Glass Polyalkenoate Cements Designed for Cranioplasty Applications: An Evaluation of Their Physical and Mechanical Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Glass Synthesis
Polyacrylic Acids (PAA)
2.2. Glass Characterization
2.2.1. Network Connectivity (NC)
2.2.2. Powder X-ray Diffraction (XRD)
2.2.3. Particle Size Analysis (PSA)
2.3. Cement Characterization
2.3.1. Cement Preparation
2.3.2. Handling Characteristics (Tw and Ts)
2.4. Scanning Electron Microscopy and Energy Dispersive X-ray Analysis (SEM-EDX)
2.5. Mechanical Properties
2.5.1. Compressive Strength
2.5.2. Biaxial Flexural Strength
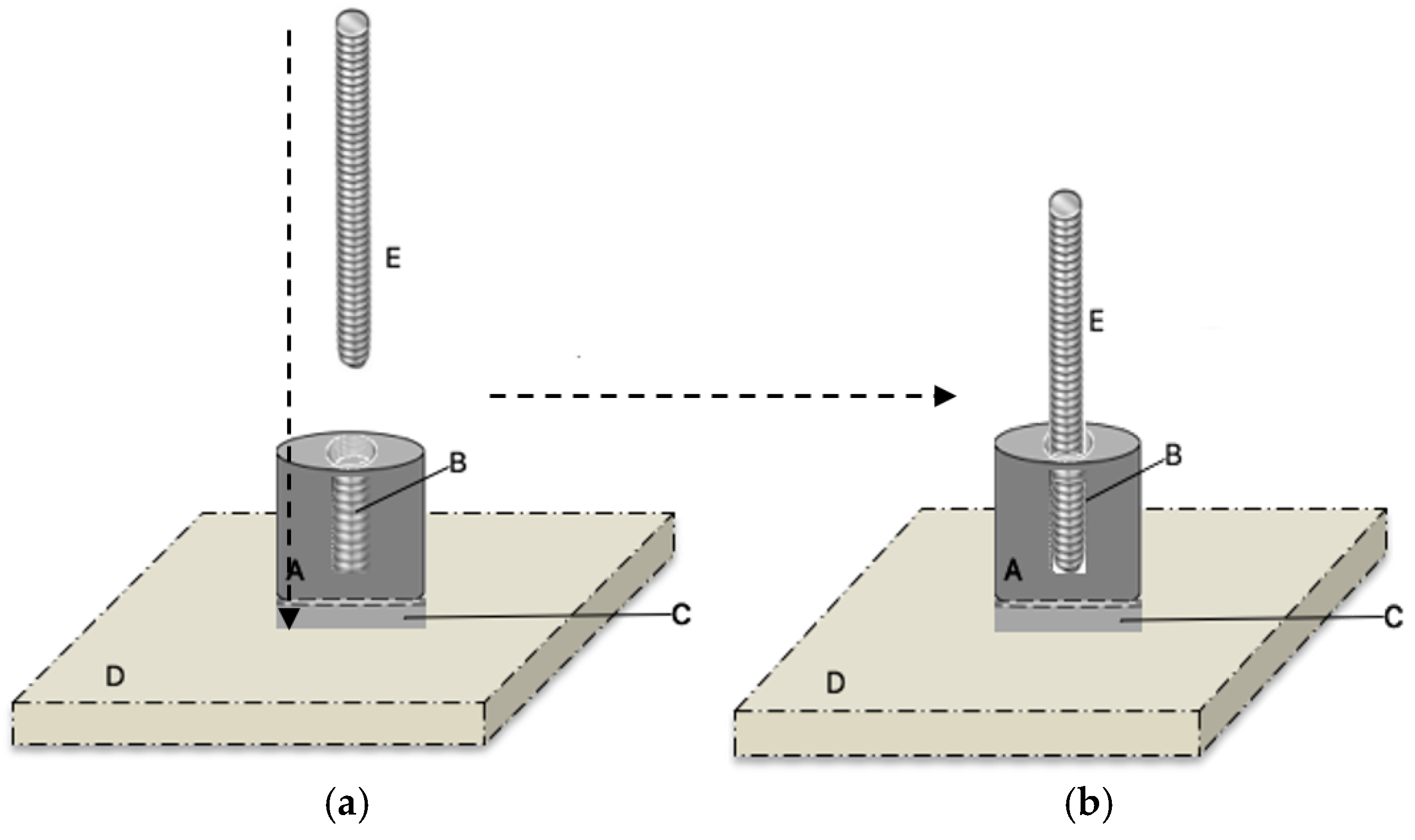
2.6. Adhesive Properties
2.6.1. Sample Preparation
2.6.2. Adhesive Test
2.7. Ion-Release
2.8. Micro-CT Analysis
2.9. Statistical Analysis
3. Results and Discussion
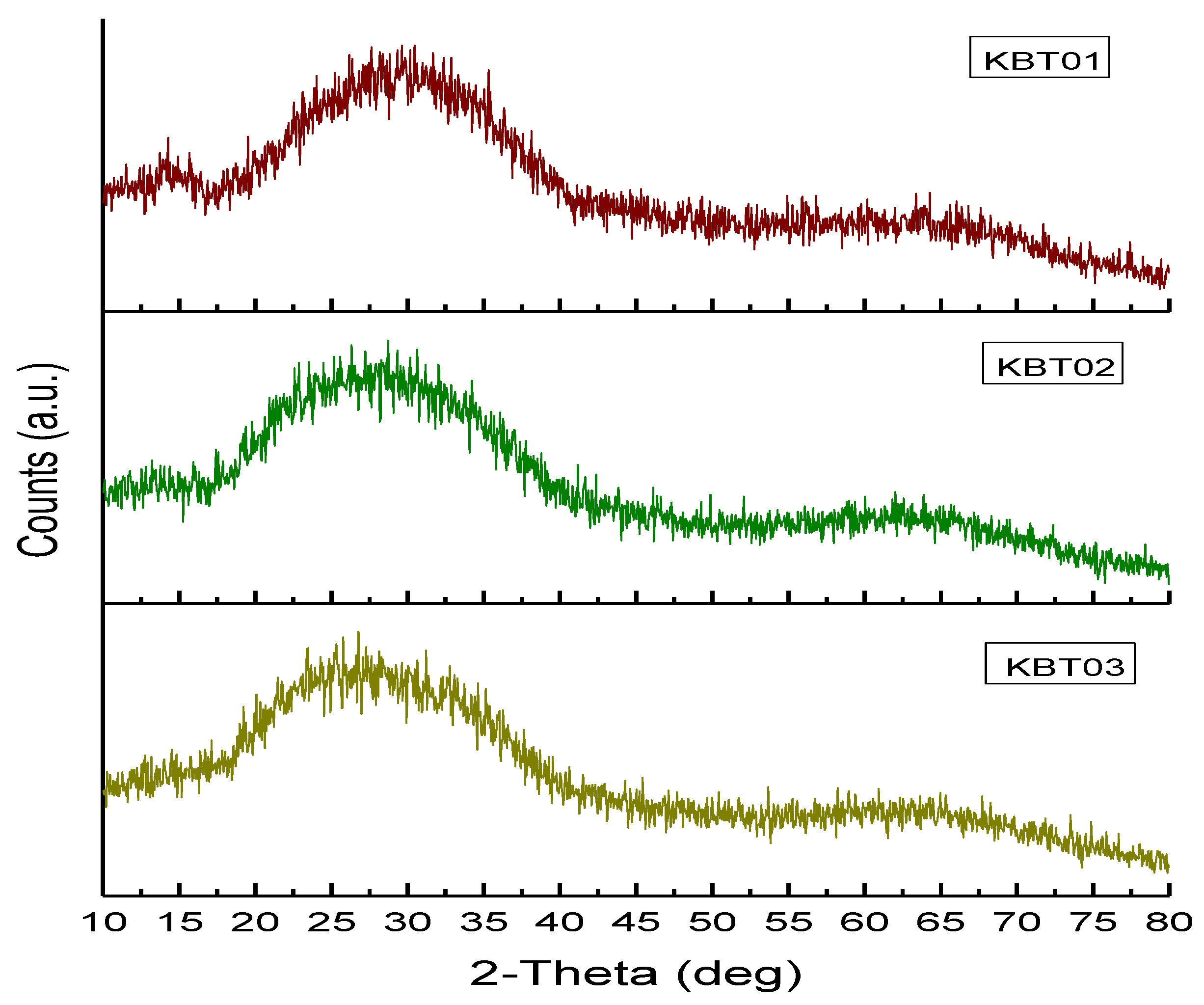
3.1. Glass Characterization
X-ray Diffraction (XRD)
3.2. Particle Size Distribution Analysis (PSA)
