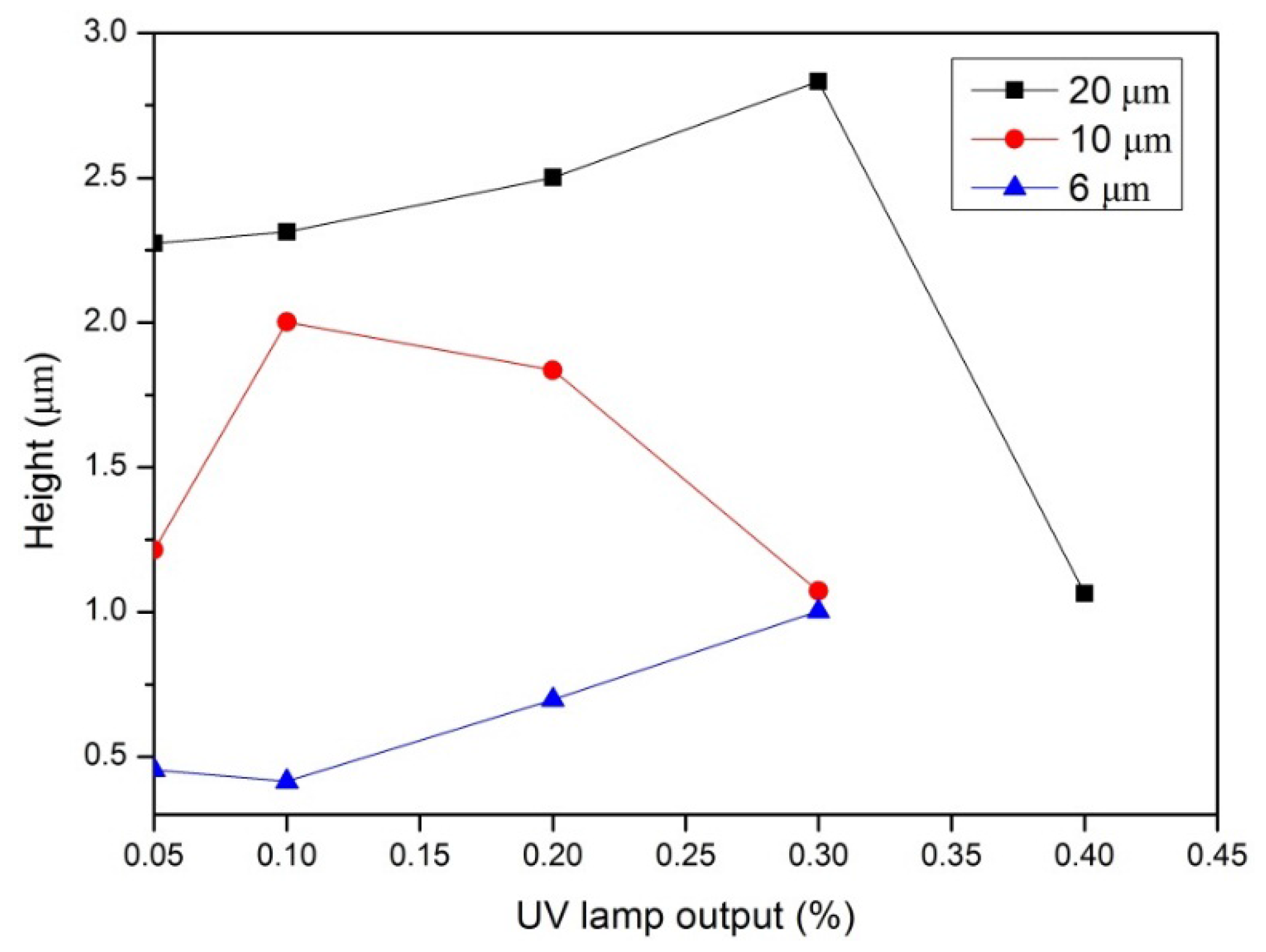
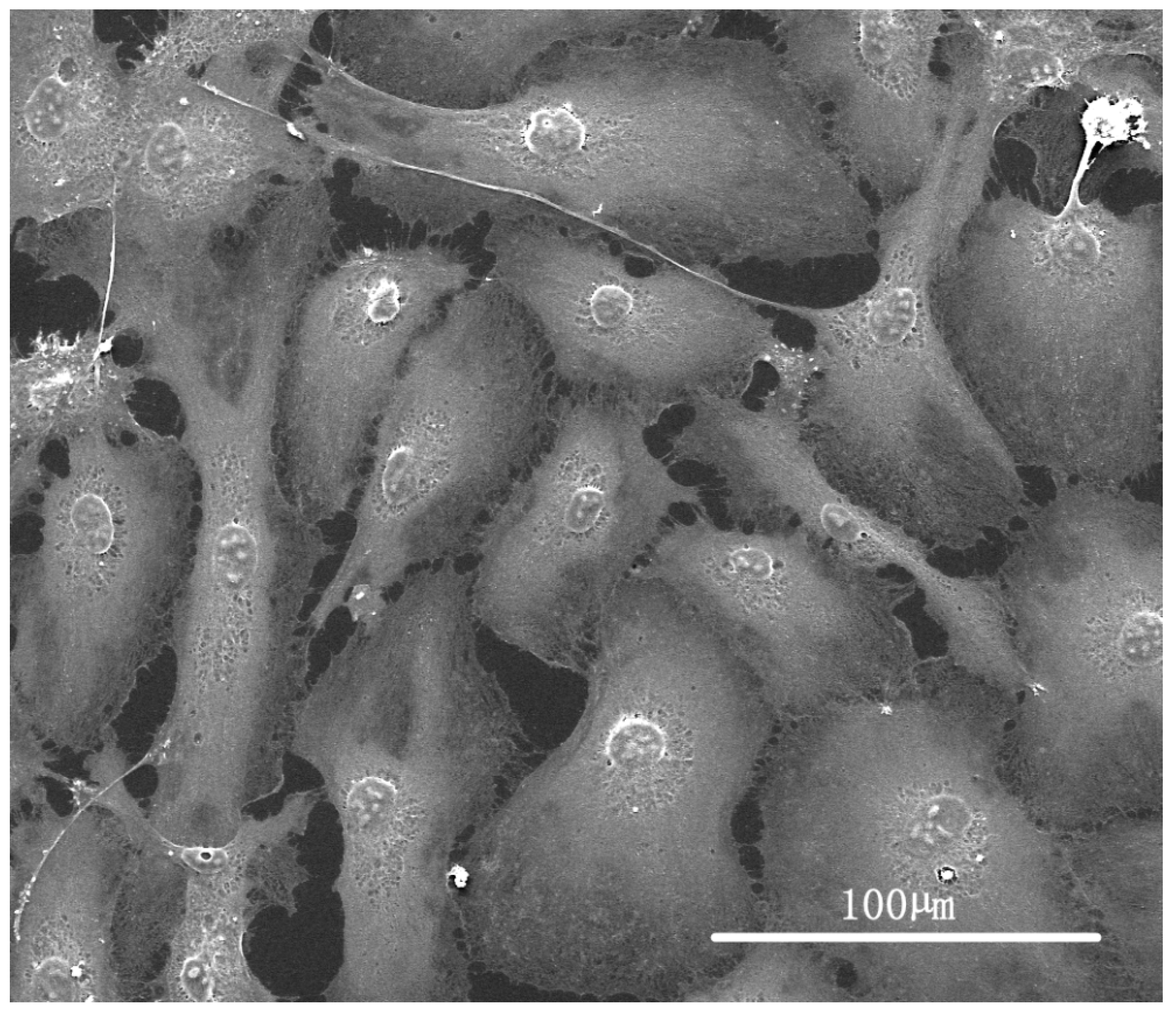
3.1. Synthesis and Characterization of Photoembossed PMMA-TPETA Films
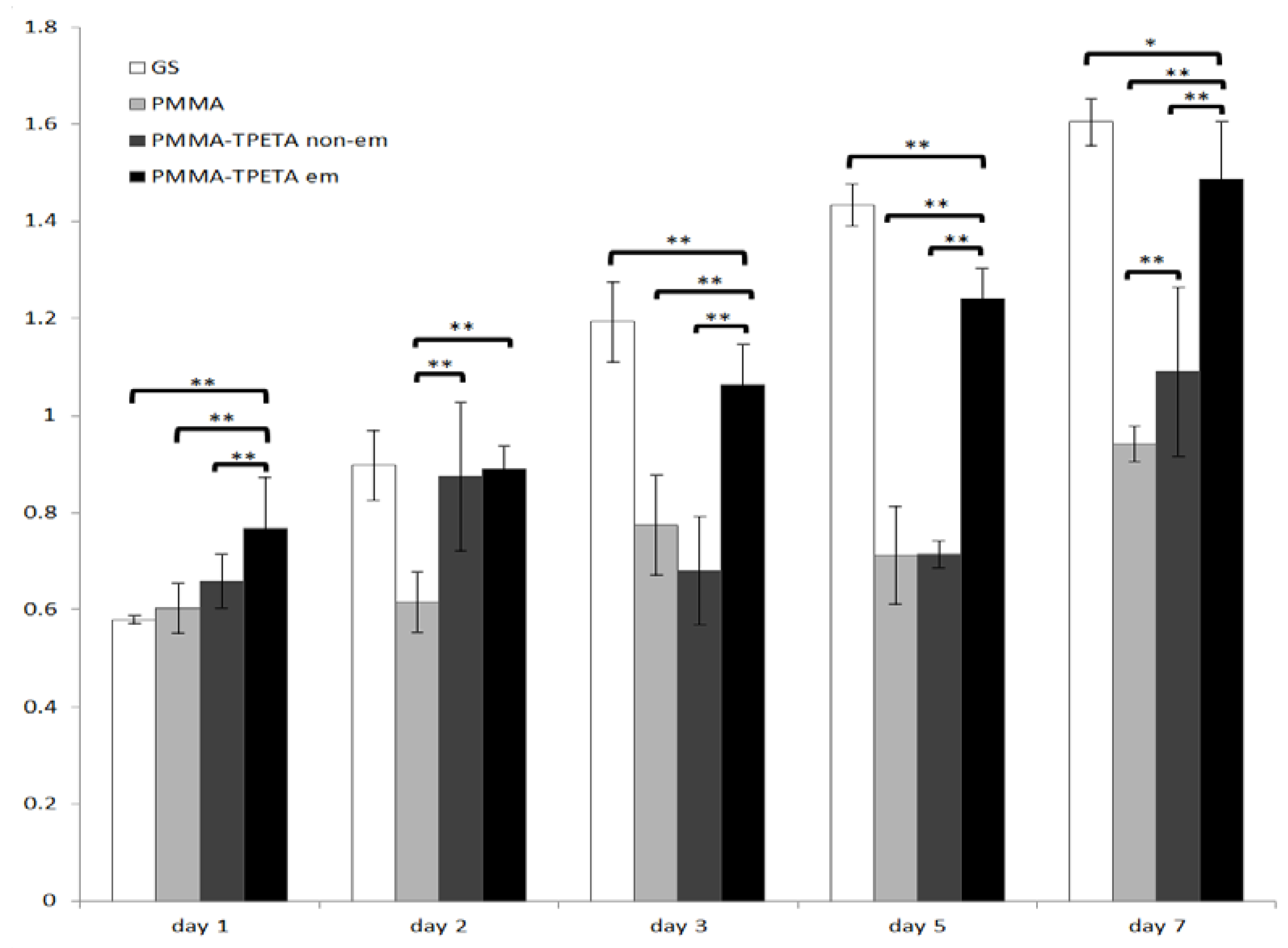
3.2. HUVEC Proliferation and Morphology
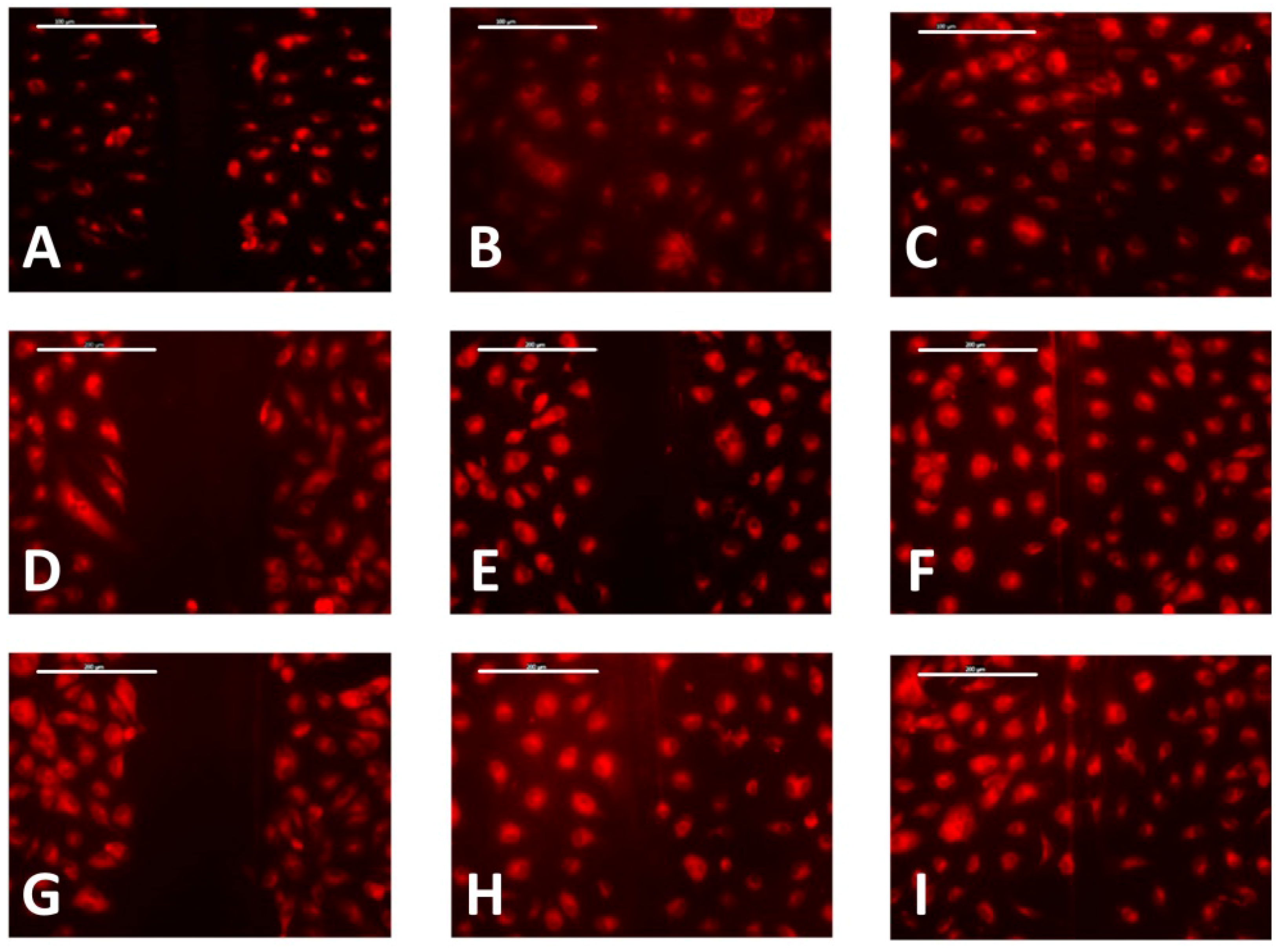
3.3. Cell Migration
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lin, H.B.; Sun, W.; Mosher, D.F.; García-Echeverría, C.; Schaufelberger, K.; Lelkes, P.I.; Cooper, S.L. Synthesis, surface, and cell-adhesion properties of polyurethanes containing covalently grafted RGD-peptides. J. Biomed. Mater. Res. 1994, 28, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Uttayarat, P.; Toworfe, G.K.; Dietrich, F.; Lelkes, P.I.; Composto, R.J. Topographic guidance of endothelial cells on silicone surfaces with micro-to nanogrooves: Orientation of actin filaments and focal adhesions. J. Biomed. Mater. Res. Part A 2005, 75, 668–680. [Google Scholar] [CrossRef] [PubMed]
- Burridge, K.; Chrzanowska-Wodnicka, M. Focal adhesions, contractility, and signaling. Annu. Rev. Cell. Dev. Biol. 1996, 12, 463–519. [Google Scholar] [CrossRef] [PubMed]
- Geiger, B.; Bershadsky, A.; Pankov, R.; Yamada, K.M. Transmembrane crosstalk between the extracellular matrix and the cytoskeleton. Nat. Rev. Mol. Cell Biol. 2001, 2, 793–805. [Google Scholar] [CrossRef] [PubMed]
