Photocrosslinkable Trehalose Derivatives Carrying Mesogenic Groups: Synthesis, Characterization, and in Vitro Evaluation for Fibroblast Attachment
Abstract
:1. Introduction
2. Results and Discussion
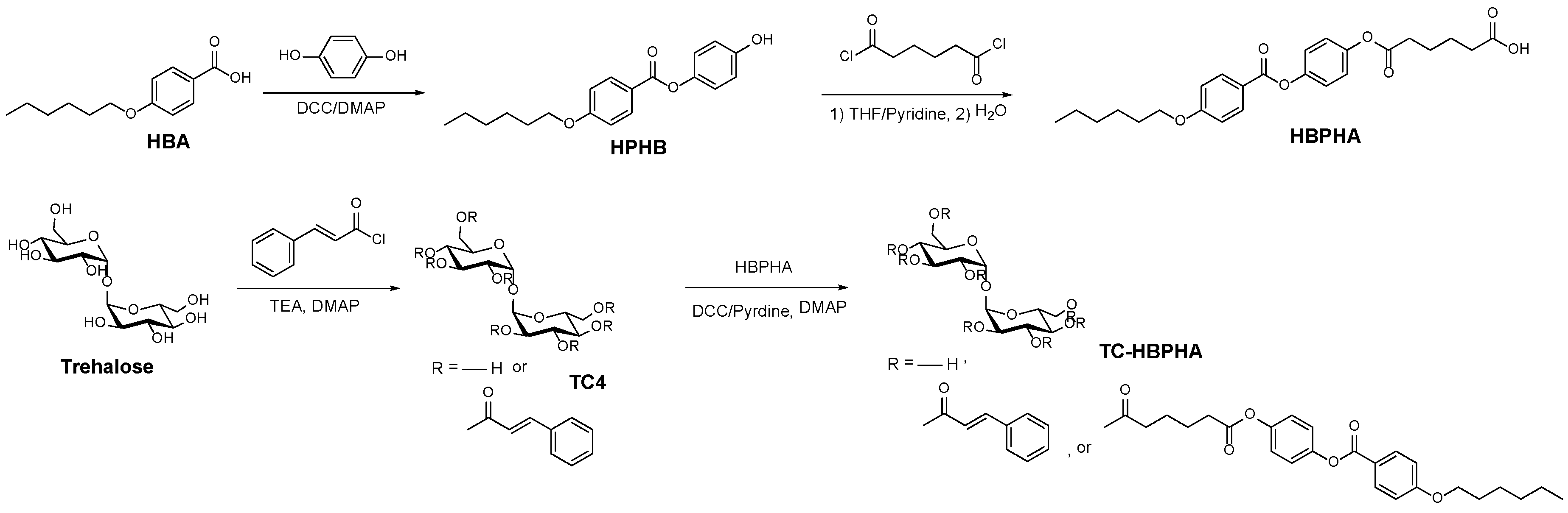
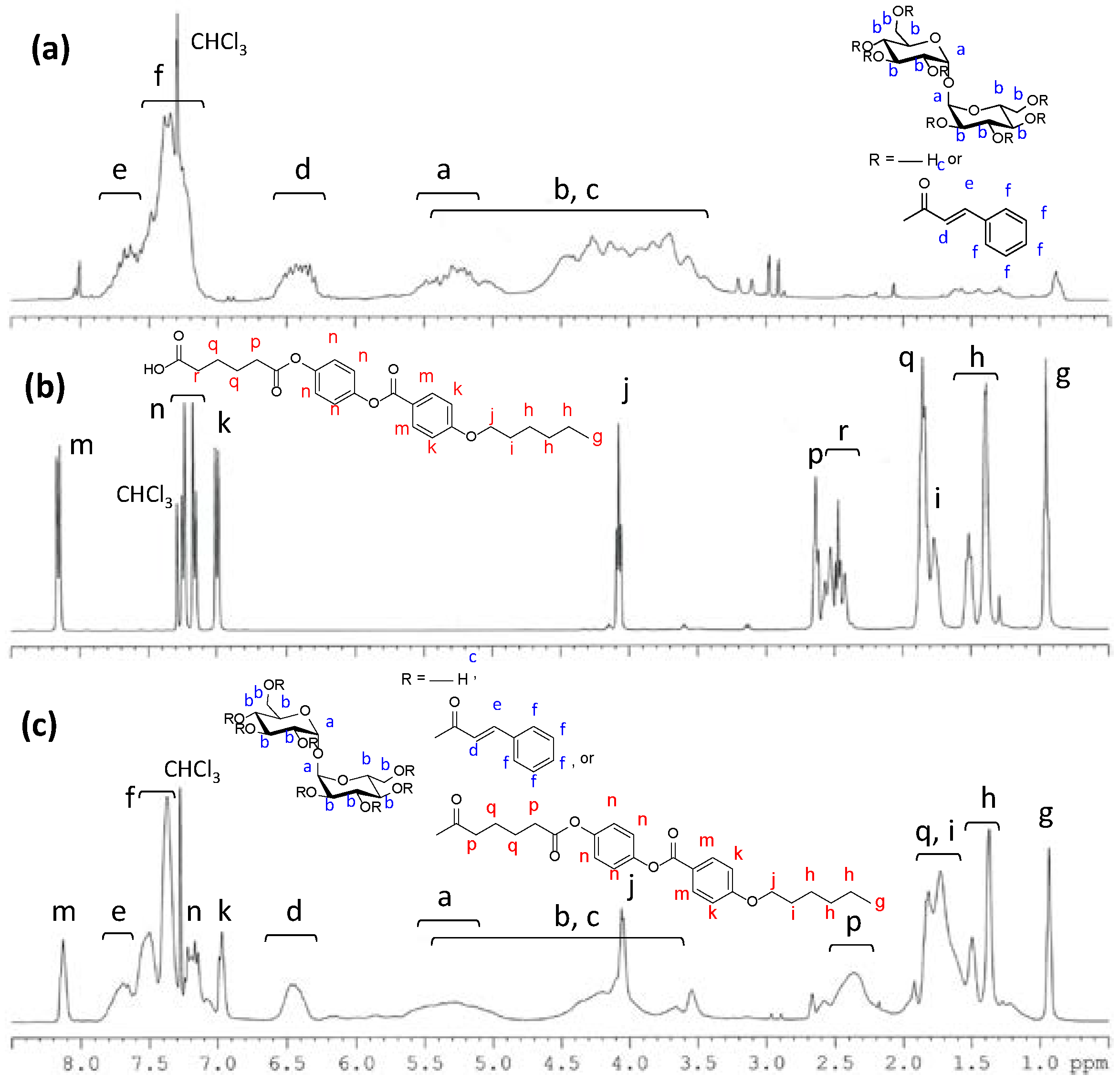
2.1. Synthesis of a Trehalose Derivative Carrying Cinnamoyl Groups and Mesogenic Groups
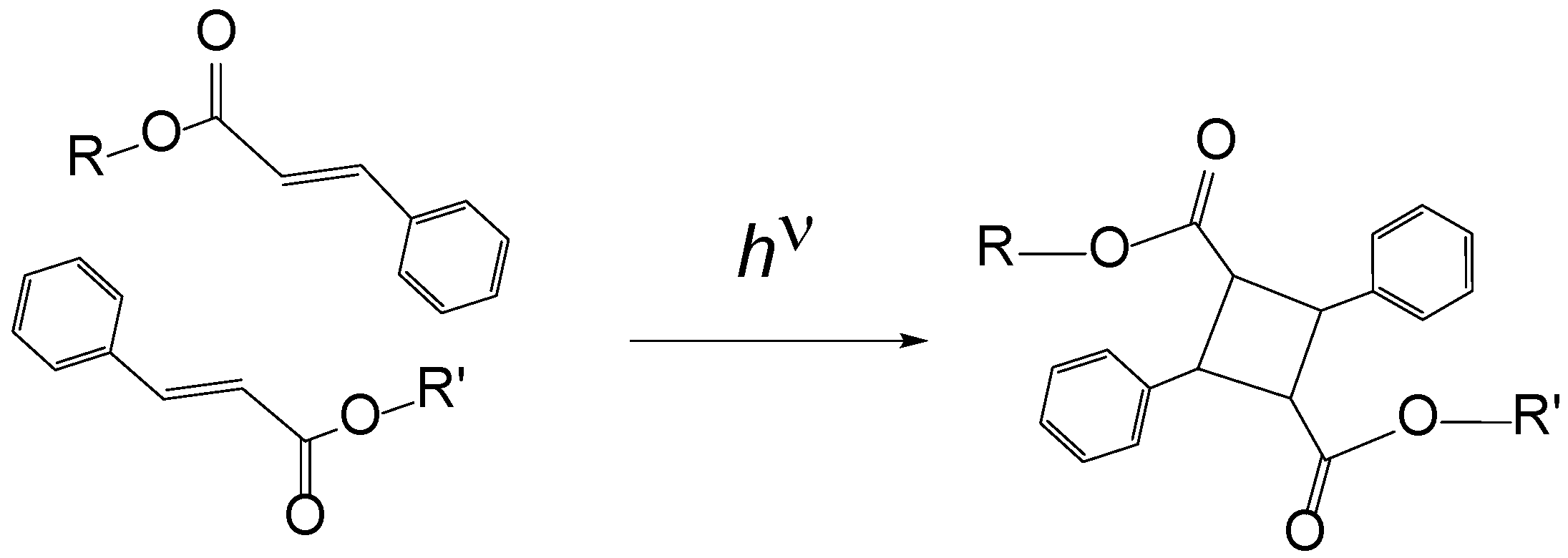
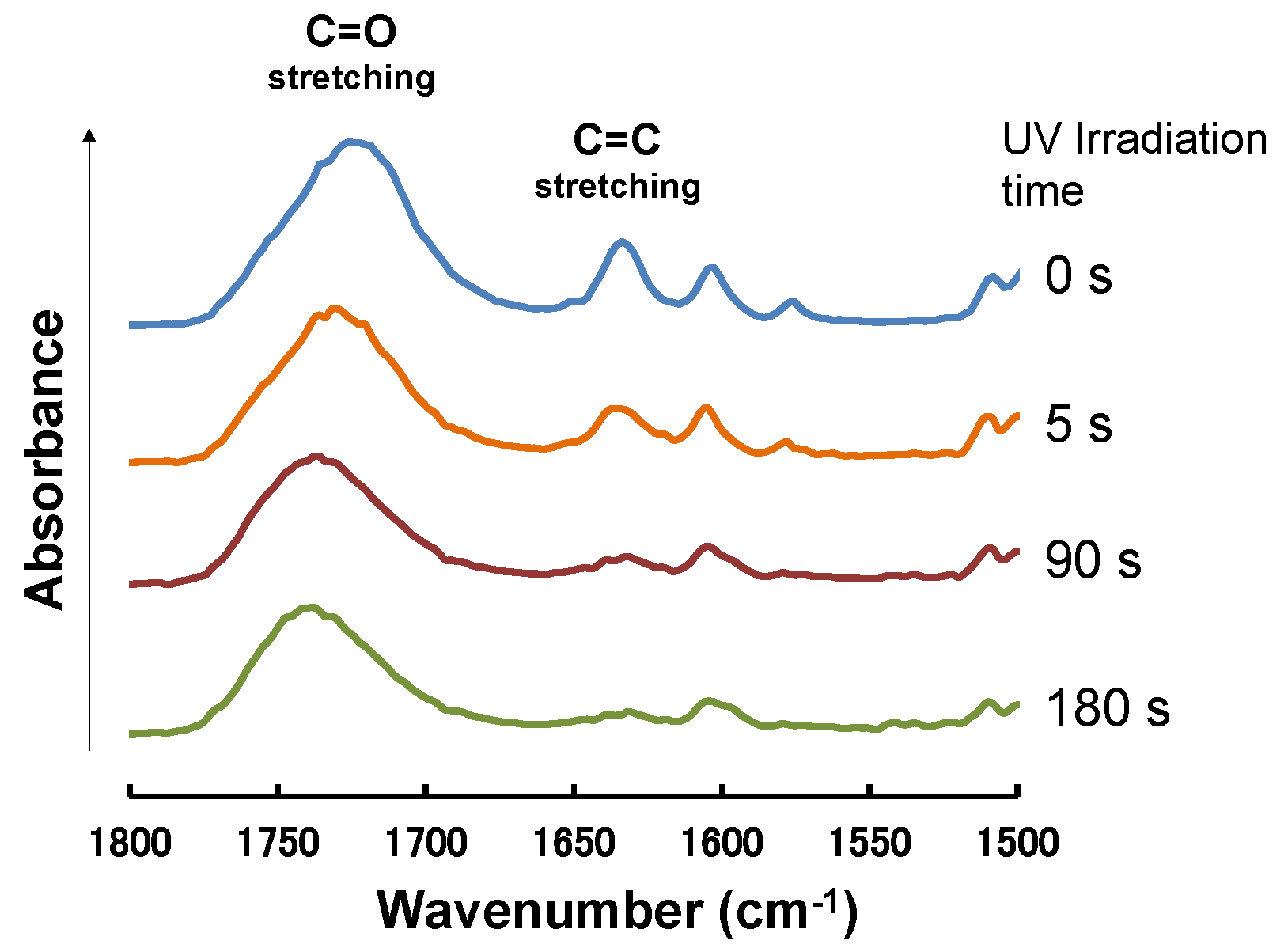
2.2. Photocrosslinking of TC-HBPHA
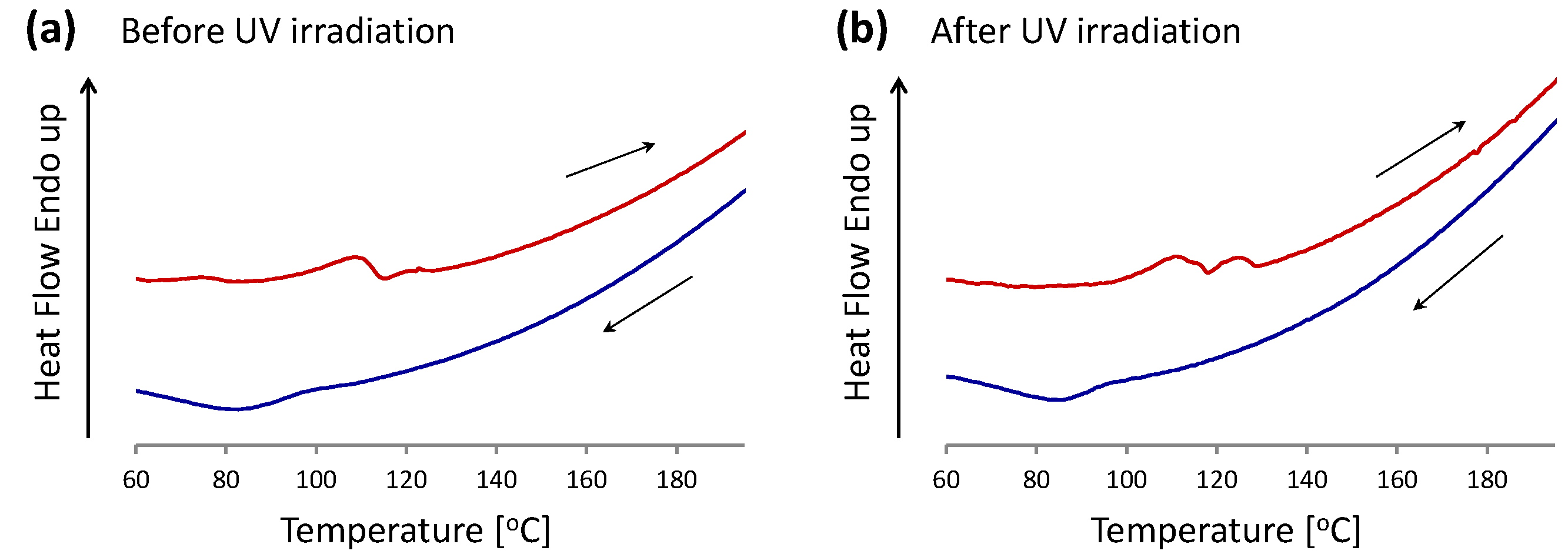
2.3. Liquid Crystalline Behavior of TC-HBPHA before and after UV Irradiation
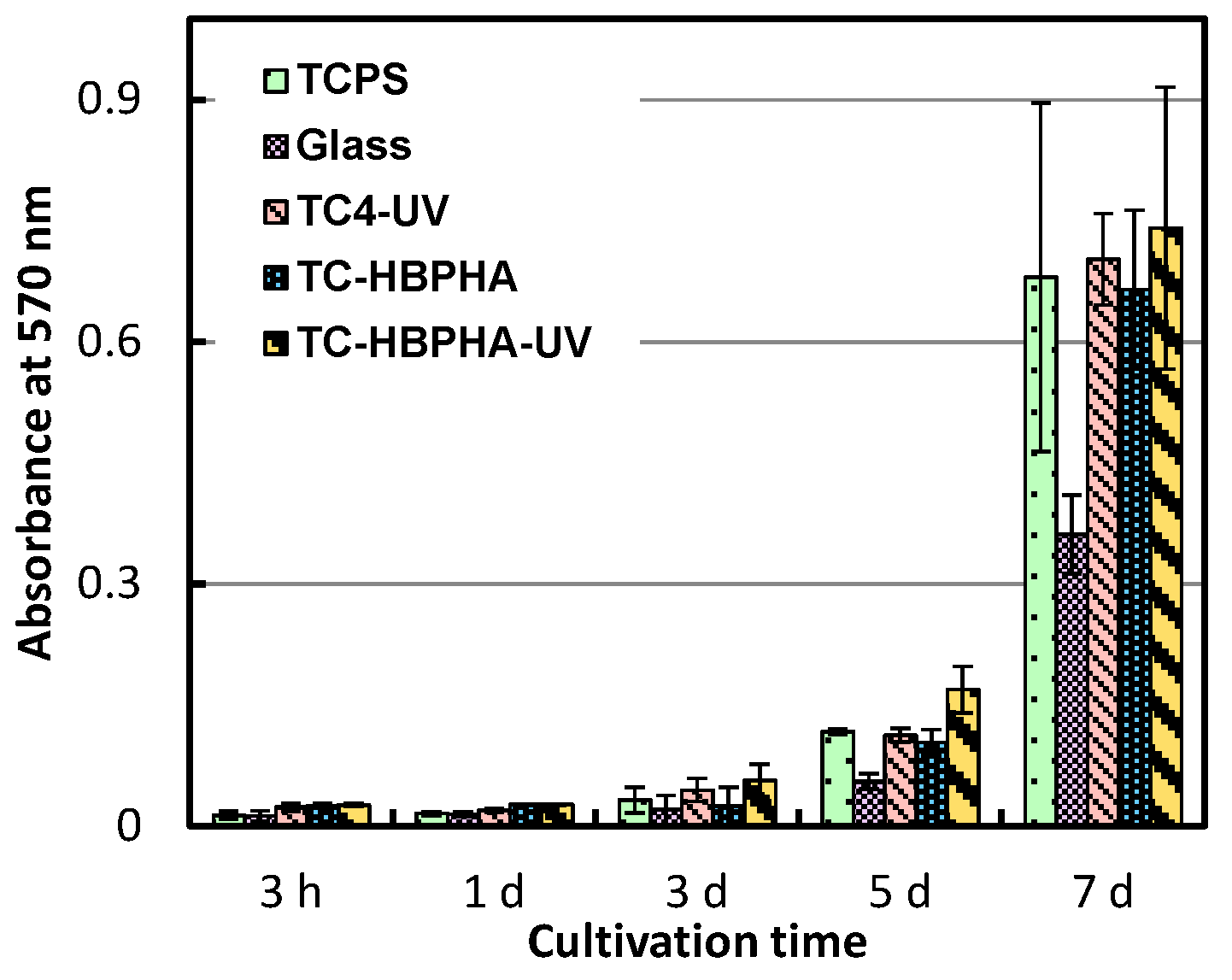
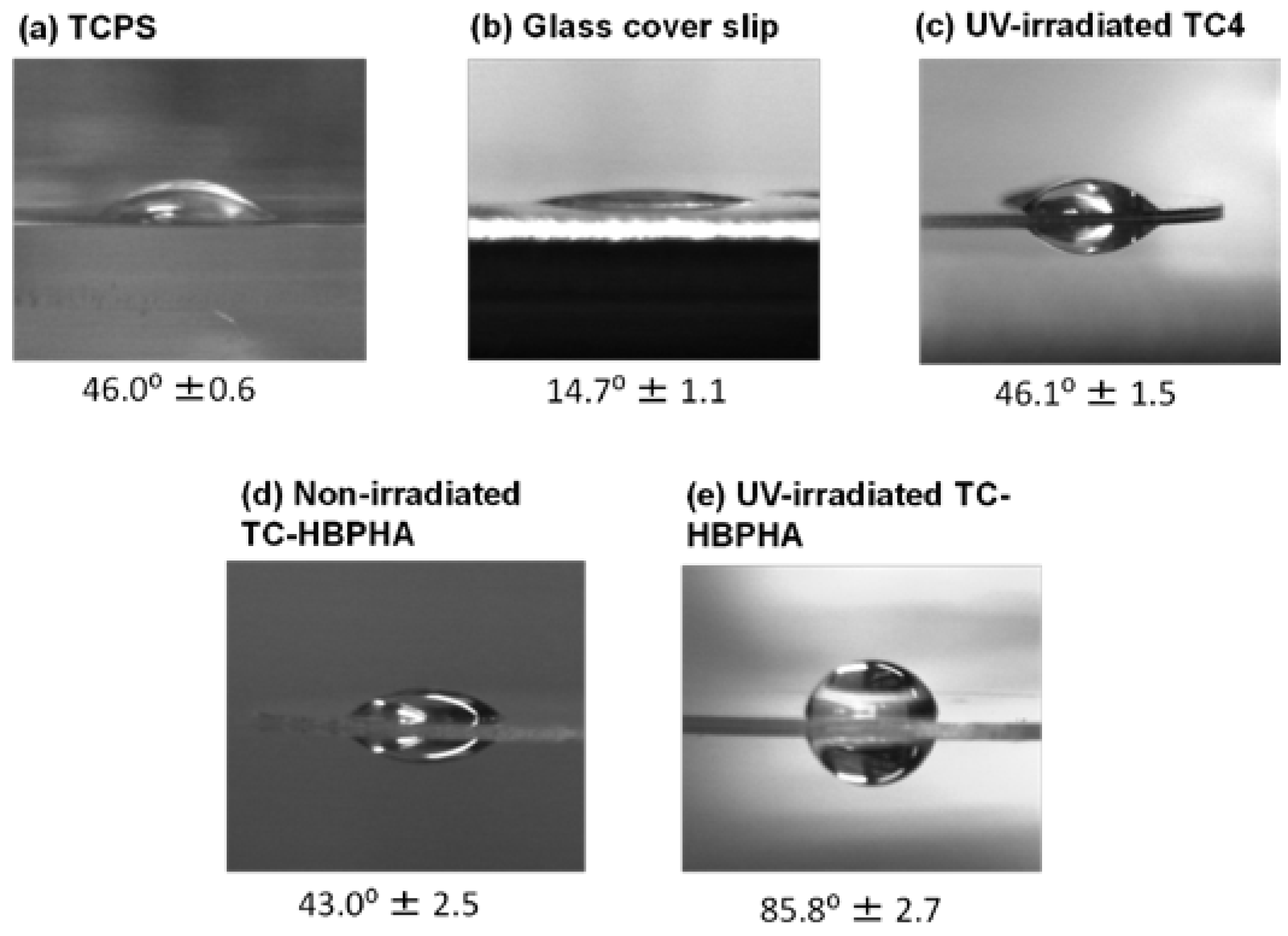
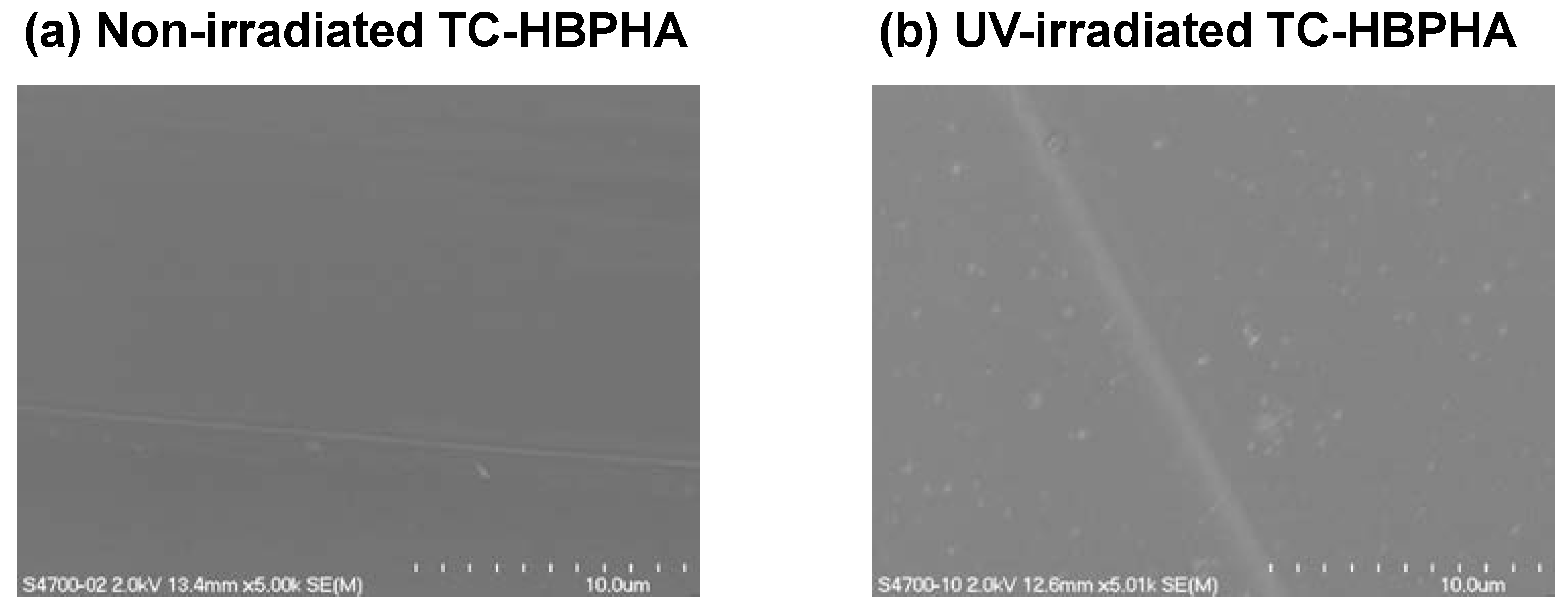
2.4. Cell Culture on UV-Irradiated TC-HBPHA and Study of Surface Hydrophobicity
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Partially Cinnamoyl-Modified Trehalose (TC4)
3.3. Synthesis of 4-Hydroxyphenyl 4'-Hexyloxybenzoate (HPHB)
3.4. Synthesis of 6-(4-Hexyloxybenzoyloxy)phenoxy-6-oxohexanoic Acid (HBPHA)
3.5. Synthesis of a Trehalose Derivative Esterified with Cinnamoyl Groups and HBPHA (TC-HBPHA)
3.6. Photocrosslinking of a Trehalose Derivative Carrying Mesogenic Groups
3.7. Characterization
3.8. Cell Culture Study
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Martin, S. Liquid crystal materials and liquid crystal displays. Ann. Rev. Mater. Sci. 1997, 27, 305–379. [Google Scholar]