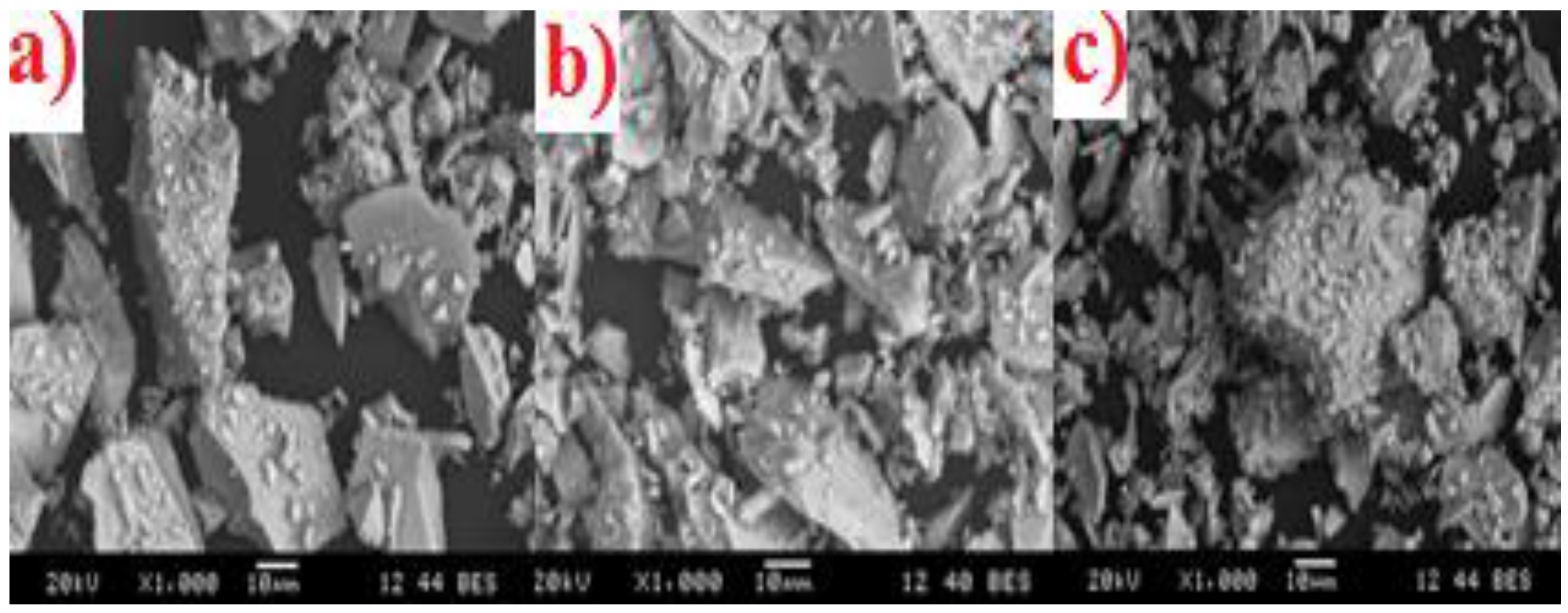
3.3. Scanning Electron Microscopy and Energy Dispersive X-ray Analysis (SEM-EDX)
3.4. Calculation of Network Connectivity
3.5. Cement Handling Characteristics (Tw and Ts)
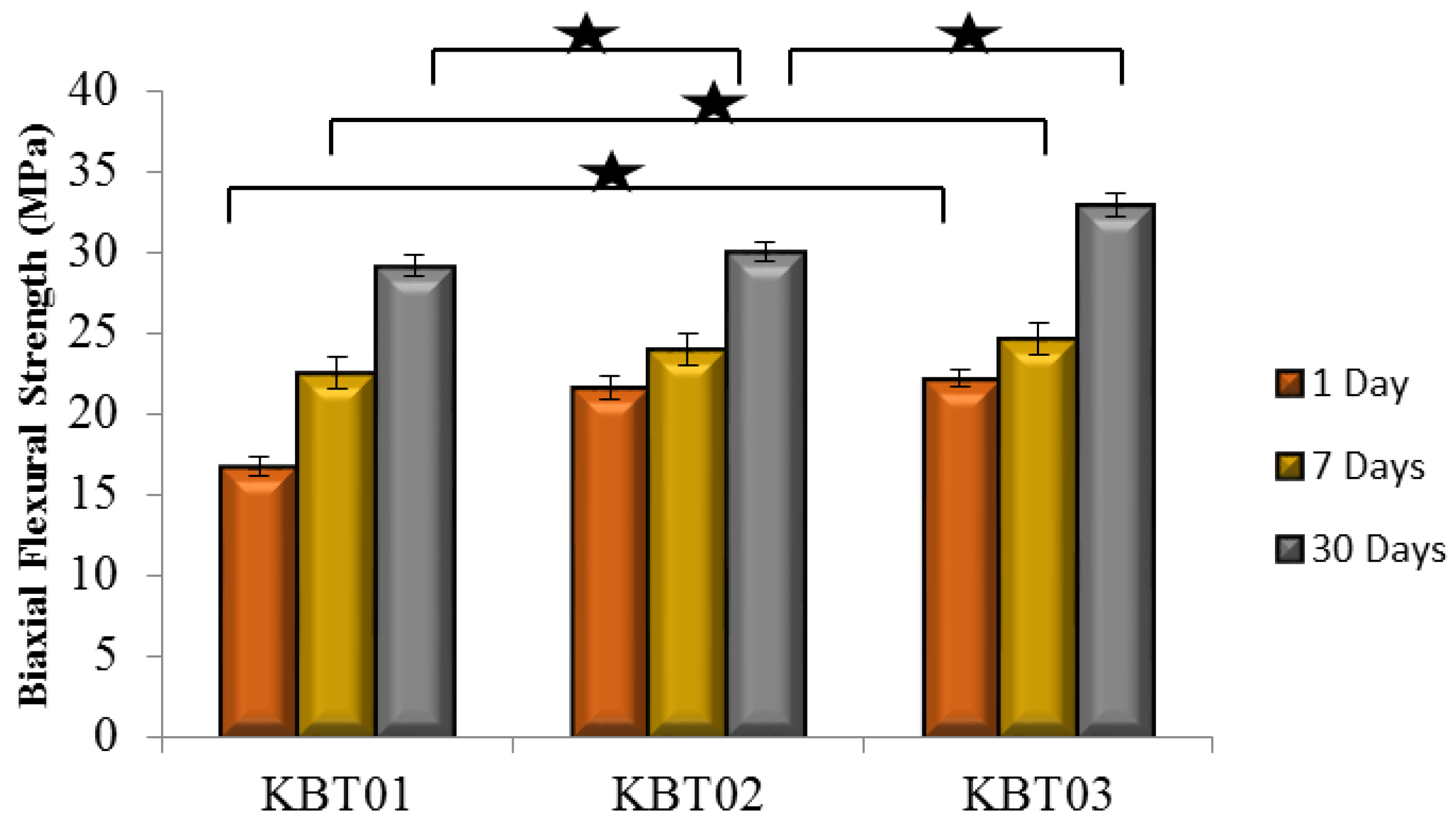
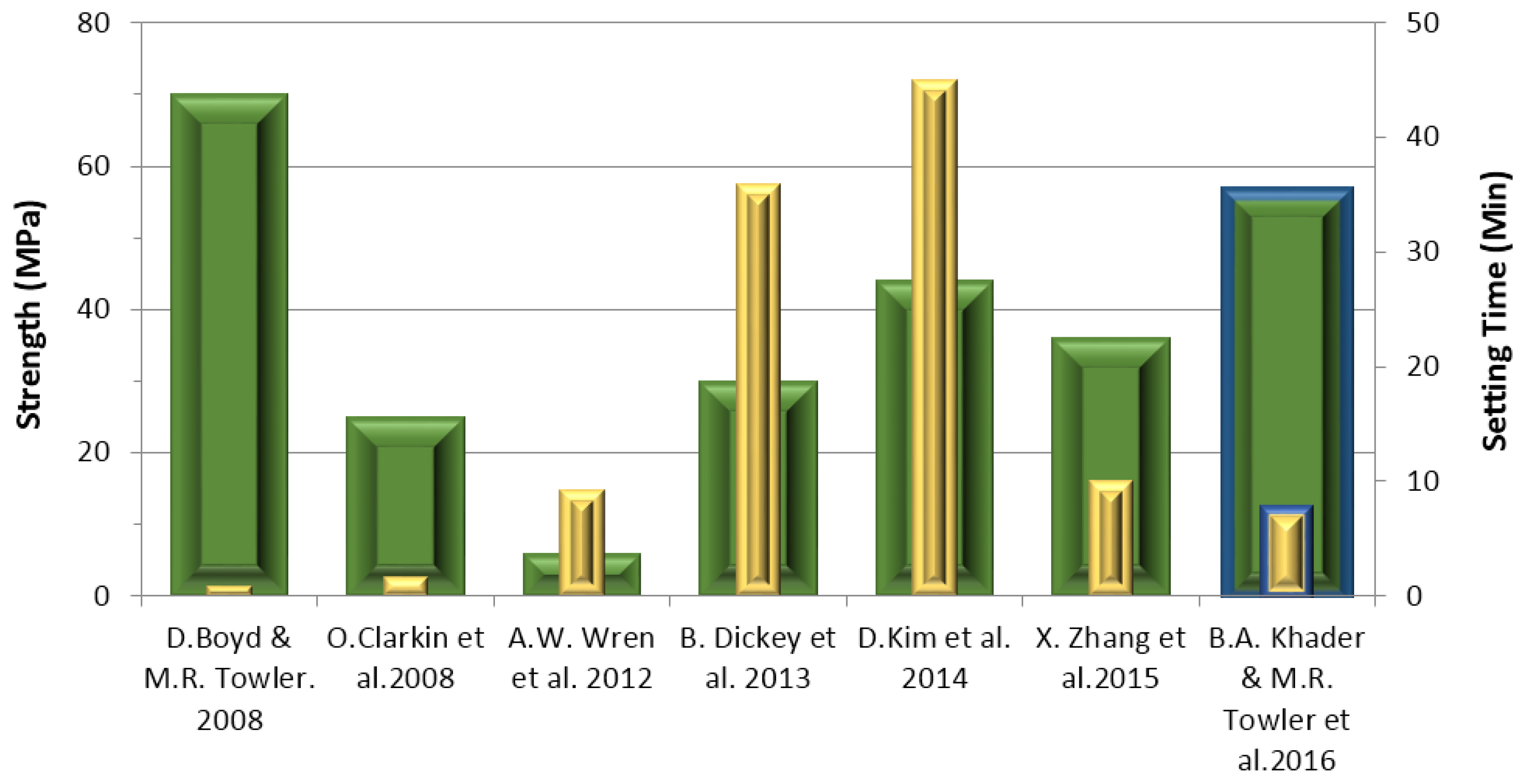
3.6. Compressive (σc) and Flexural (σf) Strengths
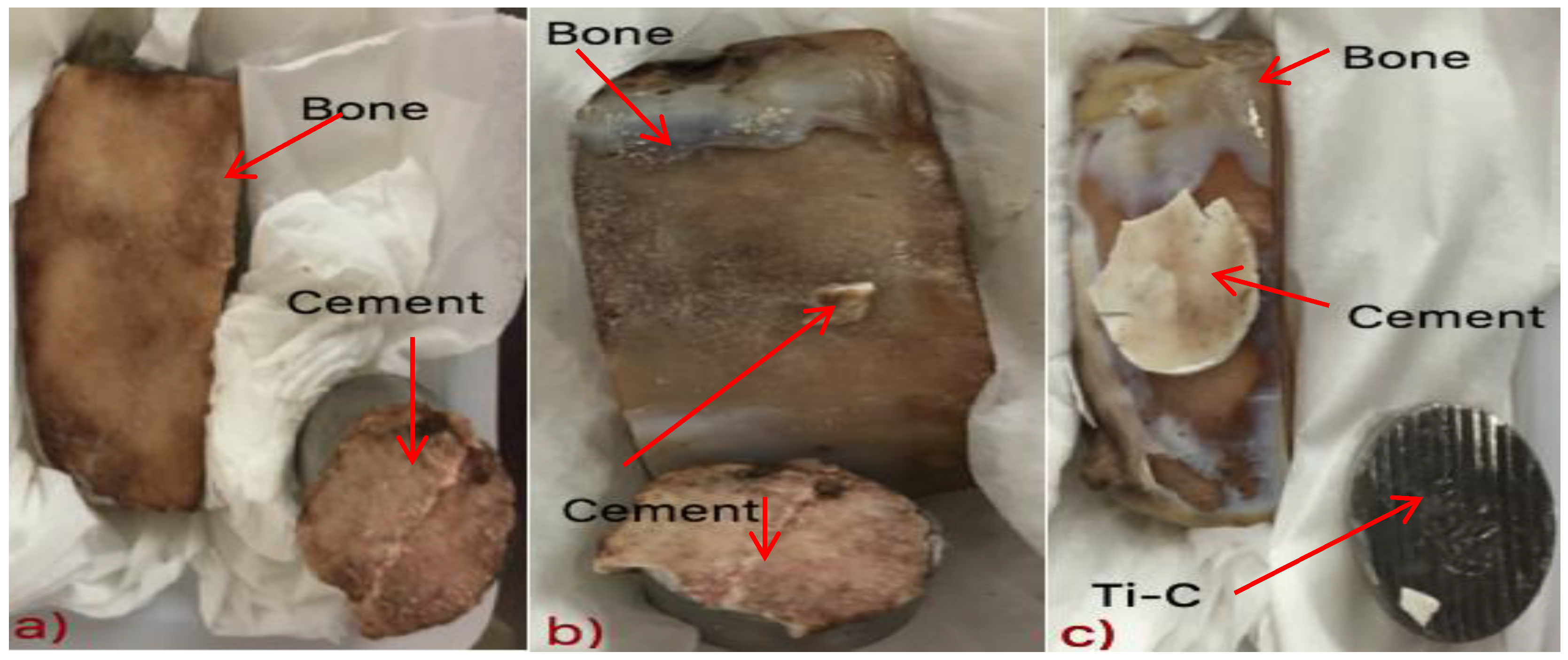
3.7. Tensile Bonding Test
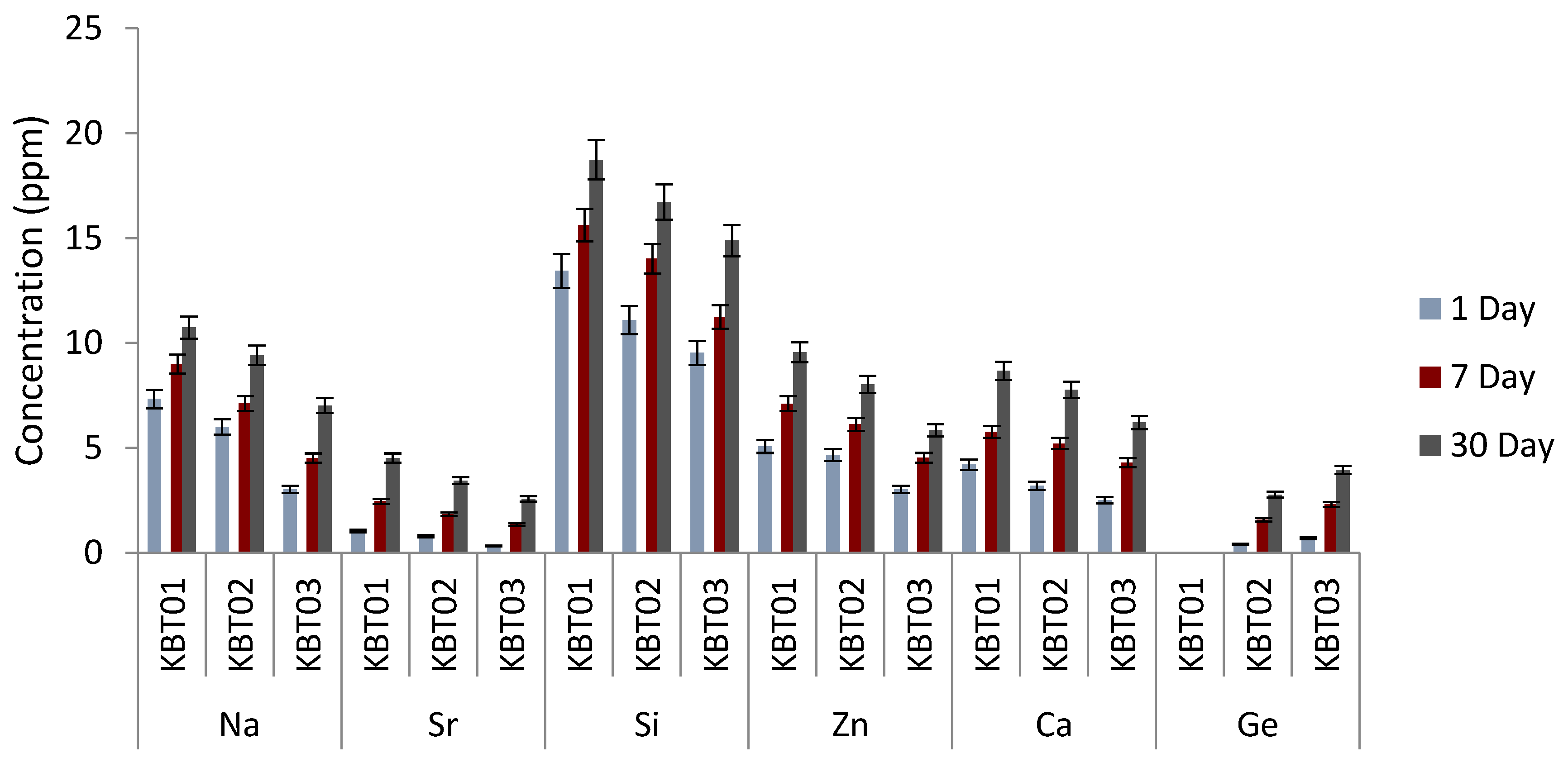
3.8. Ion Release
3.9. Radiopacity (X-ray)—MicroCT
4. Conclusions
5. Limitations of the Study
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Beals, S.P.; Munro, I.R. The use of miniplates in craniomaxillofacial surgery. Plast. Reconstr. Surg. 1987, 79, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Simpson, D. Titanium in cranioplasty. J. Neurosurg. 1965, 22, 292–293. [Google Scholar] [CrossRef] [PubMed]
