Mineralization at Titanium Surfaces is a Two-Step Process
Abstract
:1. Introduction
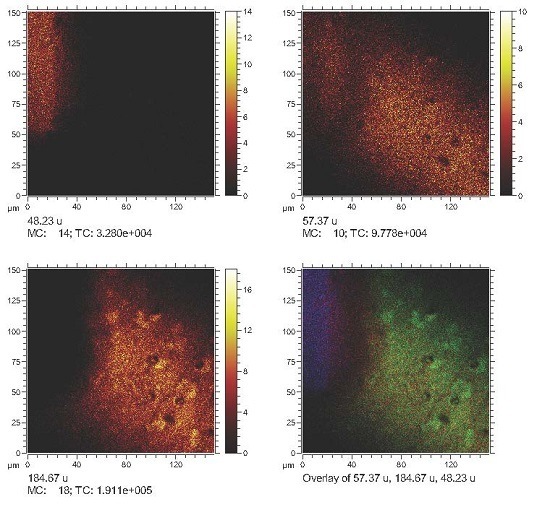
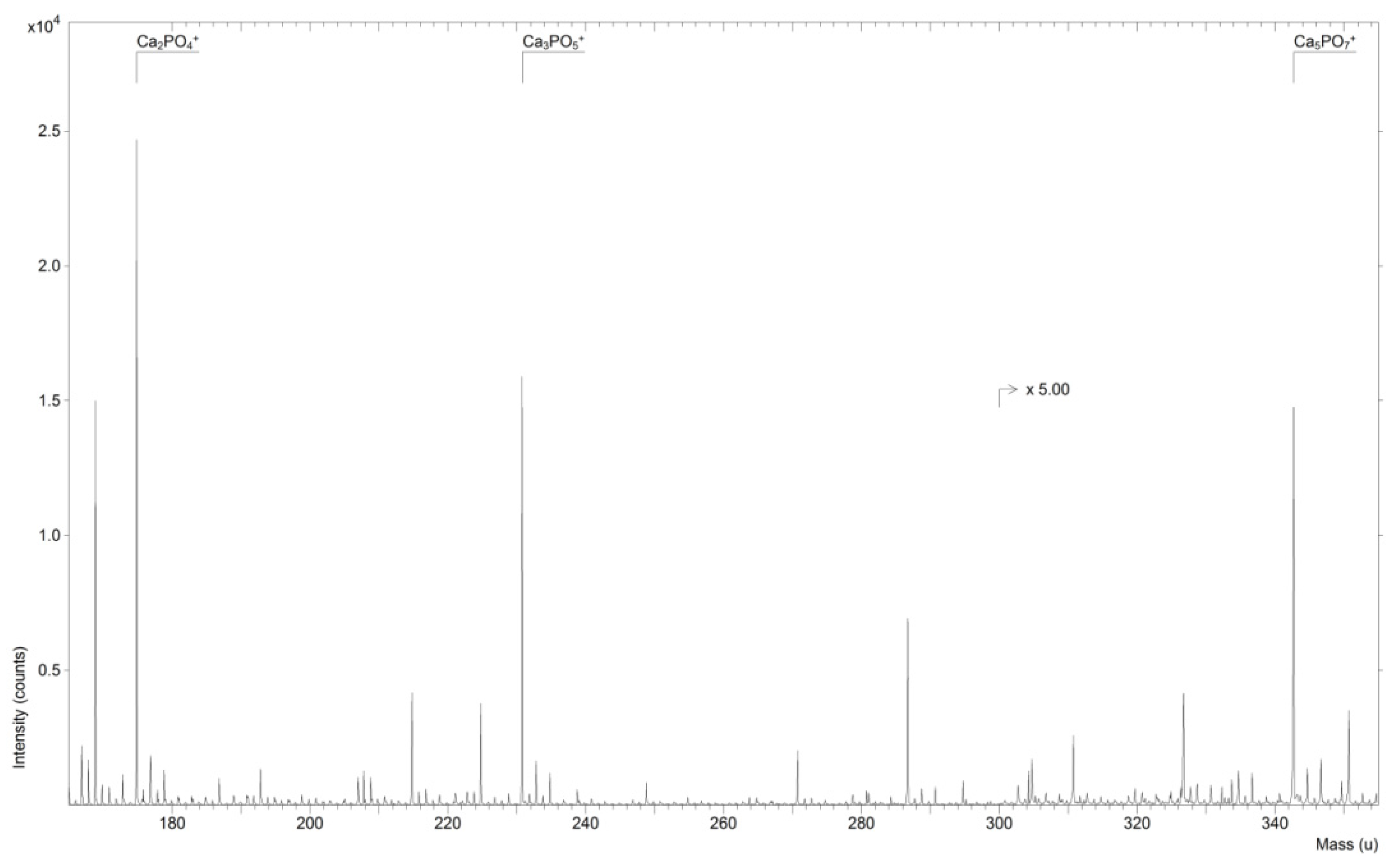
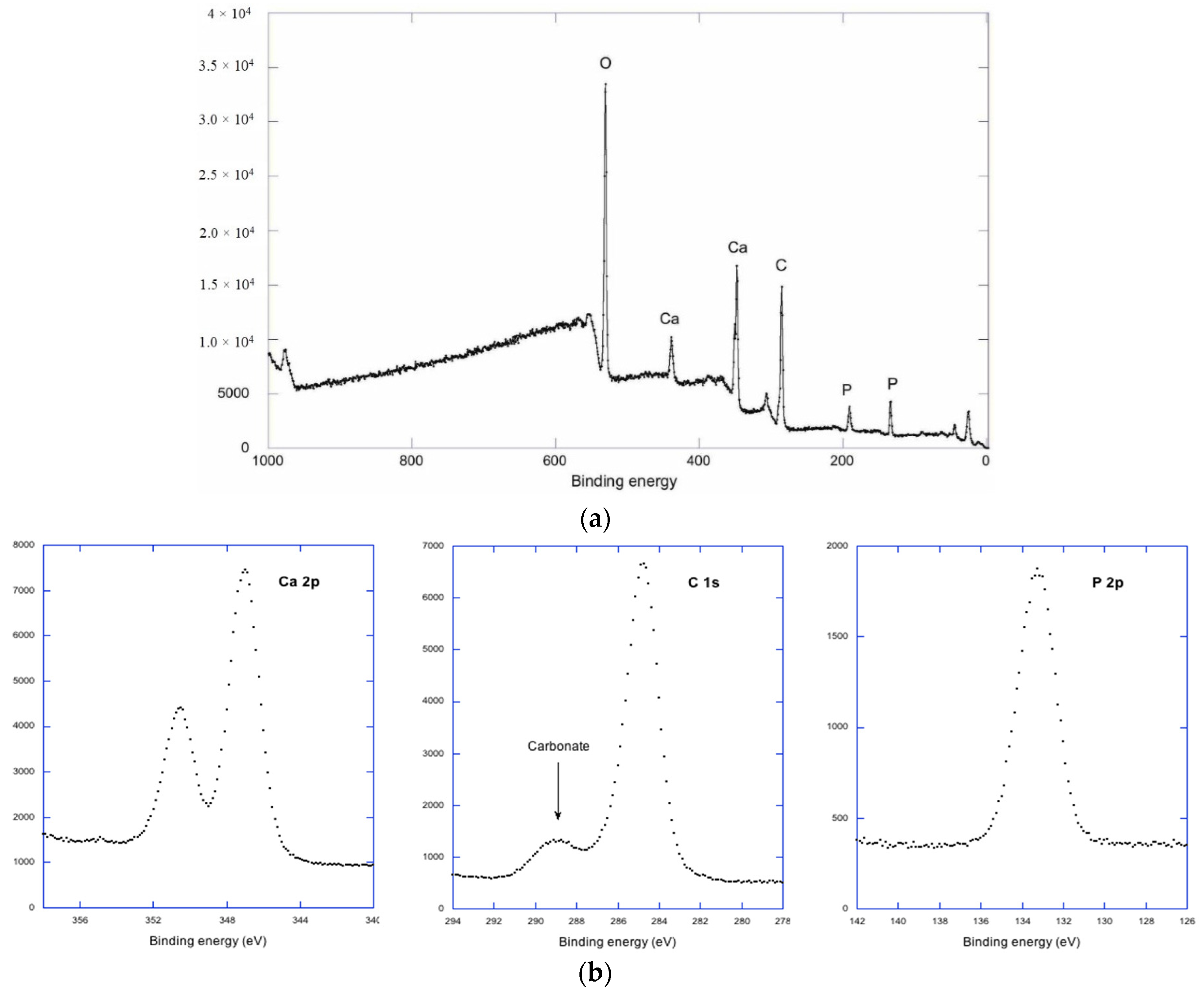
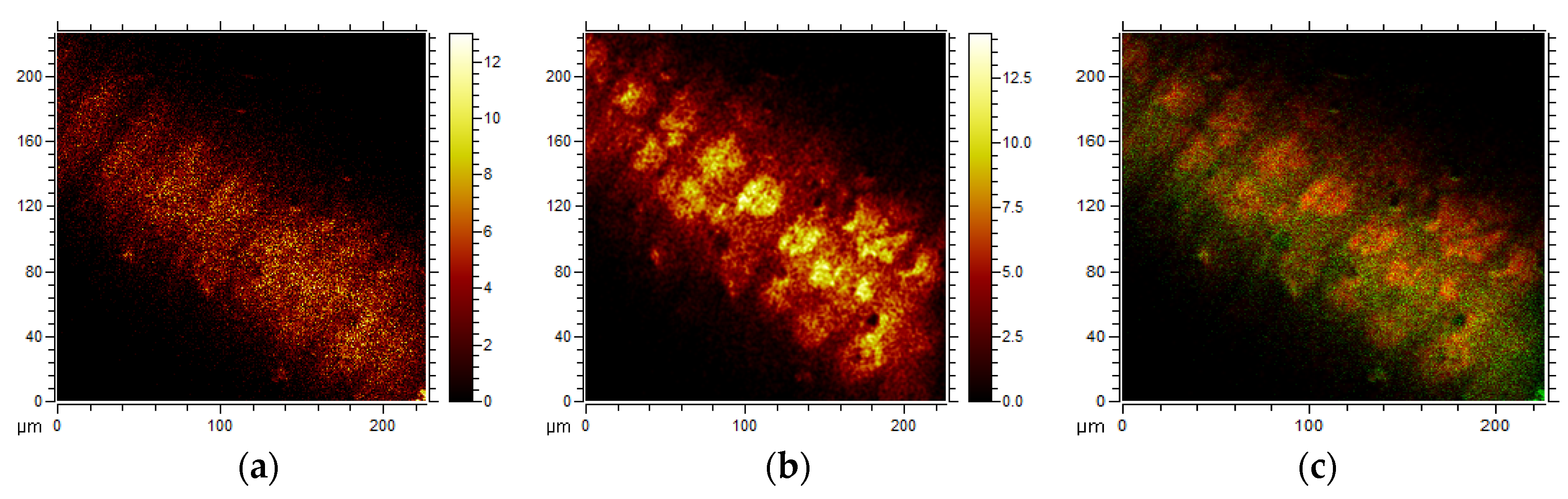
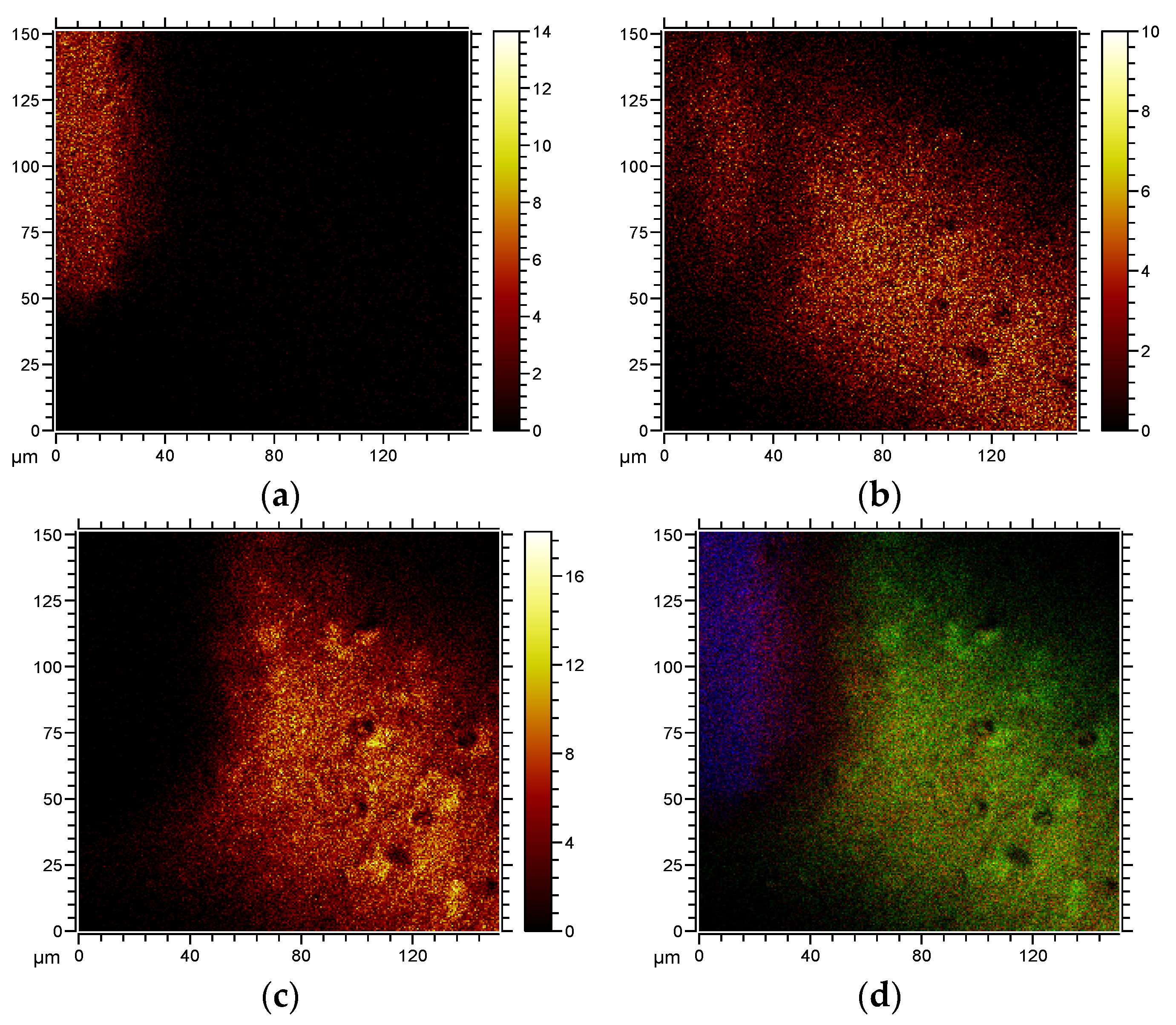
2. Results and Discussion
3. Experimental Section
Environmental Scanning Electron Microscopy and EDX
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Linder, L.; Albrektsson, T.; Brånemark, P.-I.; Hansson, H.-A.; Ivarsson, B.; Jönsson, U.; Lundström, I. Electron microscopic analysis of bone-titanium interface. Acta Orthop. Scand. 1983, 54, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.; Lowenberg, B.; Shiga, A. The bone-titanium interface in vitro. J. Biomed. Mater. Res. 1990, 24, 1289–1306. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.Q.; Nakamura, T.; Kawanabe, K.; Nishigochi, S.; Oka, M.; Kokubo, T. Apatite layer-coated titanium for use as bone bonding implants. Biomaterials 1997, 18, 1185–1190. [Google Scholar] [CrossRef]
- Peltola, T.; Patsi, M.; Rahiala, H.; Kangasniemi, I.; Yli-Urpo, A. Calcium phosphate induction by sol-gel-derived titania coatings on titanium substrates in vitro. J. Biomed. Mater. Res. 1998, 41, 504–510. [Google Scholar] [CrossRef]