- Ciferri, A.; Krigbaum, W.R.; Meyer, R.B. Polymer Liquid Crystals; Academic Press: London, UK, 1982. [Google Scholar]
- Shannon, P.J.; Gibbons, W.M.; Sun, S.T. Patterned optical properties in photopolymerized surface-aligned liquid-crystal films. Nature 1994, 368, 532–533. [Google Scholar] [CrossRef]
- Anderle, K.; Birenheide, R.; Werner, M.J.A.; Wendorff, J.H. Molecular addressing? Studies on light-induced reorientation in liquid-crystalline side chain polymers. Liq. Cryst. 1991, 9, 691–699. [Google Scholar] [CrossRef]
- Ichimura, K.; Hayashi, Y.; Akiyama, H.; Ikeda, T.; Ishizuki, N. Photo-optical liquid crystal cells driven by molecular rotors. Appl. Phys. Lett. 1993, 63, 449–451. [Google Scholar] [CrossRef]
- Woltman, S.J.; Jay, G.D.; Crawford, G.P. Liquid-crystal materials find a new order in biomedical applications. Nat. Mater. 2007, 6, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Brake, J.M.; Daschner, M.K.; Luk, Y.-Y.; Abbott, N.L. Biomolecular interactions at phospholipid-decorated surfaces of liquid crystals. Science 2003, 302, 2094–2097. [Google Scholar] [CrossRef] [PubMed]
- Sessa, G.; Weissmann, G. Phospholipid spherules (liposomes) as a model for biological membranes. J. Lipid Res. 1968, 9, 310–318. [Google Scholar] [PubMed]
- Jewell, S.A. Living systems and liquid crystals. Liq. Cryst. 2011, 38, 1699–1714. [Google Scholar] [CrossRef]
- Barclay, G.G.; Ober, C.K. Liquid crystalline and rigid-rod networks. Prog. Polym. Sci. 1993, 18, 899–945. [Google Scholar] [CrossRef]