- Dejana, E. Endothelial cell–cell junctions: Happy together. Nat. Rev. Mol. Cell Biol. 2004, 5, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Dejana, E.; Tournier-Lasserve, E.; Weinstein, B.M. The control of vascular integrity by endothelial cell junctions: Molecular basis and pathological implications. Dev. Cell 2009, 16, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Franco, D.; Milde, F.; Klingauf, M.; Orsenigo, F.; Dejana, E.; Poulikakos, D.; Cecchini, M.; Koumoutsakos, P.; Ferrari, A.; Kurtcuoglu, V. Accelerated endothelial wound healing on microstructured substrates under flow. Biomaterials 2012, 34, 1488–1497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komatsu, R.; Ueda, M.; Naruko, T.; Kojima, A.; Becker, A.E. Neointimal tissue response at sites of coronary stenting in humans macroscopic, histological, and immunohistochemical analyses. Circulation 1998, 98, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Curtis, A.S.; Wilkinson, C.D. Reactions of cells to topography. J. Biomater. Sci. Polym. Ed. 1998, 9, 1313–1329. [Google Scholar] [CrossRef] [PubMed]
- Kooten, T.G.V.; Recum, A.F.V. Cell adhesion to textured silicone surfaces: The influence of time of adhesion and texture on focal contact and fibronectin fibril formation. Tissue Eng. 1999, 5, 223–240. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Takayama, S.; Qian, X.; Ostuni, E.; Wu, H.; Bowden, N.; LeDuc, P.; Ingber, D.E.; Whitesides, G.M. Controlling mammalian cell spreading and cytoskeletal arrangement with conveniently fabricated continuous wavy features on poly (dimethylsiloxane). Langmuir 2002, 18, 3273–3280. [Google Scholar] [CrossRef]
- Uttayarat, M.C.P.; Li, M.; Allen, F.D.; Composto, R.J.; Lelkes, P.I. Microtopography and flow modulate the direction of endothelial cell migration. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H1027–H1035. [Google Scholar] [CrossRef] [PubMed]
- Recum, A.F.; Shannon, C.E.; Cannon, C.E.; Long, K.J.; Kooten, T.G.; Meyle, J. Surface roughness, porosity, and texture as modifiers of cellular adhesion. Tissue Eng. 1996, 2, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.G. The cultivation of tissues in extraneous media as a method of morpho-genetic study. Anat. Rec. 1912, 6, 181–193. [Google Scholar] [CrossRef]
- Loeb, L.; Fleisher, M.S. On the factors which determine the movements of tissues in culture media. J. Med. Res. 1917, 37, 75–99. [Google Scholar] [PubMed]