- Li, P.J.; Kangasniemi, I.; de Groot, K.; Kokubo, T. Bonelike hydroxyapatite induction by gel-derived titania on a titanium substrate. J. Am. Ceram. Soc. 1994, 77, 1307–1312. [Google Scholar] [CrossRef]
- Michel, J.; Penna, M.; Kochen, J.; Cheung, H. Recent advances in hydroxyapatite scaffolds containing mesenchymal stem cells. Stem Cells Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.R.; Bai, J.; Yuan, S.J.; Yu, C.X.; Huang, J.; Zhang, T.L.; Wang, K. Calcium phosphate nanoparticles are associated with inorganic phosphate-induced osteogenic differentiation of rat bone marrow stromal cells. Chem.-Biol. Interact. 2015, 238, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.C.; Yuan, L.J.; Lin, S.S.; Chen, L.H.; Chen, W.J. Mesenchymal stem cell and nucleus pulposus cell coculture modulates cell profile. Clin. Orthop Relat. Res. 2009, 467, 3263–3272. [Google Scholar] [CrossRef] [PubMed]
- Nagai, H.; Kobayashi-Fujioka, M.; Fujisawa, K.; Ohe, G.; Takamuru, N.; Hara, K.; Uchida, D.; Tamatani, T.; Ishikawa, K.; Miyamoto, Y. Effect of low crystalline carbonate apatite on proliferation and osteoblastic differentiation of human bone marrow cells. J. Mater. Sci. Mater. Med. 2015, 26, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Malmberg, P.; Bexell, U.; Eriksson, C.; Nygren, H.; Richter, K. Analysis of bone minerals by time-of-flight secondary ion mass spectrometry: A comparative study using monoatomic and cluster ions sources. Rapid Commun. Mass Spectrom. 2007, 21, 745–749. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, C.; Malmberg, P.; Nygren, H. Time-of-flight secondary ion mass spectrometric analysis of the interface between bone and titanium implants. Rapid Commun. Mass Spectrom. 2008, 22, 943–949. [Google Scholar] [CrossRef] [PubMed]
- De Mayer, E.A.; Veerbeeck, R.M.; Pieters, I.Y. Effect of K+ on the stoichiometry of carbonated hydroxyapatite obtained by the hydrolysis of Monetite. Inorg. Chem. 1996, 35, 857–863. [Google Scholar] [CrossRef]
- Eriksson, C.; Borner, K.; Nygren, H.; Ohlson, K.; Bexell, U.; Billerdahl, N.; Johansson, M. Studies by imaging TOF-SIMS of bone mineralization on porous titanium implants after 1 week in bone. Appl. Surf. Sci. 2006, 252, 6757–6760. [Google Scholar] [CrossRef]