- Hikmet, R.A.M.; Lub, J.; Broer, D.J. Anisotropic networks formed by photopolymerization of liquid-crystalline molecules. Adv. Mater. 1991, 3, 392–394. [Google Scholar] [CrossRef]
- Xie, P.; Zhang, R. Liquid crystal elastomers, networks and gels: Advanced smart materials. J. Mater. Chem. 2005, 15, 2529–2550. [Google Scholar] [CrossRef]
- Kelly, S.M. Anisotropic networks. J. Mater. Chem. 1995, 5, 2047–2061. [Google Scholar] [CrossRef]
- Kawatsuki, N.; Ono, H.; Takatsuka, H.; Yamamoto, T.; Sangen, O. Liquid crystal alignment on photoreactive side-chain liquid-crystalline polymer generated by linearly polarized UV light. Macromolecules 1997, 30, 6680–6682. [Google Scholar] [CrossRef]
- Schadt, M.; Schmitt, K.; Kozinkov, V.; Chigrinov, V. Surface-induced parallel alignment of liquid crystals by linearly polymerized photopolymers. Jpn. J. Appl. Phys. 1992, 31, 2155–2164. [Google Scholar] [CrossRef]
- Obi, M.; Morino, S.Y.; Ichimura, K. Factors affecting photoalignment of liquid crystals induced by polymethacrylates with coumarin side chains. Chem. Mater. 1999, 11, 656–664. [Google Scholar] [CrossRef]
- Zhou, C.; Yi, Z. Blood-compatibility of polyurethane/liquid crystal composite membranes. Biomaterials 1999, 20, 2093–2099. [Google Scholar] [CrossRef]
- Li, L.; Tu, M.; Mou, S.; Zhou, C. Preparation and blood compatibility of polysiloxane/liquid-crystal composite membranes. Biomaterials 2001, 22, 2595–2599. [Google Scholar] [CrossRef]
