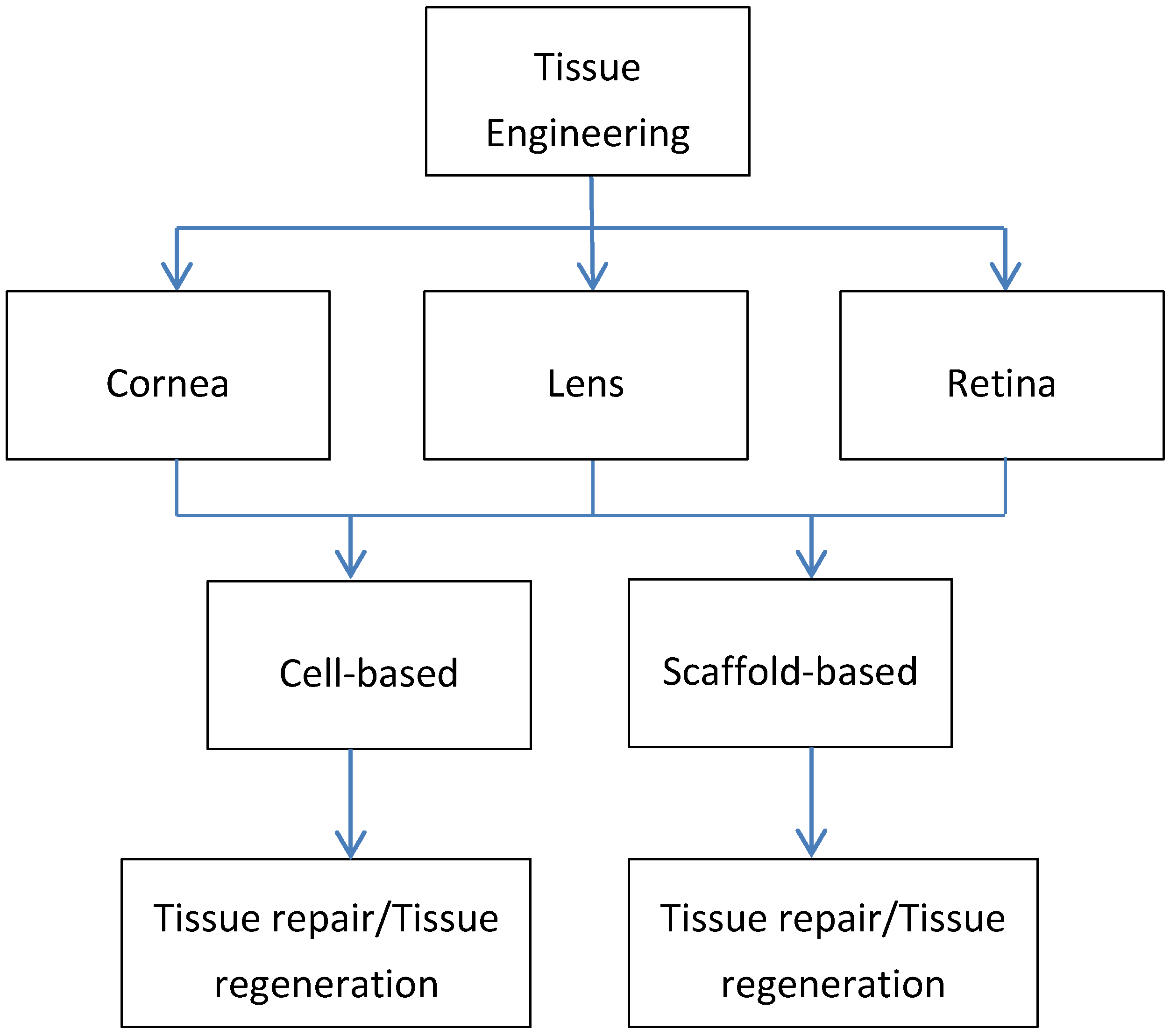
Ocular Tissue Engineering: Current and Future Directions
Conflict of Interest
Acknowledgements
References
- Sommer, F.; Brandl, F.; Gopferich, A. Ocular tissue engineering. Adv. Exp. Med. Biol. 2006, 585, 413–429. [Google Scholar]
- Pellegrini, G.; Traverso, C.E.; Franzi, A.T.; Zingirian, M.; Cancedda, R.; De Luca, M. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet 1997, 349, 990–993. [Google Scholar]
- Tsai, R.J.; Li, L.M.; Chen, J.K. Reconstruction of damaged corneas by transplantation of autologous limbal epithelial cells. New. Engl. J. Med. 2000, 343, 86–93. [Google Scholar]
- Schechter, B.A.; Rand, W.J.; Nagler, R.S.; Estrin, I.; Arnold, S.S.; Villate, N.; Velazquez, G.E. Corneal melt after amniotic membrane transplant. Cornea 2005, 24, 106–107. [Google Scholar]
- Kinoshita, S.; Nakamura, T. Development of cultivated mucosal epithelial sheet transplantation for ocular surface reconstruction. Artif. Organs 2004, 28, 22–27. [Google Scholar]
- Kinoshita, S.; Koizumi, N.; Nakamura, T. Transplantable cultivated mucosal epithelial sheet for ocular surface reconstruction. Exp. Eye Res. 2004, 78, 483–491. [Google Scholar]
- Francis, P.J.; Berry, V.; Moore, A.T.; Bhattacharya, S. Lens biology: Development and human cataractogenesis. Trends Genet. 1999, 15, 191–196. [Google Scholar]
- Apple, D.J.; Solomon, K.D.; Tetz, M.R.; Assia, E.I.; Holland, E.Y.; Legler, U.F.; Tsai, J.C.; Castaneda, V.E.; Hoggatt, J.P.; Kostick, A.M. Posterior capsule opacification. Surv. Ophthalmol. 1992, 37, 73–116. [Google Scholar]
- Tsonis, P.A.; Jang, W.; Del Rio-Tsonis, K.; Eguchi, G. A unique aged human retinal pigmented epithelial cell line useful for studying lens differentiation in vitro. Int. J. Dev. Biol. 2001, 45, 753–758. [Google Scholar]
- Algvere, P.V.; Berglin, L.; Gouras, P.; Sheng, Y.; Kopp, E.D. Transplantation of RPE in age-related macular degeneration: Observations in disciform lesions and dry RPE atrophy. Graefes. Arch. Clin. Exp. Ophthalmol. 1997, 235, 149–158. [Google Scholar]