- Weiss, P. Erzwingung elementarer Strukturverschiedenheiten am in vitro wachsenden Gewebe. Wilhelm Roux’ Archiv für Entwicklungsmechanik der Organismen 1929, 116, 438–554. [Google Scholar] [CrossRef]
- Weiss, P. Experiments on cell and axon orientation in vitro: The role of colloidal exudates in tissue organization. J. Exp. Zool. 1945, 100, 353–386. [Google Scholar] [CrossRef] [PubMed]
- Singhvi, R.; Stephanopoulos, G.; Wang, D.I. Effects of substratum morphology on cell physiology. Biotechnol. Bioeng. 1994, 43, 764–771. [Google Scholar] [CrossRef] [PubMed]
- Chesmel, K.; Clark, C.; Brighton, C.; Black, J. Cellular responses to chemical and morphologic aspects of biomaterial surfaces. II. The biosynthetic and migratory response of bone cell populations. J. Biomed. Mater. Res. 1995, 29, 1101–1110. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Brunette, D. Effect of cell shape on proteinase secretion by epithelial cells. J. Cell Sci. 1987, 87, 259–267. [Google Scholar] [PubMed]
- Wójciak-Stothard, B.; Curtis, A.; Monaghan, W.; Macdonald, K.; Wilkinson, C. Guidance and activation of murine macrophages by nanometric scale topography. Exp. Cell Res. 1996, 223, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Frohlich, E.M.; Zhang, X.; Charest, J.L. The use of controlled surface