- Shih, M.-F.; Shau, M.-D.; Chang, M.-Y.; Chiou, S.-K.; Chang, J.-K.; Cherng, J.-Y. Platelet adsorption and hemolytic properties of liquid crystal/composite polymers. Int. J. Pharm. 2006, 327, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.J.; Iyer, S.N.; Li, L.-S.; Claussen, R.; Harrington, D.A.; Stupp, S.I. Self-assembling biomaterials: Liquid crystal phases of cholesteryl oligo(l-lactic acid) and their interactions with cells. Proc. Natl. Acad. Sci. USA 2002, 99, 9662–9667. [Google Scholar] [CrossRef] [PubMed]
- Woolverton, C.J.; Gustely, E.; Li, L.; Lavrentovich, O.D. Liquid crystal effects on bacterial viability. Liq. Cryst. 2005, 32, 417–423. [Google Scholar] [CrossRef]
- Luk, Y.-Y.; Campbell, S.F.; Abbott, N.L.; Murphy, C.J. Non-toxic thermotropic liquid crystals for use with mammalian cells. Liq. Cryst. 2004, 31, 611–621. [Google Scholar] [CrossRef]
- Lockwood, N.A.; Mohr, J.C.; Ji, L.; Murphy, C.J.; Palecek, S.P.; de Pablo, J.J.; Abbott, N.L. Thermotropic liquid crystals as substrates for imaging the reorganization of matrigel by human embryonic stem cells. Adv. Funct. Mater. 2006, 16, 618–624. [Google Scholar] [CrossRef]
- John, G.; Zhu, G.; Li, J.; Dordick, J.S. Enzymatically derived sugar-containing self-assembled organogels with nanostructured morphologies. Angew. Chem. Int. Ed. 2006, 45, 4772–4775. [Google Scholar] [CrossRef] [PubMed]