- Ono, I.; Suda, K.; Tateshita, T.; Gunji, H.; Kaneko, F. Analysis of strength and bone conduction of hydroxyapatite ceramics. J. Jpn. Plast. Reconstr. Surg. 1993, 13, 561–571. [Google Scholar]
- Ono, I.; Gunji, H.; Suda, K.; Kaneko, F.; Yago, K. Orbital reconstruction with hydroxyapatite ceramic implants. Scand. J. Plast. Reconstr. Hand Surg. 1994, 28, 193–198. [Google Scholar] [CrossRef]
- Tanaka, Y. Development of titanium fixation screw for hydroxyapatite osteosynthesis (APACERAM). Surg. Neurol. 2008, 70, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Boyd, D. Zinc-Based Glass Polyalkenoate Cements for Skeletal Applications. PhD. Thesis, College of Engineering, University of Limerick, Limerick, Ireland, 2005. [Google Scholar]
- Cho, Y.R.; Gosain, A.K. Biomaterials in craniofacial reconstruction. Clin. Plast. Surg. 2004, 31, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Berry, J.P. Surface characteristics of fractured poly (methyl methacrylate). Nature 1960, 185, 91–92. [Google Scholar] [CrossRef]
- James, S.P.; Jasty, M.; Davies, J.; Piehler, H.; Harris, W.H. A fractographic investigation of PMMA bone cement focusing on the relationship between porosity reduction and increased fatigue life. J. Biomed. Mater. Res. 1992, 26, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Kricun, R.; Chovanes, G.I.; Shoemaker, E.I. CT and MR appearance of cervical acrylic struts. J. Comput. Assist. Tomogr. 1991, 15, 519–521. [Google Scholar] [CrossRef] [PubMed]
- Remsen, K.; Lawson, W.; Biller, H.F. Acrylic frontal cranioplasty. Head Neck Surg. 1986, 9, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Persing, J.A.; Cronin, A.J.; Delashaw, J.B.; Edgerton, M.T.; Henson, S.L.; Jane, J.A. Late surgical treatment of unilateral coronal synostosis using methyl methacrylate. J. Neurosurg. 1987, 66, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Wren, A.; Boyd, D.; Towler, M.R. The processing, mechanical properties and bioactivity of strontium based glass polyalkenoate cements. J. Mater. Sci. Mater. Med. 2008, 19, 1737–1743. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.; Manson, P.N. Rigid mesh fixation for alloplastic cranioplasty. J. Craniofac. Surg. 1994, 5, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Replogle, R.E.; Lanzino, G.; Francel, P.; Henson, S.; Lin, K.; Jane, J.A. Acrylic cranioplasty using miniplate struts. Neurosurgery 1996, 39, 747–749. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, K.J.; Hicks, W.L., Jr.; Guterman, L.R. A technique for rigid fixation of methyl methacrylat cranioplasty: The vault-locking method. Surg. Neurol. 1999, 52, 310–315. [Google Scholar] [CrossRef]
- Raja, A.I.; Linskey, M.E. In situ cranioplasty with methylmethacrylate and wire lattice. Br. J. Neurosurg. 2005, 19, 416–419. [Google Scholar] [CrossRef] [PubMed]
- Blum, K.S.; Schneider, S.J.; Rosenthal, A.D. Methyl methacrylate cranioplasty in children: Long-term results. Pediatr. Neurosurg. 1997, 26, 33–35. [Google Scholar] [CrossRef] [PubMed]
- De Santis, R.; Gloria, A.; Ambrosio, L. Materials and Technologies for Craniofacial Tissue Repair and Regeneration. Top. Med. 2010, 16, 1–6. [Google Scholar]
- Donkerwolcke, M.; Burny, F.; Muster, D. Tissues and bone adhesives-historical aspects. Biomaterials 1988, 19, 1461–1466. [Google Scholar] [CrossRef]
- Towler, M.R.; Kenny, S.; Boyd, D.; Pembroke, T.; Buggy, M.; Hill, R.G. Zinc ion release from novel hard tissue biomaterials. Biomed. Mater. Eng. 2004, 14, 565–572. [Google Scholar] [PubMed]
- Endotherapeutics: Serenocem Ear Cement and Granules. Available online: http://www.endotherapeutics.com.au/serenocem (accessed on 5 February 2016).
- Invotec International®: Serenocem™ Ostologic Cement. Available online: http://www.mundinc.com/uploads/serenocem_otologic_cement.pdf (accessed on 5 February 2016).