topography and flow-induced shear stress to influence renal epithelial cell function. Integr. Biol. 2012, 4, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Norman, J.J.; Desai, T.A. Methods for fabrication of nanoscale topography for tissue engineering scaffolds. Ann. Biomed. Eng. 2006, 34, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.W.; Liu, D.Z.; Wang, S.Y.; Wang, S.S. Enhancement of the growth of human endothelial cells by surface roughness at nanometer scale. Biomaterials 2003, 24, 4655–4661. [Google Scholar] [CrossRef]
- Hughes-Brittain, N.F.; Picot, O.T.; Dai, M.; Peijs, T.; Bastiaansen, C.W.M. Effect of polymer binder on surface texturing by photoembossing. Appl. Surf. Sci. 2012, 258, 8609–8612. [Google Scholar] [CrossRef]
- Sánchez, C.; de Gans, B.J.; Kozodaev, D.; Alexeev, A.; Escuti, M.J.; van Heesch, C.; Bel, T.; Schubert, U.S.; Bastiaansen, C.W.M.; Broer, D.J. Photoembossing of periodic relief structures using polymerization-induced diffusion: A Combinatorial Study. Adv. Mater. 2005, 17, 2567–2571. [Google Scholar] [CrossRef]
- Hughes-Brittain, N.F.; Qiu, L.; Wang, W.; Peijs, T.; Bastiaansen, C.W.M. Photoembossing of surface relief structures in polymer films for biomedical applications. J. Biomed. Mater. Res. B 2014, 102, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Tamada, Y.; Ikada, Y. Effect of preadsorbed proteins on cell-adhesion to polymer surfaces. J. Colloid Interface Sci. 1993, 155, 334–339. [Google Scholar] [CrossRef]
- Apple, D.J. Sir Harold Ridley and His Fight for Sight: He Changed the World So That We May Better See It; Slack: Thorofare, NJ, USA, 2006. [Google Scholar]
- Rho, J.Y.; Ashman, R.B.; Turner, C.H. Young’s modulus of trabecular and cortical bone material: Ultrasonic and microtensile measurements. J. Biomech. 1993, 26, 111–119. [Google Scholar] [CrossRef]
- Vallittu, P.K. A review of fiber-reinforced denture base resins. J. Prosthodont. 1996, 5, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Lemperle, G.; Gauthier-Hazan, N.; Lemperle, M. PMMA-microspheres (Artecoll) for long-lasting correction of wrinkles: Refinements and statistical results. Aesthetic Plast. Surg. 1998, 22, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Van Wachem, P.B.; Hogt, A.H.; Beugeling, T.; Feijen, J.; Bantjes, A.; Detmers, J.P.; Vanaken, W.G. Adhesion of cultured human-endothelial cells onto methacrylate polymers with varying surface wettability and charge. Biomaterials 1987, 8, 323–328. [Google Scholar] [CrossRef]