- Mancini, R.J.; Lee, J.; Maynard, H.D. Trehalose glycopolymers for stabilization of protein conjugates to environmental stressors. J. Am. Chem. Soc. 2012, 134, 8474–8479. [Google Scholar] [CrossRef] [PubMed]
- Burek, M.; Czuba, Z.P.; Waskiewicz, S. Novel acid-degradable and thermo-sensitive poly(N-isopropylacrylamide) hydrogels cross-linked by α,α-trehalose diacetals. Polymer 2014, 55, 6460–6470. [Google Scholar] [CrossRef]
- Yano, S.; Teramoto, N.; Miyamoto, R.; Nakajima, E.; Hashimoto, K.; Shibata, M. Fibroblast cell proliferation on photo-cured trehalose cinnamoyl ester thin films. J. Bioact. Compat. Polym. 2015, 30, 87–98. [Google Scholar] [CrossRef]
- Teramoto, N.; Sachinvala, N.; Shibata, M. Trehalose and trehalose-based polymers for environmentally benign, biocompatible and bioactive materials. Molecules 2008, 13, 1773–1816. [Google Scholar] [CrossRef] [PubMed]
- Minsk, L.M.; Smith, J.G.; van Deusen, W.P.; Wright, J.F. Photosensitive polymers. I. Cinnamate esters of poly(vinyl alcohol) and cellulose. J. Appl. Polym. Sci. 1959, 2, 302–307. [Google Scholar] [CrossRef]
- Yamashita, K.; Kyo, S.; Miyagawa, T.; Nango, M.; Tsuda, K. Synthesis and characterization of liquid crystal photoresist. J. Appl. Polym. Sci. 1994, 52, 577–581. [Google Scholar] [CrossRef]
- Nagata, M.; Hizakae, S. Synthesis and characterization of photocrosslinkable biodegradable polymers derived from 4-hydroxycinnamic acid. Macromol. Biosci. 2003, 3, 412–419. [Google Scholar] [CrossRef]