- Algvere, P.V.; Gouras, P.; Dafgard Kopp, E. Long-term outcome of RPE allografts in non-immunosuppressed patients with AMD. Eur. J. Ophthalmol. 1999, 9, 217–230. [Google Scholar]
- Binder, S.; Krebs, I.; Hilgers, R.D.; Abri, A.; Stolba, U.; Assadoulina, A.; Kellner, L.; Stanzel, B.V.; Jahn, C.; Feichtinger, H. Outcome of transplantation of autologous retinal pigment epithelium in age-related macular degeneration: A prospective trial. Invest. Ophthalmol. Vis. Sci. 2004, 45, 4151–4160. [Google Scholar]
- Binder, S.; Stolba, U.; Krebs, I.; Kellner, L.; Jahn, C.; Feichtinger, H.; Povelka, M.; Frohner, U.; Kruger, A.; Hilgers, R.D.; et al. Transplantation of autologous retinal pigment epithelium in eyes with foveal neovascularization resulting from age-related macular degeneration: A pilot study. Am. J. Ophthalmol. 2002, 133, 215–225. [Google Scholar]
- Trese, M.; Regatieri, C.V.; Young, M.J. Advances in retinal tissue engineering. Materials 2012, 5, 108–120. [Google Scholar]
- Langer, R. Biomaterials in drug delivery and tissue engineering: One laboratory’s experience. Acc. Chem. Res. 2000, 33, 94–101. [Google Scholar]
- Thomson, H.A.; Treharne, A.J.; Walker, P.; Grossel, M.C.; Lotery, A.J. Optimisation of polymer scaffolds for retinal pigment epithelium (RPE) cell transplantation. Br. J. Ophthalmol. 2011, 95, 563–568. [Google Scholar]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karamichos, D. Ocular Tissue Engineering: Current and Future Directions. J. Funct. Biomater. 2015, 6, 77-80. https://doi.org/10.3390/jfb6010077
Karamichos D. Ocular Tissue Engineering: Current and Future Directions. Journal of Functional Biomaterials. 2015; 6(1):77-80. https://doi.org/10.3390/jfb6010077
Chicago/Turabian StyleKaramichos, D. 2015. "Ocular Tissue Engineering: Current and Future Directions" Journal of Functional Biomaterials 6, no. 1: 77-80. https://doi.org/10.3390/jfb6010077