- Grinnell, F. Cellular adhesiveness and extracellular substrata. Int. Rev. Cytol. 1977, 53, 65–144. [Google Scholar]
- Baier, R.E.; Shafrin, E.G.; Zisman, W.A. Adhesion: Mechanisms that assist or impede it. Science 1968, 162, 1360–1368. [Google Scholar] [CrossRef] [PubMed]
- Coleman, D.L.; Gregonis, D.E.; Andrade, J.D. Blood-materials interactions: The minimum interfacial free energy and the optimum polar/apolar ratio hypotheses. J. Biomed. Mater. Res. 1982, 16, 381–398. [Google Scholar] [CrossRef] [PubMed]
- Horbett, T.A.; Schway, M.B.; Ratner, B.D. Hydrophilic-hydrophobic copolymers as cell substrates—Effect on 3T3 cell-growth rates. J. Colloid Interface Sci. 1985, 104, 28–39. [Google Scholar] [CrossRef]
- Lydon, M.J.; Minett, T.W.; Tighe, B.J. Cellular interactions with synthetic-polymer surfaces in culture. Biomaterials 1985, 6, 396–402. [Google Scholar] [CrossRef]
- Hattori, S.; Andrade, J.D.; Hibbs, J.B.; Gregonis, D.E.; King, R.N. Fibroblast cell-proliferation on charged hydroxyethyl methacrylate copolymers. J. Colloid Interface Sci. 1985, 104, 72–78. [Google Scholar] [CrossRef]
- Gimbrone, M.A., Jr.; Cotran, R.S.; Folkman, J. Human vascular endothelial cells in culture. Growth and DNA synthesis. J. Cell Biol. 1974, 60, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Van der Meer, A.D.; Vermeul, K.; Poot, A.A.; Feijen, J.; Vermes, I. A microfluidic wound-healing assay for quantifying endothelial cell migration. Am. J. Physiol.