- Gupta, P.; Trenor, S.R.; Long, T.E.; Wilkes, G.L. In situ photo-cross-linking of cinnamate functionalized poly(methyl methacrylate-co-2-hydroxyethyl acrylate) fibers during electrospinning. Macromolecules 2004, 37, 9211–9218. [Google Scholar] [CrossRef]
- Noller, C.R.; Rockwell, W.C. The preparation of some higher alkylglucosides. J. Am. Chem. Soc. 1938, 60, 2076–2077. [Google Scholar] [CrossRef]
- Van Doren, H.A.; Smits, E.; Pestman, J.M.; Engberts, J.B.F.N.; Kellogg, R.M. Mesogenic sugars. From aldoses to liquid crystals and surfactants. Chem. Soc. Rev. 2000, 29, 183–199. [Google Scholar] [CrossRef]
- Kohne, B.; Praefcke, K. Hexa-o-alkanoyl-scyllo-inositols, the first alicyclic, saturated, discotic liquid crystals. Angew. Chem. Int. Ed. 1984, 23, 82–83. [Google Scholar] [CrossRef]
- Tian, M.; Zhang, B.-Y.; Cong, Y.-H.; Zhang, N.; Yao, D.-S. Mesomorphic properties of multi-arm liquid crystals containing glucose and sorbitol as cores. J. Mol. Struct. 2009, 923, 39–44. [Google Scholar] [CrossRef]
- Nikkhah, M.; Edalat, F.; Manoucheri, S.; Khademhosseini, A. Engineering microscale topographies to control the cell-substrate interface. Biomaterials 2012, 33, 5230–5246. [Google Scholar] [CrossRef] [PubMed]
- Fee, T.; Surianarayanan, S.; Downs, C.; Zhou, Y.; Berry, J. Nanofiber alignment regulates NIH3T3 cell orientation and cytoskeletal gene expression on electrospun PCL+Gelatin nanofibers. PLoS ONE 2016, 11, e0154806. [Google Scholar] [CrossRef] [PubMed]