- Eriksson, C.; Broberg, M.; Nygren, H.; Oster, L. Novel in vivo method for evaluation of healing around implants in bone. J. Biomed. Mater. Res. Part A 2003, 66, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, C.; Nygren, H. Polymorphonuclear leukocytes in coagulating whole blood recognize hydrophilic and hydrophobic titanium surfaces by different adhesion receptors and show different patterns of receptor expression. J. Lab. Clin. Med. 2001, 137, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, C.; Nygren, H. Adhesion receptors of polymorphonuclear granulocytes on titanium in contact with whole blood. J. Lab. Clin. Med. 2001, 137, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, C.; Lausmaa, J.; Nygren, H. Interactions between human whole blood and modified TiO2-surfaces: Influence of surface topography and oxide thickness on leukocyte adhesion and activation. Biomaterials 2001, 22, 1987–1996. [Google Scholar] [CrossRef]
- Yahyapour, N.; Eriksson, C.; Malmberg, P.; Nygren, H. Thrombin, kallikrein and complement C5b-9 adsorption on hydrophilic and hydrophobic titanium and glass after short time exposure to blood. Biomaterials 2004, 25, 3171–3176. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, C.; Ohlson, K.; Nygren, H. Implantation of hydrophilic and hydrophobic titanium discs in rat tibia: Cellular reactions on the surfaces during the first 3 weeks in bone. Biomaterials 2004, 24, 4759–4766. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Salvi, G.E.; Offenbacher, S.; Felton, D.A.; Cooper, L.F. Cell and matrix reactions at titanium implants in surgically prepared rat tibiae. Int. J. Oral Maxillofac. Implant. 1997, 12, 472–485. [Google Scholar]
- De Bruijn, J.D.; Klein, C.P.; de Groot, K.; van Blitterswijk, C.A. The ultrastructure of the bone-hydroxyapatite interface in vitro. J. Biomed. Mater. Res. 1992, 26, 1365–1382. [Google Scholar] [CrossRef] [PubMed]
- Hunter, G.K.; Kyle, C.L.; Goldberg, H.A. Modulation of crystal formation by bone phosphoproteins: Structural specificity of the osteopontin-mediated inhibition of hydroxyapatite formation. Biochem. J. 1994, 300, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Ayukawa, Y.; Takeshita, F.; Inoue, T.; Yoshinari, M.; Shimono, M.; Suetsugu, T.; Tanaka, T. An immunoelectron microscopic localisation of non-collagenous bone proteins (osteocalcin and osteopontin) at the bone-titanium interface. J. Biomed. Mater. Res. 1998, 41, 111–119. [Google Scholar] [CrossRef]