-Heart C 2010, 298, H719–H725. [Google Scholar] [CrossRef] [PubMed]
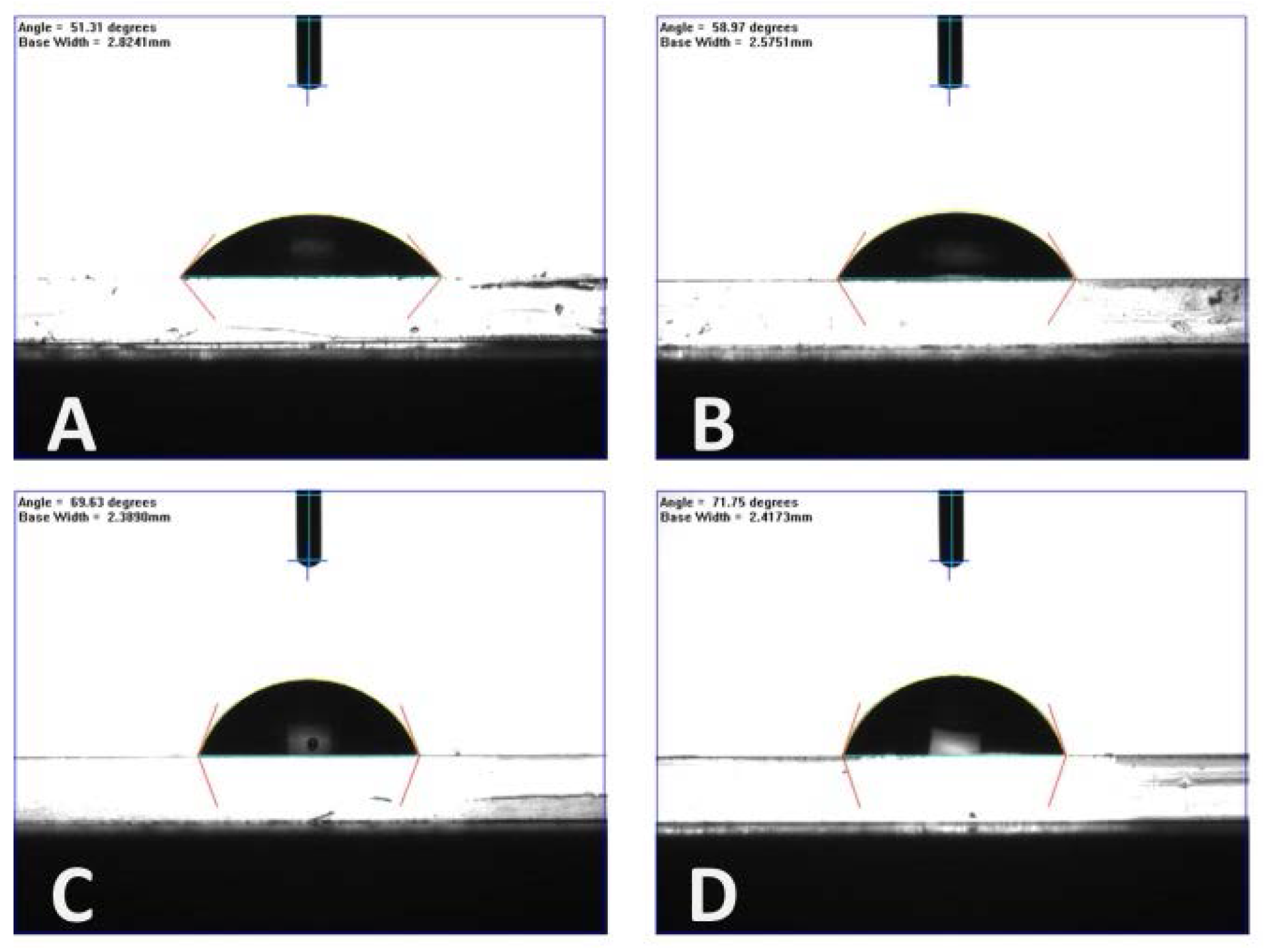

| PMMA-TPETA Films | Contact Angle (Degrees) |
|---|---|
| Smooth | 50 ± 1.6 |
| 6 µm pitch | 59 ± 1.4 |
| 10 µm pitch | 70 ± 0.3 |
| 20 µm pitch | 71 ± 0.9 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, L.; Hughes-Brittain, N.F.; Bastiaansen, C.W.M.; Peijs, T.; Wang, W. Responses of Vascular Endothelial Cells to Photoembossed Topographies on Poly(Methyl Methacrylate) Films. J. Funct. Biomater. 2016, 7, 33. https://doi.org/10.3390/jfb7040033
Qiu L, Hughes-Brittain NF, Bastiaansen CWM, Peijs T, Wang W. Responses of Vascular Endothelial Cells to Photoembossed Topographies on Poly(Methyl Methacrylate) Films. Journal of Functional Biomaterials. 2016; 7(4):33. https://doi.org/10.3390/jfb7040033
Chicago/Turabian StyleQiu, Lin, Nanayaa F. Hughes-Brittain, Cees W. M. Bastiaansen, Ton Peijs, and Wen Wang. 2016. "Responses of Vascular Endothelial Cells to Photoembossed Topographies on Poly(Methyl Methacrylate) Films" Journal of Functional Biomaterials 7, no. 4: 33. https://doi.org/10.3390/jfb7040033