- Teramoto, N.; Shibata, M. Synthesis and photocuring of cinnamoyl trehalose esters. Polym. Adv. Technol. 2007, 18, 971–977. [Google Scholar] [CrossRef]
- Ichimura, K.; Akita, Y.; Akiyama, H.; Kudo, K.; Hayashi, Y. Photoreactivity of polymers with regioisomeric cinnamate side chains and their ability to regulate liquid crystal alignment. Macromolecules 1997, 30, 903–911. [Google Scholar] [CrossRef]
- Sapich, B.; Stumpe, J.; Kricheldorf, H.R.; Fritz, A.; Schönhals, A. Synthesis, dielectric, and photochemical study of liquid crystalline main chain poly(ester imide)s containing cinnamoyl moieties. Macromolecules 2001, 34, 5694–5701. [Google Scholar] [CrossRef]
- Chae, B.; Lee, S.W.; Ree, M.; Jung, Y.M.; Kim, S.B. Photoreaction and molecular reorientation in a nanoscaled film of poly(methyl 4-(methacryloyloxy)cinnamate) studied by two-dimensional FTIR and UV correlation spectroscopy. Langmuir 2003, 19, 687–695. [Google Scholar] [CrossRef]
- Kim, W.G. Photocure properties of high-heat-resistant photoreactive polymers with cinnamate groups. J. Appl. Polym. Sci. 2008, 107, 3615–3624. [Google Scholar] [CrossRef]
- Shiota, A.; Ober, C.K. Rigid rod and liquid crystalline thermosets. Prog. Polym. Sci. 1997, 22, 975–1000. [Google Scholar] [CrossRef]