| Spectrum | C | O | P | Ca | Ti | Total |
|---|---|---|---|---|---|---|
| Spectrum 1 | 4.73 | 14.30 | 0.40 | 0.49 | 80.08 | 100.00 |
| Spectrum 2 | 4.85 | 14.60 | 0.37 | 0.54 | 79.64 | 100.00 |
| Spectrum 3 | 4.96 | 14.38 | 0.38 | 0.55 | 79.73 | 100.00 |
| Spectrum 4 | 4.68 | 14.81 | 0.39 | 0.56 | 79.57 | 100.00 |
| Mean | 4.80 | 14.52 | 0.39 | 0.53 | 79.75 | 100.00 |
| Std. deviation | 0.13 | 0.23 | 0.01 | 0.03 | 0.22 | – |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nygren, H.; Ilver, L.; Malmberg, P. Mineralization at Titanium Surfaces is a Two-Step Process. J. Funct. Biomater. 2016, 7, 7. https://doi.org/10.3390/jfb7010007
Nygren H, Ilver L, Malmberg P. Mineralization at Titanium Surfaces is a Two-Step Process. Journal of Functional Biomaterials. 2016; 7(1):7. https://doi.org/10.3390/jfb7010007
Chicago/Turabian StyleNygren, Håkan, Lars Ilver, and Per Malmberg. 2016. "Mineralization at Titanium Surfaces is a Two-Step Process" Journal of Functional Biomaterials 7, no. 1: 7. https://doi.org/10.3390/jfb7010007