- Wren, A.W.; Kidari, A.; Cummins, N.M.; Towler, M.R. A spectroscopic investigation into the setting and mechanical properties of titanium containing glass polyalkenoate cements. J. Mater. Sci. Mater. Med. 2010, 21, 2355–2364. [Google Scholar] [CrossRef] [PubMed]
- Sawai, J. Quantitative evaluation of antibacterial activities of metallic oxide powders (ZnO, MgO and CaO) by conductimetric assay. J. Microbiol. Methods 2003, 54, 177–182. [Google Scholar] [CrossRef]
- Catelan, A.; Padilha, A.C.; Salzedas, L.M.; Coclete, G.A.; dos Santos, P.H. Effect of Radiotherapy on the Radiopacity and Flexural Strength of a Composite Resin. Acta Odontol. Latinoam. 2008, 21, 159–162. [Google Scholar] [PubMed]
- Boyd, D.; Towler, M.R.; Watts, S.; Hill, R.G.; Wren, A.W.; Clarkin, O.M. The role of Sr2+ on the structure and reactivity of SrO–CaO–ZnO–SiO2 ionomer glasses. J. Mater. Sci. Mater. Med. 2008, 19, 953–957. [Google Scholar] [CrossRef] [PubMed]
- Clarkin, O.; Boyd, D.; Towler, M.R. Comparison of failure mechanisms for cements used in skeletal luting applications. J. Mater. Sci. Mater. Med. 2009, 20, 1585–1594. [Google Scholar] [CrossRef] [PubMed]
- Varshneya, A.K. Fundamentals of Inorganic Glasses, 1st ed.; Gulf Professional Publishing Academic Press: Boston, MA, USA, 1994. [Google Scholar]
- Dickey, B.; Kehoe, S.; Boyd, D. Novel adaptations to zinc-silicate glass polyalkenoate cements: The unexpected influences of germanium based glasses on handling characteristics and mechanical properties. J. Mech. Behav. Biomed. Mater. 2013, 23, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Dickey, B.; Saffary, J.; Dickinson, V.; Kehoe, S.; Abraham, R.J.; Boyd, D. Development and evaluation of an inherently radiopaque, adhesive bone cement for vertebroplasty. J. Vasc. Interv. Radiol. 2013, 4, S33. [Google Scholar] [CrossRef]
- Dickey, B.; Lee, A.; Zhang, X.; Boyd, D. The effect of composition and annealing on the properties of aluminum free GPCs: A preliminary evaluation. Funct. Mater. Lett. 2014, 129, 191–194. [Google Scholar] [CrossRef]
- Zhang, X.; Werner-Zwanziger, U.; Boyd, D. Composition-structure-property relationships for non-classical ionomer cements formulated with zinc-boron germanium-based glasses. J. Biomater. Appl. 2015, 29, 1203–1217. [Google Scholar] [CrossRef] [PubMed]
- Boyd, D.; Clarkin, O.M.; Wren, A.W.; Towler, M.R. Zinc-based glass polyalkenoate cements with improved setting times and mechanical properties. Acta Biomater. 2008, 4, 425–431. [Google Scholar] [CrossRef] [PubMed]
- ISO 9917-1 Dentistry-Water-Based Cements-Part 1: Powder/Liquid Acid-Base Cements; ISO: Geneva, Switzerland, 2007.
- ISO 9917 Dentistry-Water-Based Cements-Part 1: Powder/Liquid Acid-Base Cements; ISO: Geneva, Switzerland, 1991.
- Williams, J.A.; Billington, R.W.; Pearson, G.J. The effect of the disc support system on biaxial tensile strength of a glass ionomer cement. Dent. Mater. 2002, 18, 376–379. [Google Scholar] [CrossRef]
- ASTM B348-13 Standard Specification for Titanium and Titanium Alloy Bars and Billets; ASTM International: West Conshohocken, PA, USA, 2013.
- ASTM B265-15 Standard Specification for Titanium and Titanium Alloy Strip, Sheet, and Plate; ASTM International: West Conshohocken, PA, USA, 2015.
- Prentice, L.H.; Tyas, M.J.; Burrow, M.F. The effect of mixing time on the handling and compressive strength of an encapsulated glass- ionomer cement. Dent. Mater. 2005, 21, 704–708. [Google Scholar] [CrossRef] [PubMed]
- Wren, A.W.; Hansen, J.P.; Hayakawa, S.; Towler, M.R. Aluminium-free glass polyalkenoate cements: Ion release and in vitro antibacterial efficacy. J. Mater. Sci. Mater. Med. 2013, 24, 1167–1178. [Google Scholar] [CrossRef] [PubMed]
- Broaddus, W.C.; Holloway, K.L.; Winters, C.J.; Bullock, M.R.; Graham, R.S.; Mathern, B.E.; Ward, J.D.; Young, H.F. Titanium miniplates or stainless steel wire for cranial fixation: A prospective randomized comparison. J. Neurosurg. 2002, 96, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Boyd, D.; Towler, M.R. The Processing Mechanical Properties and Bioactivity of Zinc Based Glass ionomer Cements. J. Mater. Sci. Mater. Med. 2005, 16, 843–850. [Google Scholar] [CrossRef] [PubMed]
- DeBarra, E.; Hill, R.G. Influence of alkali metal ions on the fracture properties of glass polyalkenoate (ionomer) cements. Biomaterials 1998, 19, 495–502. [Google Scholar] [CrossRef]
- Clarkin, O.; Boyd, D.; Towler, M.R. Strontium-based Glass Polyalkenoate Cements for Luting Applications in the Skeleton. J. Biomater. Appl. 2010, 24, 483–502. [Google Scholar] [CrossRef] [PubMed]
- Wren, A.W.; Coughlan, A.; Placek, L.; Towler, M.R. Gallium containing glass polyalkenoate anti-cancerous bone cements: Glass characterization and physical properties. J. Mater. Sci. Mater. Med. 2012, 23, 1823–1833. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.D.; Nicholson, J.W. Acid-Base Cements—Their Biomedical and Industrial Applications (Chemistry of Solid State Materials); West, A.R., Baxter, H., Eds.; Cambridge University Press: Cambridge, UK, 1993. [Google Scholar]
- Hill, R.G. The fracture properties of glass polyalkenoate cements as a function of cement age. J. Mater. Sci. 1993, 28, 3851–3858. [Google Scholar] [CrossRef]
- Barkema, G.T.; Panja, D.; van Leeuwen, J.M.J. Structural modes of a polymer in the repton model. J. Chem. Phys. 2011, 134, 154901. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, M. Discretized model of entangled-polymer dynamics. Phys. Rev. Lett. 1987, 59, 1946–1949. [Google Scholar] [CrossRef] [PubMed]
- McLeish, T.C.B. Tube theory of entangled polymer dynamics. Adv. Phys. 2002, 51, 1379–1527. [Google Scholar] [CrossRef]
- ASTM F640-12: 2012 Standard Test Methods for Determining Radiopacity for Medical Use; ASTM International: West Conshohocken, PA, USA, 2012.