- Nwabunma, D.; Chiu, H.-W.; Kyu, T. Morphology development and dynamics of photopolymerization-induced phase separation in mixtures of a nematic liquid crystal and photocuratives. Macromolecules 2000, 33, 1416–1424. [Google Scholar] [CrossRef]
- Duclos, G.; Garcia, S.; Yevick, H.G.; Silberzan, P. Perfect nematic order in confined monolayers of spindle-shaped cells. Soft Matter 2014, 10, 2346–2353. [Google Scholar] [CrossRef] [PubMed]
- Clark, P.; Connolly, P.; Moores, G.R. Cell guidance by micropatterned adhesiveness in vitro. J. Cell Sci. 1992, 103, 287–292. [Google Scholar] [PubMed]



© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yano, S.; Teramoto, N.; Shimasaki, T.; Shibata, M. Photocrosslinkable Trehalose Derivatives Carrying Mesogenic Groups: Synthesis, Characterization, and in Vitro Evaluation for Fibroblast Attachment. J. Funct. Biomater. 2016, 7, 24. https://doi.org/10.3390/jfb7030024
Yano S, Teramoto N, Shimasaki T, Shibata M. Photocrosslinkable Trehalose Derivatives Carrying Mesogenic Groups: Synthesis, Characterization, and in Vitro Evaluation for Fibroblast Attachment. Journal of Functional Biomaterials. 2016; 7(3):24. https://doi.org/10.3390/jfb7030024
Chicago/Turabian StyleYano, Shinya, Naozumi Teramoto, Toshiaki Shimasaki, and Mitsuhiro Shibata. 2016. "Photocrosslinkable Trehalose Derivatives Carrying Mesogenic Groups: Synthesis, Characterization, and in Vitro Evaluation for Fibroblast Attachment" Journal of Functional Biomaterials 7, no. 3: 24. https://doi.org/10.3390/jfb7030024