- Radiology Masterclass: Basics of X-ray Physics. Available online: http://www.radiologymasterclass.co.uk/tutorials/physics/X-ray_physics_densities.html (accessed on 14 June 2015).
- Kim, D.A.; Abo-Mosallam, H.A.; Lee, H.Y.; Kim, G.R.; Kim, H.W.; Lee, H.H. Development of a novel aluminum-free glass ionomer cement based on magnesium/strontium-silicate glasses. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 42, 665–671. [Google Scholar] [CrossRef] [PubMed]
- ISO 5833:1992 Implants for Surgery Acrylic Resin Cements; ISO: Geneva, Switzerland, 1992.





| Nomenclature | SiO2 | CaO | ZnO | Na2O | GeO2 |
|---|---|---|---|---|---|
| KBT01 | 0.50 | 0.10 | 0.30 | 0.10 | 0 |
| KBT02 | 0.50 | 0.10 | 0.27 | 0.10 | 0.03 |
| KBT03 | 0.50 | 0.10 | 0.24 | 0.10 | 0.06 |
| Parameters | Si | Zn | Ca | Na | Sr | Ge |
|---|---|---|---|---|---|---|
| Lamp current (mA) | 5 | 5 | 10 | 5 | 10 | 10 |
| Wavelength (nm) | 251.6 | 213.9 | 239.9 | 330.2 | 460.7 | 265.16 |
| Composition | KBT01 | KBT02 | KBT03 |
|---|---|---|---|
| O | 44.5 | 48.1 | 44.1 |
| Si | 18.3 | 17.7 | 18.2 |
| Ca | 2.8 | 2.5 | 2.8 |
| Zn | 26.5 | 22.1 | 22.1 |
| Na | 7.9 | 7.5 | 7.6 |
| Ge | - | 2.1 | 5.2 |
| Nomenclature | SiO2 Backbone (mol %) | ||||||
|---|---|---|---|---|---|---|---|
| SiO2 (NF) | CaO (NM) | ZnO (NM) | Na2O (NM) | SrO (NM) | GeO2 (NF) | NC | |
| KBT01 | 0.50 | 0.10 | 0.30 | 0.10 | 0.00 | 0.00 | 2.0 |
| KBT02 | 0.50 | 0.10 | 0.27 | 0.10 | 0.00 | 0.03 | 2.2 |
| KBT03 | 0.50 | 0.10 | 0.24 | 0.10 | 0.00 | 0.06 | 2.4 |
| BT101 | 0.48 | 0.12 | 0.36 | 0.00 | 0.04 | 0.00 | 1.83 |
| Composition | XRD | TW/TS (s) | σc (MPa) (Min-Max) | σf (MPa) (Min-Max) | Ti*-Bone (MPa) | Radiopacity (Density g/cm2) |
|---|---|---|---|---|---|---|
| Analysis | p < 0.05 | p < 0.05 | p < 0.05 | (Min-Max) | ||
| KBT01 | Amorphous | 141/806 | 27–42 | 17–29 | 0.34–0.56 | 1.35 |
| KBT02 | Amorphous | 123/583 | 29–51 | 21–30 | 0.45–0.72 | 1.43 |
| KBT03 | Amorphous | 112/448 | 31–56 | 22–33 | 0.53–0.86 | 1.57 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khader, B.A.; Curran, D.J.; Peel, S.; Towler, M.R. Glass Polyalkenoate Cements Designed for Cranioplasty Applications: An Evaluation of Their Physical and Mechanical Properties. J. Funct. Biomater. 2016, 7, 8. https://doi.org/10.3390/jfb7020008
Khader BA, Curran DJ, Peel S, Towler MR. Glass Polyalkenoate Cements Designed for Cranioplasty Applications: An Evaluation of Their Physical and Mechanical Properties. Journal of Functional Biomaterials. 2016; 7(2):8. https://doi.org/10.3390/jfb7020008
Chicago/Turabian StyleKhader, Basel A., Declan J. Curran, Sean Peel, and Mark R. Towler. 2016. "Glass Polyalkenoate Cements Designed for Cranioplasty Applications: An Evaluation of Their Physical and Mechanical Properties" Journal of Functional Biomaterials 7, no. 2: 8. https://doi.org/10.3390/jfb7020008






